Abstract
Purpose
Intraoperative hypoglycemia is presumed to be rare, but generalizable multicentre incidence and risk factor data for adult patients are lacking. We used a multicentre registry to characterize adults with intraoperative hypoglycemia and hypothesized that intraoperative insulin administration would be associated with hypoglycemia.
Methods
We conducted a cross-sectional retrospective multicentre cohort study. We searched the Multicenter Perioperative Outcomes Group registry to identify adult patients with intraoperative hypoglycemia (glucose < 3.3 mmol·L−1 [< 60 mg·dL−1]) from 1 January 2015 to 31 December 2019. We evaluated characteristics of patients with intraoperative glucose measurements and with intraoperative hypoglycemia.
Results
Of 516,045 patients with intraoperative glucose measurements, 3,900 (0.76%) had intraoperative hypoglycemia. Diabetes mellitus and chronic kidney disease were more common in the cohort with intraoperative hypoglycemia. The odds of intraoperative hypoglycemia were higher for the youngest age category (18–30 yr) compared with the odds for every age category above 40 yr (odds ratio [OR], 1.57–3.18; P < 0.001), and were higher for underweight or normal weight patients compared with patients with obesity (OR, 1.48–2.53; P < 0.001). Parenteral nutrition was associated with lower odds of hypoglycemia (OR, 0.23; 95% confidence interval [CI], 0.11 to 0.47; P < 0.001). Intraoperative insulin use was not associated with hypoglycemia (OR, 0.996; 95% CI, 0.91 to 1.09; P = 0.93).
Conclusion
In this large cross-sectional retrospective multicentre cohort study, intraoperative hypoglycemia was a rare event. Intraoperative insulin use was not associated with hypoglycemia.
Résumé
Objectif
L’hypoglycémie peropératoire est présumée rare, mais il n’existe pas de données généralisables sur l’incidence multicentrique et les facteurs de risque chez la patientèle adulte. Nous avons utilisé un registre multicentrique pour caractériser les personnes adultes atteintes d’hypoglycémie peropératoire et émis l’hypothèse que l’administration peropératoire d’insuline serait associée à l’hypoglycémie.
Méthode
Nous avons réalisé une étude de cohorte multicentrique rétrospective transversale. Nous avons effectué des recherches dans le registre du Multicenter Perioperative Outcomes Group afin d’identifier les patient·es adultes atteint·es d’hypoglycémie peropératoire (glucose < 3,3 mmol· L−1 [< 60 mg·dL−1]) du 1er janvier 2015 au 31 décembre 2019. Nous avons évalué les caractéristiques des patient·es présentant des mesures de glucose et une hypoglycémie peropératoires.
Résultats
Sur 516 045 patient·es ayant des mesures de glucose peropératoires, 3900 (0,76 %) ont présenté une hypoglycémie peropératoire. Le diabète sucré et l’insuffisance rénale chronique étaient plus fréquents dans la cohorte présentant une hypoglycémie peropératoire. Les risques d’hypoglycémie peropératoire étaient plus élevés pour la catégorie d’âge la plus jeune (18-30 ans) par rapport aux catégories d’âge au-dessus de 40 ans (rapport des cotes [RC], 1,57-3,18; P < 0,001), et étaient plus élevés chez les patient·es de poids insuffisant ou de poids normal par rapport aux patient·es obèses (RC, 1,48-2,53; P < 0,001). La nutrition parentérale était associée à une probabilité plus faible d’hypoglycémie (RC, 0,23; intervalle de confiance [IC] à 95 %, 0,11 à 0,47; P < 0,001). L’utilisation peropératoire d’insuline n’était pas associée à l’hypoglycémie (RC, 0,996; IC 95 %, 0,91 à 1,09; P = 0,93).
Conclusion
Dans cette vaste étude de cohorte multicentrique rétrospective transversale, l’hypoglycémie peropératoire était un événement rare. L’utilisation peropératoire d’insuline n’était pas associée à l’hypoglycémie.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Improving intraoperative glucose control to reduce surgical site infections and cost has been a focus of quality improvement and clinical research for decades.1,2,3 Intensive control of glucose, while aimed at preventing hyperglycemia, involves an increased risk of hypoglycemia.4 Moderate hypoglycemia may cause altered mental status and lead to delayed emergence, while severe hypoglycemia may lead to seizures, neurologic injury, and death.5,6,7
Symptoms of hypoglycemia may be masked during anesthesia, leading to delayed diagnosis and treatment. Limited access to a patient after surgical draping or difficulty obtaining a blood sample or glucometer for intraoperative use may pose further barriers to evaluation of glucose during surgery. Long-acting insulin dosing is often reduced during perioperative fasting to reduce the risk of hypoglycemia.8
Intraoperative hypoglycemia is rare: in an observational study of various surgical procedures, the incidence was 0.1%.2 By contrast, certain subgroups are at higher risk. In cardiac surgery patients, studies have shown a frequency of 8–20%.1,9 The frequency is substantially higher (up to 29%) in clinical trials of intensive insulin therapy in the critical care context.10,11 This wide range reflects differences in patient populations, clinical settings, and whether glucose measurements were performed for all patients by protocol or selectively, based on clinical judgement. As a result, the incidence and risk factors for intraoperative hypoglycemia remain poorly understood.
While most studies of intraoperative hypoglycemia are from a single institution, we have previously used the Multicenter Perioperative Outcomes Group (MPOG; Ann Arbor, MI, USA) registry for an analysis of intraoperative hypoglycemia in a large population of children.12 In the current complementary study, the focus is on intraoperative hypoglycemia in adults. Our aims were to describe clinical features of patients who are selected for intraoperative glucose measurements, to determine the frequency of intraoperative hypoglycemia for these patients, and to identify patient and case characteristics associated with hypoglycemia. We hypothesized a priori that intraoperative insulin use would be associated with an increased risk of intraoperative hypoglycemia.
Methods
This cross-sectional retrospective multicentre cohort study had a prespecified protocol (Electronic Supplementary Material [ESM] eAppendix 1) and followed the RECORD extension of the STROBE guidelines13 (ESM eAppendix 2). The MPOG database, methods, and quality assurance standards have been described previously.14,15,16,17 Each institution participating in MPOG obtained local institutional review board approval (University of Utah, Salt Lake City, UT, USA; approval numbers: 135657 and 96411; 18 August 2020) with waiver of consent for research using anonymized data. Authors at the University of Michigan used the MPOG case viewer to review details of selected cases to guide data cleaning and to validate the robustness of the selection criteria code. The MPOG data are derived from the electronic health record at participating sites. Standardized clinical data are extracted and linked to laboratory, billing, and diagnostic coding data. The study protocol, including primary outcome, power analysis for necessary sample size, and statistical analysis plan, was presented at the MPOG Perioperative Clinical Research Committee (PCRC) and approved prior to data extraction and analysis. The MPOG database was queried for all patients ≥ 18 yr of age who had a surgical procedure between 1 January 2015 and 31 December 2019 with a case that met MPOG intraoperative research standards (ESM Appendix 3). The identity of the contributing institution was anonymized. Liver transplant cases and cases with cardiopulmonary bypass (identified on the basis of Current Procedural Terminology [CPT] codes for such surgeries) were excluded from the main analysis because aspects of these cases (e.g., liver failure, the anhepatic phase, ischemia-reperfusion associated with cardiopulmonary bypass) would be expected to impose significant physiologic impacts on glucose homeostasis. Nevertheless, the frequency of hypoglycemia in these cohorts is included for comparison with the frequency of hypoglycemia in the main analytic cohort. The intraoperative period was defined as anesthesia start to anesthesia end. Sites contributing fewer than 250 cases were excluded. Cases were excluded if the reported duration was > 24 hr (based on data cleaning rules regarding plausibility of case characteristics), or if there was no associated surgical procedure (defined as cases without associated surgical CPT codes).
Intraoperative hypoglycemia was defined as a glucose value < 3.3 mmol·L−1 (< 60 mg·dL−1) from a plasma sample on an institution laboratory instrument, an arterial blood gas analysis on an institution laboratory instrument, or a point-of-care arterial or venous blood gas analysis or a point-of-care glucometer capillary sample, documented during the intraoperative period. The hypoglycemia definition was based on the physiologic threshold at which neurologic effects are expected to have clinical manifestations.7 A glucose < 3.3 mmol·L−1 was declared valid only if there was an additional intraoperative glucose measurement—regardless of whether additional measurements were normal or low—and/or documented treatment with concentrated dextrose (a dextrose solution with a concentration of 10% or higher). Furthermore, cases with glucose measurements < 1.1 mmol·L−1 (< 20 mg·dL−1) were excluded because chart review of a sample of these cases showed that measurements in this range were spurious.
Comorbidities were defined using International Classification of Diseases 9 and 10 codes and specific Elixhauser comorbidity definitions, as appropriate. The six Elixhauser comorbidities included in the analysis were chronic pulmonary disease, obesity, neurodegenerative disorders, renal failure, weight loss, and diabetes mellitus. Chronic pulmonary disease included asthma, emphysema, bronchiectasis, and chronic obstructive pulmonary disease.18 The comorbidities were selected based on availability in the MPOG data and considerations of potential implications relevant to glucose homeostasis. Additional data included demographics (age interval [by decade], sex, American Society of Anesthesiologists [ASA] Physical Status [I or II vs ≥ III], emergency status, inpatient status, body mass index [BMI], preoperative laboratory values, and medications) and case information (anesthesia type, intraoperative medications, including intraoperative parenteral nutrition, duration of anesthesia, and intraoperative blood loss). Because of a high amount of missing data or because of absence from the database, we could not include home medications, duration of fasting, or preoperative hemoglobin A1C in the analysis.
The MPOG perioperative medication administration data include medications given within four hours prior to the start of anesthesia, including the oral glucose-lowering medications metformin, glipizide, glyburide, pioglitazone, and glimepiride. For brevity, and considering that noninsulin medications constituted only 1.67% of glucose-lowering agents, this medication category is labelled insulin hereafter. Antihypertensive medications included both peripherally-acting antihypertensive medications (nicardipine, nitroglycerin, nitroprusside, and clevidipine) as well as α2-adrenoceptor agonists (dexmedetomidine and clonidine). Intraoperative medication classes included in the multivariable model were medications for treating vasoplegia (ascorbic acid, hydrocortisone, cyanocobalamin, thiamine, methylene blue, calcium chloride, or calcium gluconate), insulin, antihypertensive medications, inotropes/vasopressors (dobutamine, dopamine, epinephrine by infusion [not bolus], milrinone, norepinephrine, or vasopressin by infusion [not bolus]), and β-blockers (labetalol, metoprolol, or esmolol).
Statistical analysis
A data analysis and statistical plan was written and filed with both the University of Utah institutional review board (Salt Lake City, UT, USA; #00135657) and the MPOG Perioperative Clinical Research Committee before data were accessed. We used basic descriptive statistics (means, medians, and interquartile range) to assess the distribution and characteristics of the data. Categorical data are presented as frequencies and percentages. We analyzed covariates by comparing those with and without glucose measurements, and, for just those with glucose measurements, comparing those with and without hypoglycemia. We used standardized mean differences (SMDs) to compare differences; a SMD > 0.2 and < 0.5 was considered a marker of a small to medium difference in the distribution of characteristics between groups.
We constructed a multivariable logistic regression model for the association between selected clinical characteristics and the odds ratios for intraoperative hypoglycemia for the noncardiac, nonliver transplant (general) cohort. We used generalized mixed effects logistic regression, with adjustment for multiple comparisons. We assessed covariates for multicollinearity using a generalized variance inflation factor, with a value less than 5 considered acceptable. In addition, the effect of clustering based on patients being nested within each MPOG site was incorporated into the model by treating the MPOG site as a random effect. The modelling strategy treats institution as a random effect, with cases nested within institutions.
To evaluate the effect of cases being nested within each MPOG site, we constructed a caterpillar plot of the intercept of the regression line for each site, treating institution as having a random effect in the model. In addition, we evaluated the adjusted and conditional intraclass correlation coefficient corresponding to the variance of hypoglycemia attributed to the MPOG site.
Measures of effect are reported as adjusted odds ratios (aORs) with 95% confidence intervals (CIs) and displayed in forest plots.
We conducted a sensitivity analysis using a hypoglycemia threshold of < 3.9 mmol·L−1 (< 70 mg·dL−1), the American Diabetes Association definition of hypoglycemia.19 We considered a P value < 0.05 statistically significant. When appropriate, the Benjamini–Hochberg method to control the false discovery rate was used to adjust for multiple testing. Specifically, this adjustment was made for CIs and P values for the multivariable model of the odds of hypoglycemia for selected clinical characteristics, both in the primary analysis (for hypoglycemia < 3.3 mmol·L−1) and for the sensitivity analysis (for hypoglycemia < 3.9 mmol·L−1). Statistical analysis was performed in R version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria) with the R packages Epi version 2.44 and emmeans version 1.7.3. To define the range of risk associated with each level of covariates in the model, marginal means (aOR) were estimated with Scheffe-adjusted P values.20 Estimated marginal means are useful in focusing on pairwise comparisons for different covariate levels while adjusting for means of other factors in the model. The α threshold for P values was set at 0.05.
Results
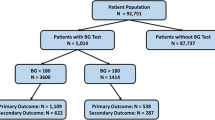
A total of 6,536,227 cases from 48 MPOG sites met the inclusion criteria for the general cohort. We excluded 69,185 cases because of missing sex, BMI, or emergency status. Intraoperative glucose was measured in 516,045 cases (7.9%). There were 3,900 cases of hypoglycemia, constituting 0.76 % of the cases with glucose measurements (Fig. 1). Point-of-care assays (glucose assays from point-of-care arterial blood gas, capillary fingerstick, and unspecified point-of-care testing) made up 74% of the measurements. Of the hypoglycemic measurements, 85% were in the moderate hypoglycemia range (2.2–3.3 mmol·L−1 [40–59 mg·dL−1]) and 15% were in the severe range (1.1–2.1 mmol·L−1 [20–39 mg·dL−1]) (Fig. 2).
Of 69,253 patients in the cardiac cohort with intraoperative glucose measurements, 189 (0.27%) had intraoperative hypoglycemia. Of 5,448 patients in the liver transplant cohort with intraoperative glucose measurements, 107 (2%) had intraoperative hypoglycemia (Fig. 1).
We compared the characteristics of patients with intraoperative glucose measurements with those of patients without intraoperative glucose measurements (Table 1). Compared with patients without glucose measurements, those with measurements were older and had a higher ASA Physical Status, a higher frequency of emergency case status, and a longer duration of anesthesia. General anesthesia was used in 86% of the cases with intraoperative glucose measurements, compared with 54% of cases without measurements. Diabetes mellitus and obesity were more common in cases with glucose measurements (31% vs 9% and 22% vs 12%, respectively), as were the other comorbidities. Intraoperative insulin was administered in 13% of cases with glucose measurements, compared with 0.5% of cases without glucose measurements.
Characteristics of patients with hypoglycemia were compared with those of patients without hypoglycemia, without adjustment (Table 2). Characteristics of hypoglycemic patients with a SMD > 0.2 compared with those of nonhypoglycemic patients were younger age, higher number of Elixhauser comorbidities, higher frequency of diabetes and chronic kidney disease, and lower frequency of general anesthesia.
Factors associated with hypoglycemia in multivariable analysis
Evaluation of the intercept of the regression lines of the multivariable model of clinical characteristics and odds of hypoglycemia by institution revealed that there was broad overlap for most of the sites (ESM eFig. 1). The conditional intraclass correlation coefficient for MPOG site was 0.044, indicating about 4% of the variation of the model was explained by clustering of cases by contributing site.
Six clinical characteristics were associated with a significantly higher adjusted odds of hypoglycemia: female sex (aOR, 1.24; 95% CI, 1.16 to 1.32; P < 0.001), ASA Physical Status > II (aOR, 1.32; 95% CI, 1.20 to 1.44; P < 0.001), emergency case status (aOR, 1.23; 95% CI, 1.12 to 1.35; P < 0.001), increasing Elixhauser comorbidity count (aOR, 1.30; 95% CI, 1.26 to 1.34; P < 0.001), intraoperative medication to treat vasoplegia (aOR, 1.38; 95% CI, 1.25 to 1.51; P < 0.001), and intraoperative inotrope or vasopressor (aOR, 1.34; 95% CI, 1.22 to 1.48; P < 0.001) (Fig. 3).
Odds of hypoglycemia for each clinical characteristic in multivariable model, OR, and 95% confidence interval, using a definition threshold of hypoglycemia of < 3.3 mmol·L−1. The dot is the adjusted odds ratio of hypoglycemia associated with the specified covariate. The line around the dot indicates the 95% confidence interval. *P < 0.05; ***P < 0.0005. aBMI classification: underweight, < 18.5 kg·m−2; normal weight, 18.5–24.9 kg·m−2; overweight, 25.0–29.9 kg·m−2; class 1 obesity, 30.0–34.9 kg·m−2; class 2 obesity, 35.0–39.9 kg·m−2; class 3 obesity, > 40.0 kg·m−2. Emergency case (yes): ASA Physical Status (I–V) included the emergency case designation. Inpatient vs outpatient: the patient was already admitted to the hospital prior to the day of surgery, rather than admitted on the day of surgery (e.g., for ambulatory surgery cases, a patient admitted to the short stay or day surgery unit would not have an inpatient status). Total number of Elixhauser comorbidities present from the following: diabetes (either uncomplicated or complicated), chronic pulmonary disease, obesity, neurodegenerative disorders, renal failure, and weight loss. Medication for vasoplegia: administration of any of the following medications during the intraoperative period: ascorbic acid, hydrocortisone, cyanocobalamin, thiamine, methylene blue, calcium chloride, or calcium gluconate. Parenteral nutrition: administration of any of the following during the intraoperative period: total parenteral nutrition, peripheral parenteral nutrition, or parenteral nutrition—type not specified. Intraoperative insulin: administration of any of the following glucose-lowering medications during the intraoperative period: insulin (aspart, glargine, NPH, regular, unspecified insulin type, lispro, detemir), or an oral hypoglycemic—glimepiride, pioglitazone, glyburide, glipizide, or metformin. For brevity, and considering that noninsulin medications were used in only 1.67% of patients, this medication category is labelled insulin in the risk analysis models and figures. Antihypertensive: administration of any of the following during the intraoperative period: clonidine, nicardipine, nitroglycerin, nitroprusside, clevidipine, or dexmedetomidine. Inotrope: administration of any of the following during the intraoperative period: dobutamine, dopamine, epinephrine by infusion (not bolus), milrinone, norepinephrine, or vasopressin by infusion (not bolus). Beta blocker: administration of any of the following during the intraoperative period: labetalol, metoprolol, esmolol. ASA = American Society of Anesthesiology Physical Status
Six clinical characteristics were associated with lower odds of hypoglycemia (Fig. 3). Intraoperative parenteral nutrition had the lowest associated odds (aOR, 0.23; 95% CI, 0.11 to 0.47; P < 0.001). As age class increased, the associated odds of hypoglycemia decreased. Higher BMI correlated with progressively lower odds of hypoglycemia. Inpatient status, rather than admission on the day-of-surgery, was associated with lower odds of hypoglycemia (aOR, 0.87; 95% CI, 0.80 to 0.94; P = 0.001). Medication categories associated with lower odds of hypoglycemia were beta blockers (aOR, 0.74; 95% CI, 0.67 to 0.81; P < 0.001) and antihypertensives (aOR, 0.67; 95% CI, 0.60 to 0.75; P < 0.001).
Four clinical characteristics had no statistically significant association with intraoperative hypoglycemia. Intraoperative insulin use was not associated with hypoglycemia (aOR, 0.996; 95% CI, 0.91 to 1.09; P = 0.93). The other three clinical characteristics without an association with hypoglycemia were duration of anesthesia, estimated blood loss class, and red blood cell transfusion class.
In the sensitivity analysis, using a definition of hypoglycemia of < 3.9 mmol·L−1, there were 9,432 cases of hypoglycemia (1.8% out of the 516,045 cases with intraoperative glucose measurements). Intraoperative parenteral nutrition remained the model predictor associated with the lowest odds of hypoglycemia (aOR, 0.23; 95% CI, 0.14 to 0.38; P < 0.001) (ESM eFig. 2). Intraoperative insulin was associated with lower odds of hypoglycemia (aOR, 0.80; 95% CI, 0.75 to 0.86; P < 0.001). The same trends of progressively lower odds with increasing age and with increasing obesity class were present. All six of the clinical characteristics associated with higher odds of hypoglycemia in the primary multivariable analysis were also associated with higher odds of hypoglycemia in the sensitivity analysis.
Increasing Elixhauser comorbidity count was associated with a higher risk of hypoglycemia (OR for each point of increasing count, 1.3). For every increase in the Elixhauser comorbidity count, the probability of hypoglycemia increased significantly, ranging from a probability of 0.3% at a count of 0 to a probability of 1.3% at a count of 6 (ESM eAppendix 4 and 5). Other clinical characteristics associated with a higher probability of hypoglycemia (use of a medication to treat vasoplegia, use of an inotrope or vasopressor, ASA Physical Status III–V, female sex, and emergency case status) had odds ratios ranging from 1.23 to 1.38, and the associated differences in the probability of hypoglycemia were substantially lower (0.08–0.12%) (ESM eAppendices 4 and 5).
Three characteristics (use of parenteral nutrition, older age class, and higher BMI class) were associated with significantly reduced odds of hypoglycemia (ESM eAppendices 4 and 6). The probability of hypoglycemia for patients with parenteral nutrition (0.17%) was less than one quarter the probability of hypoglycemia for patients without parenteral nutrition (0.77%). The probability of hypoglycemia in the oldest age class (0.21%) was one third the probability of hypoglycemia for patients in the youngest age class (0.67%). The marginal means analysis showed a consistent finding, from the inverse comparison: the adjusted odds of hypoglycemia were higher for the youngest age category (18–30 yr) compared with the odds for every age category > 40 yr (odds ratio [OR], 1.57–3.18; P < 0.001) (eAppendix 4). The probability of hypoglycemia for patients with class 3 obesity (0.23%) was less than half the probability of hypoglycemia for underweight patients (0.59%) (ESM Supplementary Material 4). The marginal means analysis demonstrated a consistent finding, from the inverse comparison: the adjusted odds of hypoglycemia were higher for underweight or normal weight patients compared with patients with every class of obesity (OR, 1.48–2.53; P < 0.001) (eAppendix 4). Finally, intraoperative insulin use, anesthesia duration, and estimated blood loss had associated odds of hypoglycemia with CIs overlapping unity (Fig. 3 and ESM eAppendix 4).
Discussion
In this large cross-sectional retrospective multicentre cohort study, we found hypoglycemia to be a rare event: among the 516,045 general cases with intraoperative glucose measurements, hypoglycemia occurred in 3,900 (0.76%) cases. Considering that the probability of hypoglycemia was higher for age classes 18–40 yr than for age classes > 40 yr, and higher for underweight, normal weight, and preobese BMI categories than for any category of obesity, it is noteworthy that patients with intraoperative glucose measurements were, on average, older and within higher obesity classes than patients without measurements.
Parenteral nutrition was associated with lower odds of intraoperative hypoglycemia. Given that labs are frequently monitored for patients dependent on parenteral nutrition, it may be the case that any trend in the direction of hypoglycemia may be detected before the onset of significant hypoglycemia. Another consideration is that patients being treated with parenteral nutrition often have hyperglycemia.21
Intraoperative glucose measurements were reported in only 8% of general cases, compared with 79% of cardiac cases and 81% of liver transplant cases, reflecting the common use of arterial lines in cardiac and transplant surgery, as well as the frequency of intraoperative labs. Compared with the general cohort, cardiac surgery patients with intraoperative glucose measurements had a lower frequency of hypoglycemia, and liver transplant patients had a higher frequency of hypoglycemia (Fig. 1). Patients undergoing liver transplantation may be vulnerable to hypoglycemia because of advanced liver disease; furthermore, gluconeogenesis and glycolysis may be limited during the anhepatic phase and reperfusion periods. Another consideration is that hyperkalemia during liver transplantation may require treatment with insulin. By contrast, the decreased frequency of hypoglycemia for cardiac surgery patients may be related to the prevalence of diabetes and metabolic syndrome in this patient population.
Characteristics associated with slightly higher odds of intraoperative hypoglycemia included use of inotropes or vasopressors, use of medications to treat vasoplegia, and emergency case status. These features are consistent with a single-centre case series of 17 patients with severe intraoperative hypoglycemia.22 Because catecholamines directly stimulate hepatic glucose production and decrease insulin sensitivity, the finding of increased odds of hypoglycemia related to intraoperative administration of inotropic medications may seem counterintuitive. One possible explanation is that fasting patients in shock who require pressor support may have depleted metabolic reserves. Another consideration is that patients with inotropic support may have a higher metabolic demand because of increased myocardial work, heart rate, and blood pressure. By contrast, intraoperative use of a β-blocker was associated with slightly decreased odds of hypoglycemia. Because β-blockers decrease heart rate, blood pressure, and myocardial work, glucose consumption would be expected to decrease. Furthermore, β-blockers reduce pancreatic insulin secretion and decrease insulin sensitivity—actions expected to lead to increased glucose levels.
Implications for anesthesiologists
Fear of causing hypoglycemia during anesthesia is an explanation for the common practice phenomenon labelled “permissive hyperglycemia.”2 Nevertheless, our finding across multiple institutions and a wide variety of patients that hypoglycemia is rare, and that severe hypoglycemia is even more rare, corroborates reports of very few cases of hypoglycemia in clinical trials aimed at aggressive control of intraoperative glucose.2,23 Further research is needed to clarify under what circumstances hypoglycemia is associated with adverse events during anesthesia. When weighing the risk of hypoglycemia against the benefit of treating perioperative hyperglycemia, a clinically relevant observation is that patients with characteristics correlating with insulin resistance (namely, older age and obesity) were at lower risk of hypoglycemia.24,25,26
An unexpected observation was that intraoperative insulin was not associated with an increased risk of hypoglycemia. Anesthesiologists are experts in titrating medications with narrow therapeutic windows to effect and avoiding overdose, and precise dosing of insulin with careful monitoring of glucose may be an explanation for this observation. Thus, several clinically relevant results of our study call for a critical reappraisal of the basis for perioperative “permissive hyperglycemia.”
Limitations
The decision to measure intraoperative glucose was at the discretion of the clinician and not based on any specified criteria. Because glucose was not measured in every case, the true frequency of intraoperative hypoglycemia is underestimated in this study. We cannot determine how often glucose was measured but the result not recorded in the medical record or not transmitted to the database. Furthermore, our validity criteria required documentation of at least two glucose measurements in a case for a low reading to be considered valid, but some of the single low-glucose measurements may have been true hypoglycemia episodes. It is likely that many of the clinicians measured glucose to diagnose or track perioperative hyperglycemia. Some of the model covariates associated with higher or lower odds of hypoglycemia may be influenced by practical case characteristics affecting the opportunity to measure glucose in the intraoperative setting. While it would be expected that patients with type 1 diabetes mellitus would be at elevated risk of intraoperative hypoglycemia, the distinction between type 1 and type 2 diabetes mellitus was not available in the comorbidity dictionary.18 The Multicenter Perioperative Outcomes Group sites are located in the USA and are large, academic hospitals—this has implications for the generalizability (external validity) of the results. Finally, we cannot exclude the possibility that some of the hypoglycemic measurements were laboratory errors. We did not investigate whether patients with hypoglycemia experienced symptoms or adverse effects from the hypoglycemia—this is an area for future research. Despite these limitations, our study is an important contribution to the literature in that it involved multiple sites and a large sample of patients and met stringent data quality standards.14,15 Our patient population included patients with and without diabetes mellitus, a wide span of ages, and a spectrum of case intensity (inpatients and outpatients, general anesthesia and monitored anesthesia care cases, and ASA Physical Status I–V).
Conclusion
In summary, in a large multicentre observational study, we found that intraoperative use of insulin was not associated with an increased risk of hypoglycemia. Further research is needed to define adverse consequences of intraoperative hypoglycemia, as well as the risk of hypoglycemia associated with protocols for the management of perioperative hyperglycemia.
References
Gandhi GY, Nuttall GA, Abel MD, et al. Intensive intraoperative insulin therapy versus conventional glucose management during cardiac surgery: a randomized trial. Ann Intern Med 2007; 146: 233–43. https://doi.org/10.7326/0003-4819-146-4-200702200-00002
Grunzweig K, Nair BG, Peterson GN, et al. Decisional practices and patterns of intraoperative glucose management in an academic medical center. J Clin Anesth 2016; 32: 214–23. https://doi.org/10.1016/j.jclinane.2016.02.027
Berríos-Torres SI, Umscheid CA, Bratzler DW, et al. Centers for Disease Control and prevention guideline for the prevention of surgical site infection, 2017. JAMA Surg 2017; 152: 784–91. https://doi.org/10.1001/jamasurg.2017.0904
NICE-SUGAR Study Investigators, Finfer S, Liu B, et al. Hypoglycemia and risk of death in critically ill patients. N Engl J Med 2012; 367: 1108–18. https://doi.org/10.1056/nejmoa1204942
Misal US, Joshi SA, Shaikh MM. Delayed recovery from anesthesia: a postgraduate educational review. Anesth Essays Res 2016; 10: 164–72. https://doi.org/10.4103/0259-1162.165506
Cryer PE, Davis SN, Shamoon H. Hypoglycemia in diabetes. Diabetes Care 2003; 26: 1902–12. https://doi.org/10.2337/diacare.26.6.1902
Suh SW, Hamby AM, Swanson RA. Hypoglycemia, brain energetics, and hypoglycemic neuronal death. Glia 2007; 55: 1280–86. https://doi.org/10.1002/glia.20440
Simha V, Shah P. Perioperative glucose control in patients with diabetes undergoing elective surgery. JAMA 2019; 321: 399–400. https://doi.org/10.1001/jama.2018.20922
Umpierrez G, Cardona S, Pasquel F, et al. Randomized controlled trial of intensive versus conservative glucose control in patients undergoing coronary artery bypass graft surgery: GLUCO-CABG trial. Diabetes Care 2015; 38: 1665–72. https://doi.org/10.2337/dc15-0303
Van den Berghe G, Wilmer A, Hermans G, et al. Intensive insulin therapy in the medical ICU. N Engl J Med 2006; 354: 449–61. https://doi.org/10.1056/nejmoa052521
Arabi YM, Dabbagh OC, Tamim HM, et al. Intensive versus conventional insulin therapy: a randomized controlled trial in medical and surgical critically ill patients. Crit Care Med 2008; 36: 3190–7. https://doi.org/10.1097/ccm.0b013e31818f21aa
Riegger LQ, Leis AM, Golmirzaie KH, Malviya S. Risk factors for hypoglycemia in children: a multicenter retrospective cohort study. Anesth Analg 2021; 132: 1075–83. https://doi.org/10.1213/ane.0000000000004979
Benchimol El, Smeeth L, Guttmann A, et al. The reporting of studies conducted using observational routinely-collected health data (RECORD) statement. PLoS Med 2015; 12: e1001885. https://doi.org/10.1371/journal.pmed.1001885
Kheterpal S. Clinical research using an information system: the multicenter intraoperative outcomes group. Anesthesiol Clin 2011; 29: 377–88. https://doi.org/10.1016/j.anclin.2011.06.002
Colquhoun DA, Shanks AM, Kapeles SR, et al. Considerations for integration of perioperative electronic health records across institutions for research and quality improvement: the approach taken by the Multicenter Perioperative Outcomes Group. Anesth Analg 2020; 130: 1133–46. https://doi.org/10.1213/ane.0000000000004489
Shah NJ, Mentz G, Kheterpal S. The incidence of intraoperative hypotension in moderate to high risk patients undergoing non-cardiac surgery: a retrospective multicenter observational analysis. J Clin Anesth 2020; 66: 109961. https://doi.org/10.1016/j.jclinane.2020.109961
Kheterpal S, Healy D, Aziz MF, et al. Incidence, predictors, and outcome of difficult mask ventilation combined with difficult laryngoscopy: a report from the multicenter perioperative outcomes group. Anesthesiology 2013; 119: 136–9. https://doi.org/10.1097/aln.0000435832.39353.20
Multicenter Perioperative Outcomes Group. Phenotypes. Available from URL: https://phenotypes.mpog.org (accessed May 2024).
Childs BP, Clark NG, Cox DJ, et al. Defining and reporting hypoglycemia in diabetes: a report from the ADA workgroup on hypoglycemia. Diabetes Care 2005; 28: 124–49.
Searle SR, Speed FM, Miliken GA. Population marginal means in the linear model: an alternative to least square means. Am Stat 1980; 34: 216–21. https://doi.org/10.1080/00031305.1980.10483031
Vennard KC, Selen DJ, Gilbert MP. The management of hyperglycemia in noncritically ill patients treated with continuous enteral or parenteral nutrition. Endocr Pract 2018; 24: 900–6. https://doi.org/10.4158/ep-2018-0150
Schwenk ES, Mraovic B, Maxwell RP, Kim GS, Ehrenfeld JM, Epstein RH. Root causes of intraoperative hypoglycemia: a case series. J Clin Anesth 2012; 24: 625–30. https://doi.org/10.1016/j.jclinane.2012.04.009
Abdelmalak B, Maheshwari A, Kovaci B, et al. Validation of the DeLiT Trial intravenous insulin infusion algorithm for intraoperative glucose control in noncardiac surgery: a randomized controlled trial. Can J Anesth 2011; 58: 606–16. https://doi.org/10.1007/s12630-011-9509-3
Kurauti MA, Soares GM, Marmentini C, Bronczek GA, Souto Branco RC, Boschero AC. Insulin and aging. Vitam Horm 2021; 115: 185–219. https://doi.org/10.1016/bs.vh.2020.12.010
Zhao Y, Yue R. Aging adipose tissue, insulin resistance, and type 2 diabetes. Biogerontology 2024; 25: 53–69. https://doi.org/10.1007/s10522-023-10067-6
Longo M, Zatterale F, Naderi J, et al. Adipose tissue dysfunction as determinant of obesity-associated metabolic complications. Int J Mol Sci 2019; 20: 2358. https://doi.org/10.3390/ijms20092358
Author contributions
Matthew Griffee helped with study design, chart review, submission of the data query to the Multicenter Perioperative Outcomes Group (MPOG), data cleaning rules, analysis of results, and drafting and revising the manuscript. Aleda Leis, Nirav Shah, and Sathish Kumar helped with study design, submission of the data query to MPOG, analysis of results, and drafting and revising the manuscript. Nathan Pace helped with study design and submission of the data query to MPOG, performed the statistical analysis, contributed to analysis of results, edited the manuscript, and composed the statistical methods section. Graciela Mentz helped with analysis of results, revising the manuscript, and interpretation of statistical results. Lori Riegger helped with study concept, study design, submission of the data query to MPOG, analysis of results, and drafting and revising the manuscript.
Acknowledgements
The authors gratefully acknowledge the valuable contributions to protocol and final manuscript review by the Multicenter Perioperative Outcomes Group (MPOG) collaborators, including Robert B. Schonberger, MD, MHS (Department of Anesthesiology, Yale School of Medicine, New Haven, CT, USA) for substantial contributions to the analysis and interpretation of data for the work, and assistance in critically revising the work for important intellectual content); Robert E. Freundlich, MD, MS (Department of Anesthesiology, Vanderbilt University Medical Center, Nashville, TN, USA) for substantial contributions to the conception or design of the work, substantial contributions to data analysis and interpretation, and assistance in critically revising the work for important intellectual content; Bhiken I. Naik, MBBCh, MSCR (University of Virginia, Charlottesville, VA, USA) for substantial contributions to the conception and design of the work); Robert H. Coleman, BSCS (Department of Anesthesiology, University of Michigan, Ann Arbor, MI, USA) for data access and query development; David Clark (MPOG Administrative Coordinator, Department of Anesthesiology, University of Michigan) for data and statistical server access; Robli Kennedy (Department of Anesthesiology, University of Utah, Salt Lake City, UT, USA) for administrative assistance; and Michelle T. Vaughn, MPH (MPOG Lead Research Facilitator, University of Michigan) for administrative assistance. Sachin Kheterpal, MD, MBA (Department of Anesthesiology, University of Michigan) critically revised the manuscript.
Disclosures
None.
Funding statement
This work was supported by departmental and institutional resources at each contributing site. In addition, partial funding to support underlying electronic health record data collection into the Multicenter Perioperative Outcomes Group registry was provided by Blue Cross Blue Shield of Michigan/Blue Care Network as part of the Blue Cross Blue Shield of Michigan/Blue Care Network Value Partnerships program. Although Blue Cross Blue Shield of Michigan/Blue Care Network and Multicenter Perioperative Outcomes Group work collaboratively, the opinions, beliefs, and viewpoints expressed by the authors do not necessarily reflect the opinions, beliefs, and viewpoints of Blue Cross Blue Shield of Michigan/Blue Care Network or any of its employees. This research was supported in part through computational resources and services provided by Advanced Research Computing (ARC), a division of Information and Technology Services at the University of Michigan, Ann Arbor, MI, USA.
Editorial responsibility
This submission was handled by Dr. Stephan K. W. Schwarz, Editor-in-Chief, Canadian Journal of Anesthesia/Journal canadien d’anesthésie.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Griffee, M.J., Leis, A.M., Pace, N.L. et al. Intraoperative hypoglycemia among adults with intraoperative glucose measurements: a cross-sectional multicentre retrospective cohort study. Can J Anesth/J Can Anesth (2024). https://doi.org/10.1007/s12630-024-02816-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12630-024-02816-z