Abstract
We present the first reported case of a patient with intraoperative hypoglycemia, with no predisposing factors, that was indicative of post-hepatectomy liver failure due to liver injury. A 56-year-old man was hospitalized to undergo left lateral segmentectomy, cholecystectomy and T-tube choledocholithotripsy due to calculi in the intrahepatic and common bile ducts. His medical history was unremarkable. Three hours after surgery initiation, his glucose level decreased from 84 mg/dL to below detectable levels. We infused 20 % dextrose repeatedly until his glucose level returned to within normal limits. His aspartate aminotransferase and alanine aminotransferase levels increased to over 10,000 IU/L, and his blood urea nitrogen and creatinine levels increased postoperatively. Thus, we diagnosed post-hepatectomy liver failure and hepatorenal syndrome and treated the patient conservatively. This case illustrates that, if no other causative factors for severe hypoglycemia occurring during liver resection are present, the anesthesiologist should predict post-hepatectomy liver failure due to liver injury and inform the surgeon in order to enable rapid evaluation and treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Post-hepatectomy liver failure may occur due to liver resection and can generally be diagnosed from the patient’s symptoms and follow-up laboratory findings after surgery. Although hyperglycemia commonly develops during surgery, intraoperative hypoglycemia is rarely encountered. When it does, it is associated with certain causative factors. We present the first reported case of a patient without predisposing factors who developed intraoperative hypoglycemia indicative of post-hepatectomy liver failure due to liver injury. The patient demonstrated severe hypoglycemia detected by glucose testing during surgery. He was diagnosed postoperatively with acute liver failure, which was conservatively treated.
Case report
A 56-year-old man (weight 64 kg; height 163 cm) was diagnosed with cholecystitis and calculi in the intrahepatic and common bile ducts. He was hospitalized to undergo left lateral segmentectomy, cholecystectomy and T-tube choledocholithotripsy. While his medical history was unremarkable, jaundice without any pitting edema was observed on physical examination. His vital signs before surgery were stable. Preoperative laboratory data revealed that the total bilirubin level was increased to 18.4 mg/dL and the aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels were increased to 80 and 92 IU/L, respectively. Other laboratory findings were within the normal range.
A solution of 5 % dextrose in 0.9 % saline was continuously administered at a rate of 120 mL/h during a fasting period without any premedication. After the patient arrived in the operating room, he was intravenously administered 20 mg of famotidine. His initial blood pressure (BP) was 110/70 mmHg, with a heart rate (HR) of 70 beats/min and oxygen saturation by pulse oximetry (SpO2) of 95 %. Anesthesia was induced by continuous intravenous infusion of 0.1 µg/kg/min remifentanil. After intravenous administration of 130 mg propofol and 50 mg rocuronium, endotracheal intubation was performed, and anesthesia was maintained with 4–5 vol% desflurane and 50 % nitrous oxide to maintain a bispectral index under 50. The patient was infused with Hartmann’s solution and 0.9 % saline. Arterial blood gas analysis (ABGA) findings before surgery were normal, and the patient’s glucose level was 84 mg/dL. ABGA was performed at hourly intervals.
Two hours after surgery was begun, a hepatic artery was injured during dissection of Calot’s triangle for T-tube drainage. The surgeon performed temporary clamping of the portal triad to control excessive bleeding because he experienced difficulty in repairing the injured hepatic artery. Ultimately, the surgeon failed to repair the injured artery, so he ligated the right hepatic artery. The total ischemic time by clamping the portal triad was about 30 min, during which the patient’s BP was 80–90/45–50 mmHg, with a HR of 95–105 beats/min and SpO2 of 100 %.
The ABGA findings taken 3 h after the start of surgery were normal, but the glucometer signaled a decrease in glucose level to below the detectable limit. We assumed that this signal was an instrumental error and repeated the measurement, but the same results were observed. We rapidly injected 100 mL of 20 % dextrose solution, and the glucose level was measured as 42 mg/dL. Another 200 mL of 20 % dextrose solution was administered, and the patient’s glucose level increased to 109 mg/dL. Subsequently, 5 % dextrose solution was continuously infused. One hour later, the surgery was completed. The total operation time was 4 h and 30 min, and the total anesthesia time was 5 h and 20 min. Total input was 8500 mL, including the dextrose solution, Hartmann’s solution, 0.9 % saline and 500 mL of hydroxyethyl starch (Voluven, Fresenius Kabi, Germany). Additionally, seven units of packed red blood cells and two units of fresh frozen plasma were transfused during surgery. Blood loss during surgery was approximately 3500 mL, and urine output was 700 mL. The patient was extubated and transferred to the intensive care unit (ICU). When the patient arrived in the ICU, his BP was 110/50 mmHg, his HR was 112 beats/min, and his SpO2 was 100 % with O2 mask 5 L/min. His mental status was drowsy and Glasgow coma scale score was 13. His glucose level was measured as 48 mg/dL after 1 h in the ICU, so he was infused with 100 mL of 50 % dextrose solution. His glucose level increased to 265 mg/dL after infusion and was maintained between 79 and 172 mg/dL by continuous infusion of 10 % dextrose solution.
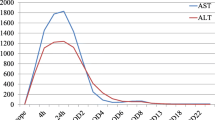
Laboratory results on the day of surgery showed a rapid increase in the AST and ALT levels to 14,641 and 4086 IU/L, respectively, which further increased to 21,850 and 1225 IU/L, respectively, on the following day. The patient’s AST and ALT levels then began to decrease, returning to within normal limits on the sixth day after surgery, and slightly increased afterwards (Fig. 1). Levels of other enzymes, namely lactate dehydrogenase and creatine phosphokinase, were also increased, to 21,850 and 1225 IU/L, respectively, on the day of surgery. Blood urea nitrogen and creatinine levels increased slightly after surgery to 36.5 and 1.84 mg/dL, respectively, and then steadily increased, as seen in Fig. 2. The patient’s urine output was reduced to 5–10 mL/h.
We considered these changes to indicate liver failure and diagnosed the patient with post-hepatectomy liver failure and hepatorenal syndrome. Continuous renal replacement therapy was initiated beginning on postoperative day (POD) 1. The patient was switched to intermittent hemodialysis on POD 10, which ended on POD 44. In addition, the patient’s total bilirubin level increased to 20 mg/dL on POD 9, and plasmapheresis was performed twice as symptomatic treatment. The patient’s mental status was nearly fully alert while he was receiving intensive care and returned to normal on POD 50. He was discharged without any specific findings.
Discussion
It is common for patients to become hyperglycemic during surgery. Hyperglycemia is a common response to critical illness and metabolic stress [1]. Stress-induced release of counter-regulatory hormones, such as cortisol, glucagon, epinephrine and growth hormones, leads to upregulation of hepatic gluconeogenesis and glycogenolysis despite hyperinsulinemia and compromised insulin-regulated peripheral glucose uptake. Intraoperative hypoglycemia does not occur often, and may develop when the nil per os (NPO) time is prolonged in children, fluids that do not contain dextrose are administered during long surgical procedures, intensive insulin treatment or peritoneal dialysis is administered due to diabetes mellitus [2], or the patient has certain disease states (e.g., pancreatic islet cell adenoma, carcinoma, hepatoma, fibroma or adrenal insufficiency) [3, 4].
Post-hepatectomy liver failure is defined as a postoperative acquired deterioration in the ability of the liver to maintain its synthetic, excretory and detoxifying functions. It is characterized by an increased international normalized ratio (INR) >1.5 and hyperbilirubinemia >3 mg/dL on or after POD 5. The reported incidence of post-hepatectomy liver failure ranges from 1.2 to 32 %, partly as a result of differences in the patient populations studied and procedures performed [5, 6].
The patient in this case had no underlying disease and his NPO time was 24 h. He was continuously supplied with dextrose-containing fluids during the fasting period, and his glucose level was 84 mg/dL at the start of the operation.
He had multiple risk factors for post-hepatectomy liver failure, including cholangitis, male sex, excessive intraoperative blood loss (approximately 3500 mL) and a prolonged operation time (Table 1) [7]. Although this patient only underwent a two-segment resection, he met the criteria for post-hepatectomy liver failure. We believe that the patient developed liver failure because of the massive bleeding, long ischemia time from the portal triad clamping, and insufficient hepatic blood flow due to hepatic artery ligation. As a result, the unexpectedly severe hypoglycemia during the surgery was expressed as a sign of liver failure. We believe that this was due to reduced gluconeogenesis in the dysfunctional liver, because ischemic injuries occurred as the hepatic blood flow decreased, leading to liver failure and hepatic glucose, protein and lipid metabolism defects that could cause either hyperglycemia or hypoglycemia [8, 9]. Mellinkoff et al. [10] reported unexpected hypoglycemia due to decreased hepatic blood flow in a non-diabetic patient with congestive heart failure. Numura et al. [11] reported severe hypoglycemia in ischemic hepatitis, and they hypothesized that the hypoglycemia was due to reduced gluconeogenesis in the exhausted liver.
Here, we presented the first reported case of a patient with intraoperative hypoglycemia and no predisposing factors that was indicative of post-hepatectomy liver failure due to liver injury. In conclusion, if hypoglycemia is noted during surgery, the measurement should be repeated for confirmation, and possible causative factors for the hypoglycemia should be assessed. If there are no causative factors for severe hypoglycemia while performing liver resection, the anesthesiologist should predict post-hepatectomy liver failure due to liver injury and inform the surgeon in order to enable rapid evaluation and treatment.
References
Lipshutz AK, Gropper MA. Perioperative glycemic control: an evidence-based review. Anesthesiology. 2009;110:408–21.
Cho E, Lee S. Severe hypoglycemia due to falsely elevated capillary blood glucose levels in a peritoneal dialysis patient using icodextrin. Korean J Anesthesiol. 2009;56:221–4.
Sieber FE, Smith DS, Traystman RJ, Wollman H. Glucose: a reevaluation of its intraoperative use. Anesthesiology. 1987;67:72–81.
Schiff RL, Welsh GA. Perioperative evaluation and management of the patient with endocrine dysfunction. Med Clin North Am. 2003;87:175–92.
Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, Koch M, Makuuchi M, Dematteo RP, Christophi C, Banting S, Usatoff V, Nagino M, Maddern G, Hugh TJ, Vauthey JN, Greig P, Rees M, Yokoyama Y, Fan ST, Nimura Y, Figueras J, Capussotti L, Buchler MW, Weitz J. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery. 2011;149:713–24.
Schreckenbach T, Liese J, Bechstein WO, Moench C. Posthepatectomy liver failure. Dig Surg. 2012;29:79–85.
van den Broek MA, Olde Damink SW, Dejong CH, Lang H, Malago M, Jalan R, Saner FH. Liver failure after partial hepatic resection: definition, pathophysiology, risk factors and treatment. Liver Int. 2008;28:767–80.
Postic C, Dentin R, Girard J. Role of the liver in the control of carbohydrate and lipid homeostasis. Diabetes Metab. 2004;30:398–408.
Cotrozzi G. Casini Raggi V, Relli P, Buzzelli G. Role of the liver in the regulation of glucose metabolism in diabetes and chronic liver disease. Ann Ital Med Int. 1997;12:84–91.
Mellinkoff SM, Tumulty PA. Hepatic hypoglycemia; its occurrence in congestive heart failure. N Engl J Med. 1952;247:745–50.
Nomura T, Keira N, Urakabe Y, Naito D, Nakayama M, Kido A, Kanemasa H, Matsubara H, Tatsumi T. Chronic pericardial constriction induced severe ischemic hepatitis manifesting as hypoglycemic attack. Circ J. 2009;73:183–6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors had conflicts of interest in relation to this study or was provided funding by the manufacturer.
About this article
Cite this article
Chung, K., Bang, S., Kim, Y. et al. Intraoperative severe hypoglycemia indicative of post-hepatectomy liver failure. J Anesth 30, 148–151 (2016). https://doi.org/10.1007/s00540-015-2070-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-015-2070-4