Abstract
This paper describes selective recovery of Au(III), Pd(II), and Ag(I) from waste printed circuit boards (WPCBs) by CFP-g-PHCTMA, an adsorbent based on cellulose filter paper (CFP) grafted with polymer chains bearing thiocarbamate moieties. As a preliminary study, the adsorption kinetics and the effect of acid concentration on the adsorption processes were studied in detail. The adsorption capacity for Au(III) and Pd(II) was not affected significantly by acid concentration, whereas that for Ag(I) was decreased under higher acid concentration. The adsorption proceeded smoothly, and reached equilibria for Pd(II) and Ag(I) within 90 min, and for Au(III) within 150 min. The selective adsorption toward these precious metal ions was maintained even in multi-element solutions. Only Ag(I), Pd(II), and Au(III) were adsorbed from a mixture containing Ag(I), Cu(II), Zn(II), Ni(II), Co(III), V(V), Cr(III), Fe(III), Mn(II), Cd(II), Au(III), Pd(II), Pt(IV), Ir(III), Os(III), Ru(III), and Rh(III). Encouraged by the fast and selective adsorption ability, the recovery of Au(III), Pd(II), and Ag(I) by CFP-g-PHCTMA was studied for a WPCB leachate in aqua regia. These precious metals were selectively adsorbed with negligible adsorption of coexisting ions contained in excess. This study demonstrates that this cellulose-based CFP-g-PHCTMA is an eco-friendly and economically viable adsorbent for the selective adsorption of precious metals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In recent decades, the technological innovations on electric and electronics equipment (EEE) and life style changes cause production of a large amount of waste electric and electronics equipment (WEEE) in a fast rate (Rahmani et al. 2014; Wang and Xu 2015; Yoshida et al. 2016). Precious metals like gold, silver, palladium are extensively used in manufacturing of EEE. Hence, WEEE are the vital sources for recovering precious metals (Akcil et al. 2015; Chancerel et al. 2009; Cui and Zhang 2008; Gurung et al. 2013). Printed circuit boards (PCBs) are the key components of EEE and are considered as the most valuable parts among them, since they contain precious metals in higher concentrations than natural high-grade ores (Chen et al. 2013; Hageluken and Corti 2010; Park and Fray 2009; Van Eygen et al. 2016). In addition, natural occurrences of these precious metals are limited and, in some mines, already depleted. Hence, the effective recovery of these precious metals from secondary sources like WPCBs is quite important from economic and environmental points of view.
Hydrometallurgical and pyrometallurgical techniques have been widely used to recover precious metals from waste materials. Hydrometallurgical treatments are basically preferred over pyrometallurgical treatments for the recovery of precious metals from WPCBs due to the higher selectivity and the better environmental friendliness originating from the lower waste gas, no volatile metals, and the lower energy consumption (Akcil et al. 2015). In recent years, the impact of environmental friendliness is increasing in recovery of precious metals (Adhikari et al. 2008; Gurung et al. 2014; He and Xu 2015; Monier et al. 2014a, b; Navarro et al. 1999; Sharma and Rajesh 2014; Zhou et al. 2013). The leaching course is the first step in given recycling chains through hydrometallurgical routes. The common leaching agents used for recovery of precious metals are aqua regia (Oh et al. 2003), alkaline cyanide (Parga et al. 2007; Warshawsky et al. 2001), chlorine (Kim et al. 2011), thiosulphate (Vinh et al. 2010), and thiourea (Orgul and Atalay 2002). Among them, aqua regia is a better leaching agent than others due to its ability for complete metal leaching from whole WPCBs along with the production of lesser toxic substances, while its strong acidity limits available adsorbents used in the next steps (Jadhav and Hocheng 2015). The leached solutions containing base and precious metals are subjected to the course of separation and purification, such as precipitation of impurities, solvent extraction, adsorption, and ion exchange to isolate and concentrate the metal of interest. The application of commercially available ion exchange resins and chelating resins is limited owing to their non-selective nature and low uptake capacity of precious metals (Chen et al. 2009; Lee et al. 2001; Ni et al. 2001). Therefore, it is important from technical, commercial, and environmental considerations that the precious metals are separated not only from the base metals with a high efficiency of recovery but also in a cost-effective method. From these points of view, it is desired to develop a new low cost metal adsorbent with high selectivity for these metals. Thus, the design of selective scavengers for precious metals would benefit from synthetic procedures involving accessible materials and facile protocols.
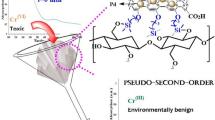
The selectivity to specific metal ions depends on the nature of chelating agents incorporated on the matrix substances. On account of high affinity of sulfur atoms to soft metal ions such as Au(III), Pd(II), and Ag(I), the selective recovery of these precious metals by chelating agents containing sulfur atoms was reported by the authors (Hyder and Ochiai 2017; Ochiai et al. 2009) and other (Nagai et al. 2010). Our adsorbent, CFP-g-PHCTMA (Fig. 1) based on accessible cellulose filter paper (CFP) and sulfur, is highly selective to precious metals (Hyder and Ochiai 2017). We subjected CFP-g-PHCTMA towards selective recovery of precious metals from a WPCB leachate. The adsorption behavior toward Au(III), Pd(II), and Ag(I) were studied in detail in order to understand the selective adsorption ability. Based on the results of these fundamental experiments, we demonstrate the practical recovery of Ag(I), Pd(II), and Au(III) from the WPCB leachate in aqua regia using CFP-g-PHCTMA.
2 Experimental
2.1 Materials
All the chemicals used were of analytical reagent grade. CFP (Adventec 5C) (Toyo Roshi, Tokyo, Japan) was used for modification. Nitric acid, and Cu(II) and Ni(II) standard solution for ICP (1000 mg L−1) were purchased from Wako Chemicals (Tokyo, Japan). Pd(II), Au(III), and Ag(I) standard solution for ICP (1000 mg L−1), and multi-elemental solution (1) (Cu(II), V(V), Ni(II), Co(II), Fe(III), Mn(II), Cr(III), Ag(I), Zn(II) and Cd(II) and multi-elemental solution (2) (Pd(II), Au(III), Pt(IV), Ru(III), Rd(III), Os(III), Ir(III)) for ICP (100 mg/L) were purchased from Sigma-Aldrich (St. Louis, Missouri, USA). Water was purified with MINIPURE TW-300RU (Nomura Micro Science, Kanagawa, Japan). The scavenger, CFP-g-PHTCMA (Hyder and Ochiai 2017), and the precursors, O-1-mercapto-3-phenoxypropan-2-yl N-2-hydroxyethylcarbamothioate (HCT) (Hyder and Ochiai 2017) and 4-(phenoxymethyl)-1,3-oxathiolane-2-thione (DTC) (Kihara et al. 1995; Ochiai and Endo 2005) was prepared per the literatures.
2.2 Adsorption experiment (typical procedure)
The adsorption experiment was carried out in batch mode. For adsorption experiments, CFP-g-PHCTMA (5 mg) was placed in a 10 mL plastic bottle containing 100 mg L−1 of a metal ion solution (5 mL) at a determined acid concentration. The adsorption experiments for Au(III) were conducted using 200 mg L−1 Au(III) solutions. The bottles were equilibrated at 25 °C on a thermostated shaker. Then the mixture was filtrated and the filtrate metal solution was analyzed for its residual concentration. The concentration of metal ions was determined by Perkin Elmer ELAN DRC II inductively coupled plasma (ICP) mass spectrophotometer.
The percentages of adsorption of metals were evaluated using the relations of initial and equilibrium concentrations of metal ions in solutions indicated in Eq. 1.
C i (mg L−1) is the initial metal ion concentration; and C e (mg L−1) is the metal ion concentration in equilibrium.
The adsorption amounts were calculated from residual amounts of metal ions in solutions per Eq. 2.
Q e (mg g−1) is the adsorption capacity; V (L) is the volume of metal solution; W (g) is the mass of dry adsorbent, respectively.
2.3 WPCB leaching in aqua regia
The sample of WPCB leachate was obtained as follows. Connectors were collected from WPCB, and treated with aqua regia (Fig. 2). The resulting mixture was filtrated and the filtrate was analyzed by ICP-MS after proper dilution. Detected metal ions are V(V), Fe(III), Ni(II), Cu(II), Mn(II), Zn(II), Pd(II), Au(III), and Ag(I), and the concentrations are indicated in Table 1.
3 Result and discussion
3.1 Effect of H+ ion concentration on adsorption processes
Selective adsorption of precious metals in strong acid is an important requirement for a metal scavenger applied in the practical hydrometallurgical collection of precious metals, as concentrated acids are used in metal leaching. Hence, adsorption isotherm studies under different acid concentrations were carried out for Au(III) and Pd(II) in aqueous HCl, and Ag(I) in aqueous HNO3 (Fig. 3). The high adsorption capacity of CFP-g-PHCTMA toward Au(III) and Pd(II) was maintained at a wide range of HCl concentration, though with slight decrements.
The trend of the decrement of the adsorption capacity for Ag(I) upon increasing the HNO3 concentration was found to be significant. The decrease of the adsorption capacities can be explained by the slight protonation of sulfur atoms in the thione functional groups of CFP-g-PHCTMA as observed in adsorption of Au(III) and Pd(II) by phosphine sulphide-type chelating polymers (Sanchez et al. 2001) and the competition between the proton ion and Au(III), Pd(II), and Ag(I) for the exchange sites on the CFP-g-PHCTMA (Monier et al. 2014a).
3.2 Kinetics of Adsorption of Au(III), Pd (II), and Ag(I) by CFP-g-PHCTMA
Fast uptake ability is an important requirement on collection of metals from waste stream. In order to investigate the kinetics of the adsorption process, an adsorption experiment was performed using 1 g L−1 CFP-g-PHCTMA for Ag(I) (100 mg L−1), Pd(II) (100 mg L−1), and Au(III) (200 mg L−1) solutions under 0.1 M acid concentrations at 25 °C (Fig. 4). The adsorption of these precious metals proceeded smoothly and the adsorbed amounts almost reached to plateaus after 50 min. The adsorption kinetics was studied using a pseudo-first order kinetic model (Eq. 3) along with its linear form (Eq. 4) and a pseudo-second model (Eq. 5) along with its linear form (Eq. 6).
Q e (mg g−1) and Q t (mg g−1) are the amounts of metal ions adsorbed per unit mass of the adsorbent at equilibrium and time (t) respectively. k 1 (g mg−1 min−1) and k 2 (dm−3 mg−1 min−1) are the pseudo-first order and pseudo-second order rate constants, respectively. The results of the kinetic were obtained analyzing Fig. 4. Table 2 lists the kinetic parameters of the pseudo-first order and pseudo-second order models.
The linear plots of the pseudo-first order (Fig. 5a) and the pseudo-second order (Fig. 5b) kinetic models for the adsorption of Ag(I), Pd(II), and Au(III) on CFP-g-PHCTMA indicate that the second-order plots have better linearity, as can be confirmed by the R 2 values (Table 2). As the adsorption of Ag(I), Pd(II), and Au(III) fitted the pseudo-second order model, the rate determining step of this adsorption is the chemical reaction between the ions and the adsorbent (Azizian 2004; Ho 2004, 2006; Ho and McKay 1999).
3.3 Selective adsorption of Ag(I), Pd(II), and Au(III) from multi-elemental solutions
Practical recovery processes of precious metals ions are carried out from multi-elemental solutions containing various base metals such as copper, zinc, and nickel in high concentration of acidic media. Accordingly, the adsorption experiments were carried out using two types of multi-elemental solutions with various acid concentrations to determine the selective adsorption behavior of CFP-g-PHCTMA even in highly acidic media (Fig. 6). Figure 6a shows the selective adsorption of Ag(I) from a multi-elemental solution (1) containing Ag(I), Cu(II), Zn(II), Ni(II), Co(III), V(V), Cr(III), Fe(III), Mn(II), and Cd(II) in HNO3 aq. with the acid concentration varied from 1 to 6 M. This result indicates that CFP-g-PHCTMA adsorbs only Ag(I) from the mixture of various base metal ions without co-adsorption of other metals. Figure 6b presents the metal adsorption behavior of CFP-g-PHCTMA from a multi-elemental solution (2) containing Au(III), Pd(II), Pt(IV), Ir(III), Os(III), Ru(III) and Rh(III) in HCl aq. with the acid concentration varied from 1 to 6 M. It was observed that only Au(III) and Pd(II) was bound to the adsorbent, whereas the trace amounts of Os(III) and Pt(IV) was adsorbed and no other precious metals were captured at all by CFP-g-PHCTMA. The adsorption of Os(III) and Pt(IV) was suppressed under high acid concentrations.
As PCB metals are often leached by aqua regia, 1 in HNO3 media and 2 in HCl media were mixed together and the mixture was employed for the adsorption experiment with CFP-g-PHCTMA (total acid concentration 1.53 M) (Fig. 7). CFP-g-PHCTMA selectively and efficiently adsorbed Au(III), Pd(II), and Ag(I) even from the mixture of the two multi-elemental solutions as well as the individual and the original multi-metal solutions.
3.4 Selective recovery of Au(III), Pd (II), and Ag(I) from WPCB leachate
On the basis of the excellent adsorptive recovery of Au(III), Pd(II), and Ag(I), we carried out collection of the precious metals from WPCB leachate with aqua regia by CFP-g-PHCTMA, which involves difficulty in selective adsorption due to the presence of various organic components and base metals such as Cu(II), Ni(II), V(V), Fe(II), and Zn(II). The leachate consists of Pd(II), Au(III), and Ag(I), and significantly higher amounts of base metals as indicated in Table 1. Figure 8 represents the adsorption behavior of metals in the WPCB metal leachate with CFP-g-PHCTMA. It was observed that Cu(II), Ni(II) Zn(II), V(V), and Fe(II) was negligibly adsorbed onto the adsorbent CFP-g-PHCTMA. By contrast, Au(III), Pd(II), and Ag(I) were completely adsorbed. The concentrations of CFP-g-PHCTMA in the metal leachate in aqua regia for the complete recovery of Au(III) and Pd(II) are 3 g L−1, while that for Ag(I) is 7 g L−1. This differences in the efficiencies agrees with the ability of adsorption of Cell-g-PHCTMA towards Au(III), Pd(II) and Ag(I) observed in the single ion experiments at higher acid concentration. These amounts of CFP-g-PHCTMA for entire recovery of Au(III), Pd(II), and Ag(I) from the WPCB leachate is not so high considering the expensiveness of these precious metals than higher than organic materials accessible from inexpensive resources. Figure 9 shows that the color of CFP-g-PHCTMA was changed from white to yellow in a similar manner with the adsorption of individual metal ions (Hyder and Ochiai 2017). These results clearly demonstrated that the precious metals Au(III), Pd(II), and Ag(I) were successfully recovered by CFP-g-PHCTMA from the metal leachate from WPCBs. In other words, CFP-g-PHCTMA is a promising candidate for practical recovering of precious metals.
4 Conclusion
The adsorbent CFP-g-PHCTMA was found to be effective for the selective recovery of Au(III), Pd(II), and Ag(I) from WPCB. The successful result originates from the specific adsorption ability of the thiocarbamate ligand and the stability under very high concentration of acid media. The high selectivity was manifested by the negligible adsorption of base metals, namely Cu(II), Zn(II), Ni(II), Co(III), V(V), Cr(III), Fe(III), Mn(II), and Cd(II), and other precious metals, namely Pt(IV), Ir(III), Os(III), Ru(III), and Rh(III). The kinetic study revealed that the adsorption rates are fast and the experimental data well fit the pseudo-second order model. This features are vital for practical recovery of precious metals, and CFP-g-PHCTMA is a promising adsorbent for a wide range of hydrometallurgical recovery of precious metals from various wastes.
References
Adhikari CR, Parajuli D, Kawakita H, Inoue K, Ohto K, Harada H (2008) Dimethylamine-modified waste paper for the recovery of precious metals. Environ Sci Technol 42:5486–5491
Akcil A, Erust C, Gahan CS, Ozgun M, Sahin M, Tuncuk A (2015) Precious metal recovery from waste printed circuit boards using cyanide and non-cyanide lixiviants—a review. Waste Manag 45:258–271
Azizian S (2004) Kinetic models of sorption: a theoretical analysis. J Colloid Interf Sci 276:47–52
Chancerel P, Meskers CEM, Hageluken C, Rotter VS (2009) Assessment of precious metal flows during preprocessing of waste electrical and electronic equipment. J Ind Ecol 13:791–810
Chen AH, Yang CY, Chen CY, Chen CY, Chen CW (2009) The chemically crosslinked metal-complexed chitosans for comparative adsorptions of Cu(II), Zn(II), Ni(II) and Pb(II) ions in aqueous medium. J Hazard Mater 163:1068–1075
Chen MJ, Wang JB, Chen HY, Ogunseitan OA, Zhang MX, Zang HB, Hu JK (2013) Electronic waste disassembly with industrial waste heat. Environ Sci Technol 47:12409–12416
Cui JR, Zhang LF (2008) Metallurgical recovery of metals from electronic waste: a review. J Hazard Mater 158:228–256
Gurung M, Adhikari BB, Kawakita H, Ohto K, Inoue K, Alam S (2013) Recovery of gold and silver from spent mobile phones by means of acidothiourea leaching followed by adsorption using biosorbent prepared from persimmon tannin. Hydrometallurgy 133:84–93
Gurung M, Adhikari BB, Gao XP, Alam S, Inoue K (2014) Sustainability in the metallurgical industry: chemically modified cellulose for selective biosorption of gold from mixtures of base metals in chloride media. Ind Eng Chem Res 53:8565–8576
Hageluken C, Corti CW (2010) Recycling of gold from electronics: cost-effective use through ‘Design for Recycling’. Gold Bull 43:209–220
He YC, Xu ZM (2015) Recycling gold and copper from waste printed circuit boards using chlorination process. Rsc Adv 5:8957–8964
Ho YS (2004) Citation review of Lagergren kinetic rate equation on adsorption reactions. Scientometrics 59:171–177
Ho YS (2006) Review of second-order models for adsorption systems. J Hazard Mater 136:681–689
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Hyder MKMZ, Ochiai B (2017) Synthesis of a selective scavenger for Ag(I), Pd(II), and Au(III) based on cellulose filter paper grafted with polymer chains bearing thiocarbamate moieties. Chem Lett (Accepted)
Jadhav U, Hocheng HC (2015) Hydrometallurgical recovery of metals from large printed circuit board pieces. Sci Rep 5:14574
Kihara N, Nakawaki Y, Endo T (1995) Preparation of 1,3-oxathiolane-2-thiones by the reaction of oxirane and carbon disulfide. J Org Chem 60:473–475
Kim EY, Kim MS, Lee JC, Pandey BD (2011) Selective recovery of gold from waste mobile phone PCBs by hydrometallurgical process. J Hazard Mater 198:206–215
Lee ST, Mi FL, Shen YJ, Shyu SS (2001) Equilibrium and kinetic studies of copper(II) ion uptake by chitosan-tripolyphosphate chelating resin. Polymer 42:1879–1892
Monier M, Akl MA, Ali WM (2014a) Modification and characterization of cellulose cotton fibers for fast extraction of some precious metal ions. Int J Biol Macromol 66:125–134
Monier M, Kenawy IM, Hashem MA (2014b) Synthesis and characterization of selective thiourea modified Hg(II) ion-imprinted cellulosic cotton fibers. Carbohydr Polym 106:49–59
Nagai D, Imazeki T, Morinaga H, Oku H, Kasuya KI (2010) Three-component polyaddition of diamines, carbon disulfide, and diacrylates in water. J Polym Sci Polym Chem 48:845–851
Navarro RR, Sumi K, Matsumura M (1999) Improved metal affinity of chelating adsorbents through graft polymerization. Water Res 33:2037–2044
Ni CH, Yi CH, Feng ZY (2001) Studies of syntheses and adsorption properties of chelating resin from thiourea and formaldehyde. J Appl Polym Sci 82:3127–3132
Ochiai B, Endo T (2005) Carbon dioxide and carbon disulfide as resources for functional polymers. Prog Polym Sci 30:183–215
Ochiai B, Ogihara T, Mashiko M, Endo T (2009) Synthesis of rare-metal absorbing polymer by three-component polyaddition through combination of chemo-selective nucleophilic and radical additions. J Am Chem Soc 131:1636–1637
Oh CJ, Lee SO, Yang HS, Ha TJ, Kim MJ (2003) Selective leaching of valuable metals from waste printed circuit boards. J Air Waste Manag 53:897–902
Orgul S, Atalay U (2002) Reaction chemistry of gold leaching in thiourea solution for a Turkish gold ore. Hydrometallurgy 67:71–77
Parga JR, Valenzuela JL, Cepeda F (2007) Pressure cyanide leaching for precious metals recovery. JOM 59:43–47
Park YJ, Fray DJ (2009) Recovery of high purity precious metals from printed circuit boards. J Hazard Mater 164:1152–1158
Rahmani M, Nabizadeh R, Yaghmaeian K, Mahvi AH, Yunesian M (2014) Estimation of waste from computers and mobile phones in Iran. Resour Conserv Recycl 87:21–29
Sanchez JM, Hidalgo M, Salvado V (2001) Synthesised phosphine sulphide-type macroporous polymers for the preconcentration and separation of gold(III) and palladium(II) in a column system. React Funct Polym 49:215–224
Sharma S, Rajesh N (2014) 2-Mercaptobenzothiazole impregnated cellulose prepared by ultrasonication for the effective adsorption of precious metal palladium. Chem Eng J 241:112–121
Van Eygen E, De Meester S, Tran HP, Dewulf J (2016) Resource savings by urban mining: the case of desktop and laptop computers in Belgium. Resour Conserv Recycl 107:53–64
Vinh HH, Lee JC, Jeong J, Huynh TH, Jha MK (2010) Thiosulfate leaching of gold from waste mobile phones. J Hazard Mater 178:1115–1119
Wang JB, Xu ZM (2015) Disposing and recycling waste printed circuit boards: disconnecting, resource recovery, and pollution control. Environ Sci Technol 49:721–733
Warshawsky A, Kahana N, Kampel V, Rogachev I, Kautzmann RM, Cortina JL, Sampaio CH (2001) Ion exchange resins for gold cyanide extraction containing a piperazine functionality, 2 study of the gold extraction reaction. Macromol Mater Eng 286:285–295
Yoshida A, Terazono A, Ballesteros FC, Nguyen DQ, Sukandar S, Kojima M, Sakata S (2016) E-waste recycling processes in Indonesia, the Philippines, and Vietnam: a case study of cathode ray tube TVs and monitors. Resour Conserv Recycl 106:48–58
Zhou YM, Hu XY, Zhang M, Zhuo XF, Niu JY (2013) Preparation and characterization of modified cellulose for adsorption of Cd(II), Hg(II), and acid fuchsin from aqueous solutions. Ind Eng Chem Res 52:876–884
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hyder, M.K.M.Z., Ochiai, B. Selective recovery of Au(III), Pd(II), and Ag(I) from printed circuit boards using cellulose filter paper grafted with polymer chains bearing thiocarbamate moieties. Microsyst Technol 24, 683–690 (2018). https://doi.org/10.1007/s00542-017-3277-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00542-017-3277-0