Abstract
Purpose
A classic general anesthesia is performed by induction with an intravenous hypnotic (such as propofol) and maintenance with a volatile anesthetic (such as sevoflurane). The aim of the present study was to compare the effects of a propofol/sevoflurane maintenance regimen with that of a sevoflurane regimen on recovery profiles.
Methods
One hundred and sixty patients, who were ASA 1 or 2, 45–65 years of age, and scheduled for elective gastrointestinal surgery under combined general/epidural anesthesia, were allocated randomly to receive the sevoflurane maintenance regimen (group S, n = 80) or sevoflurane/propofol regimen (group SP, n = 80). After induction, anesthesia was maintained with sevoflurane in group S and sevoflurane with propofol (1.2 μg/ml target plasma concentration) in group SP. Bispectral index (BIS) values were maintained within 40–60 during the maintenance. Time to extubation, incidence of serious coughing and agitation, and other recovery characteristics were evaluated during emergence.
Results
The time to awakening and extubation in group SP were 7.2 ± 2 min and 8.0 ± 1.8 min, respectively, which were shorter than those results in group S (12.3 ± 1.5 and 12.8 ± 1.6 min, respectively) (P < 0.05). The incidence of serious coughing and agitation in SP (30 % and 25 %) was lower than that of group S (68 % and 53 %) (P < 0.05). BIS value, pain score, requirements of analgesics and antiemetics in the PACU, and length of stay in the PACU were similar in the two groups.
Conclusions
Compared to sevoflurane maintenance, coadministration of propofol and sevoflurane provides faster awakening and extubation with a low incidence of emergence coughing and agitation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A classic general anesthesia is performed by induction with an intravenous hypnotic (such as propofol) and maintenance with a volatile anesthetic (such as sevoflurane), with intermittent or continuous opioids and muscle relaxants. Coadministration of propofol and sevoflurane for maintenance has been suggested recently [1, 2] because of the antiemetic effect of propofol [3], the myocardial protective effects of sevoflurane [4, 5], and possible smooth emergence resulting from low administered amounts of each anesthetic. Moreover, it has been frequently reported that propofol can reduce the incidence of emergence agitation in children undergoing sevoflurane anesthesia [6–8]. However, the effects of coadministration of propofol and sevoflurane on the recovery profiles from general anesthesia in adults have not been fully investigated.
For a proper coadministration of propofol and sevoflurane, the type and extent of their interaction has been described in recent studies [2, 9], in which an additive approach was demonstrated. The additive effect of these two drugs was partly attributed to their separate binding sites and converging pathways of action on the GABAA receptor [9]. Furthermore, Schumacher et al. [1] reported the ratios between effect-site concentrations with 50 % of effect (Ce50) of sevoflurane and propofol at different clinical endpoints (tolerance of shaking and shouting, tetanic stimulation, laryngeal mask airway insertion, and laryngoscopy) are identical, averaging 0.43 vol%/ml/μg, which gave us a reference to calculate the combined drug potency in steady state.
We hypothesized that the propofol/sevoflurane maintenance regimen may provide a faster and smoother emergence than sevoflurane alone. This prospective, randomized, double-blinded study was conducted to verify this hypothesis in patients undergoing elective abdominal surgery.
Materials and methods
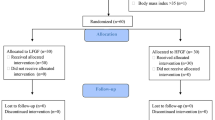
The study was registered at http://www.chictr.org/cn/ (ChiCTR-TRC-13003577) and was approved by the Ethics Committee of Zhongshan Hospital, Fudan University, China. Between August 2013 and November 2013, we enrolled 160 consecutive patients, 45–65 years of age and ASA I–II, who were scheduled for elective gastrointestinal surgery under combined general/epidural anesthesia. Written informed consent was obtained from all patients before randomization. Exclusion criteria were cognitive impairment; history of uncontrolled hypertension; a recent respiratory tract infection; history of respiratory disease such as asthma or chronic obstructive lung disease; heart block greater than first degree; kidney or liver disease; chronic use of antipsychotic medications; and body mass index ≥30 kg/m2.
Patients were randomly assigned into two groups by the program of SPSS16.0 software. No preoperative sedatives or analgesics were administered. The sevoflurane group (group S, n = 80) received sevoflurane for anesthesia maintenance, and sevoflurane/propofol group (group SP, n = 80) received sevoflurane and propofol (1.2 μg/ml target plasma concentration) for maintenance. After epidural and central vein catheter placement, electrocardiogram, pulse oximetry, invasive arterial pressure, and end-tidal CO2 (EtCO2) were applied. Before induction, 3 ml 2 % lidocaine was given through the epidural catheter as a test dose, then 10 ml 0.375 % bupivacaine was given as the first dose and followed by 5 ml 0.375 % bupivacaine supply per hour. Patients were excluded from the study if the epidural anesthesia could not be successfully performed.
Propofol was administered via a target-controlled infusion (TCI) device (Cardinal Health, Basingstoke, UK), which used a three-compartment population pharmacokinetic model defined by Schnider et al. [10]. Induction was performed with propofol TCI (target plasma concentration was set at 4.0 μg/ml), remifentanil (0.15 μg/kg/min) fentanyl 1 μg/kg, and rocuronium bromide 0.6–0.8 mg/kg. After tracheal intubation, ventilation was controlled artificially and the ventilatory parameters were adjusted for EtCO2 between 35 and 45 mmHg in 50 % O2/air and a flow rate of 1 l/min. After induction, remifentanil infusion was stopped in the two groups; propofol TCI was turned off in group S. In group SP, the target plasma concentration of propofol was decreased to 1.2 μg/ml, which would give an equivalent of 0.52 vol% sevoflurane or approximately 0.3 MAC (the Ce50 ratio of sevoflurane to propofol equals 0.43 vol%/ml/μg [1]).
During the operation, sevoflurane concentration in the two groups was regulated to maintain the bispectral index value (BIS; A-2000TM SP, Aspect Medical System, Norwood, MA, USA) between 40 and 60. The MAC and BIS values of each patient from the time of propofol at 1.2 μg/ml to the time of “time zero” were recorded every half hour. Intermittent intravenous fentanyl (50 μg) and rocuronium (10 mg) were given to the patients as needed, and antiemetic drugs or nonsteroidal antiinflammatory drugs were avoided. Tachycardia (heart rate >100 beats/min) was treated with i.v. esmolol in 10-mg increments. Bradycardia (heart rate <40 beats/min) was treated with i.v. atropine 0.5 mg. Hypotension (MAP <60 mmHg) was treated with i.v. phenylephrine at 0.1-mg increments or continuous i.v. norepinephrine infusion and i.v. fluid administration.
Oral suction was performed when the surgery was complete, and reversal agents (neostigmine 0.04 mg/kg and atropine 20 mg/kg) were given after the return of neuromuscular function. Following these steps, sevoflurane and/or propofol were turned off in the two groups. The ‘turn off’ was defined as ‘time zero’ of the emergence process, and the ventilation flow rate was then increased to 8 l/min. The emergence period is defined from ‘time zero’ to 2 min after tracheal extubation. All patients were awakened by continual verbal commands to open their eyes. Tracheal extubation was performed when patients began breathing spontaneously and were able to follow verbal commands with a BIS value >70. All investigators and patients were blinded to group assignment. During emergence, recovery profiles were observed at different time points by the same anesthesiologist blinded to the study protocol. The patient monitor screen and syringes were hidden before the observer entered the operating room at the time of ‘time zero.’
During emergence, the widely used Ricker sedation–agitation [11] scale was used to evaluate the level of agitation: 1 = minimal or no response to noxious stimuli; 2 = arouses to physical stimuli but does not communicate; 3 = difficult to arouse but awakens to verbal stimuli or gentle shaking; 4 = calm and follows commands; 5 = anxious or physically agitated and calms to verbal instructions; 6 = requiring restraint and frequent verbal reminding of limits; and 7 = pulling at tracheal tube, trying to remove catheters, or striking at staff. Any score on the sedation–agitation scale ≥5 was defined as emergence agitation.
The grade of coughing during emergence was assessed by a four-point scale [12]: grade 0 = no cough, grade 1 = light (single) cough, grade 2 = moderate cough (more than one episode of nonsustained coughing), and grade 3 = sustained and repetitive cough movements with head lift. Grade 3 was defined as serious coughing. The time to verbal response and extubation, BIS value at the time of extubation, and the intraoperative hemodynamic events (hypotension, tachycardia, and bradycardia) were assessed and recorded in all patients. Nausea and vomiting score (0 = no nausea; 1 = mild nausea; 2 = severe nausea requiring antiemetics; and 3 = retching, vomiting, or both), and numerical rating scale (NRS) for pain (0 = no pain and 10 = worst pain imaginable) were evaluated in the postanesthesia care unit (PACU). When NRS was ≥5, additional i.v. fentanyl 1 μg/kg was given, and i.v. tropisetron 6 mg was given if the nausea and vomiting score was ≥2.
Statistical analysis
The sample size calculation was based on the main endpoint of recovery profiles, the time to extubation. The reported time to extubation of sevoflurane maintenance is 13 min [13]. Based on our preliminary study, with the assumption that propofol/sevoflurane would shorten the time to extubation by 40 % (α of 0.05 and a power of 80 %) compared to sevoflurane regimen, 77 subjects were required in each group, respectively. Therefore, we included 80 patients per group to compensate for dropouts.
The values were expressed as mean (SD), median (range), or the number of patients (%). Statistical analyses were performed using SPSS16.0 (SPSS, Chicago, IL, USA). The independent t test was used to analyze parametric data, and nonparametric data were analyzed by Mann–Whitney U test. Repeated-measure variables were analyzed using linear mixed models with a Bonferroni correction. Categorical variables were evaluated by the Fisher’s exact test. P < 0.05 was considered statistically significant.
Results
There were no differences among two groups with respect to age, gender, height, weight, smoking history, or type and duration of surgery (Table 1).
The time to verbal response of group SP (7.2 ± 2 min) was shorter than that of group S (12.3 ± 1.5 min) (P < 0.05). The time to extubation in group SP was 8.0 ± 1.8 min, which was shorter than that of group S (12.8 ± 1.6 min) (P < 0.05) (Table 2). Incidences of agitation and serious coughing in SP (25 % and 30 %) were lower than these of group S (53 and 68 %) (P < 0.05) (Fig. 1). The requirements of fentanyl and rocuronium were comparable in group S (170.6 ± 22.5 and 45.6 ± 7.5 mg) and group SP (175.6 ± 15.8 and 48.3 ± 6.4 mg). The average MAC/BIS values during maintenance in group S and group SP was 0.98/48 and 0.6/47, respectively.
Incidence of serious coughing and agitation during emergence. Time from end of surgery to 2 min after extubation is defined as the emergence period. Agitation is defined as a sedation–agitation scale score ≥5. Serious coughing is defined as sustained and repetitive cough movements with head lift. ▲● P < 0.05 vs. group S
BIS value, pain score, intraoperative MAP and HR, the requirements of analgesics and antiemetics in PACU, and the length of stay in PACU were similar in the two groups (Table 3). The number of patients who required intervention for intraoperative hypotension in groups S and SP was 28 and 31, respectively. Four patients in group SP and 6 patients in group S complained of nausea and vomiting; all were successfully treated with tropisetron (6 mg). No other severe adverse effects were observed.
Discussion
In patients undergoing abdominal surgery, combined general/epidural anesthesia is usually performed. Given the analgesic and muscle relaxant effects of epidural anesthesia, only a few opioids and muscle relaxants were given during the maintenance period [14]. In addition to hypnosis, the main function of maintenance drugs is to inhibit the cough reflex elicited by the tracheal tube on patient’s larynx and trachea. Therefore, it seems reasonable to perform and investigate the sevoflurane/propofol maintenance regimen in these patients. The results from the present study suggest that the coadministration regimen seems applicable in combined general/epidural anesthesia. The time to awakening and extubation in the coadministration group was shorter than that in the sevoflurane group. Furthermore, continuous propofol infusion with sevoflurane reduced the incidence of emergence coughing and agitation induced by sevoflurane without increasing the incidence of other complications.
Additive interactions occur when the effect of coadministration of two drugs equals the effect of either alone in an amount equal to the sum of the two drugs [15]. The additive effect of propofol and sevoflurane has been reported in many studies [2, 9], and the Ce50 ratio(0.43 vol%/ml/μg) of sevoflurane to propofol at different clinical endpoints reported by Schumacher et al. [1] can be used to calculate the combined drug potency in steady state. Based on these findings, our study used the same pharmacokinetic variables for propofol and TCI software as Schnider et al. [10], a propofol target plasma concentration of 1.2 μg/ml would give an equivalent of 0.52 vol% sevoflurane or approximately sevoflurane 0.3 MAC.
Sevoflurane has a rapid emergence time after discontinuation, which is the result of its low blood gas partition coefficient [16]. By metabolic elimination and redistribution, propofol also has shown a rapid recovery from anesthesia [17]. In anesthesia maintained with propofol/remifentanil or sevoflurane/remifentanil, the time to tracheal extubation in the propofol group was shorter than that of the sevoflurane group [12, 18]. Importantly, because the recovery profiles of coadministration of sevoflurane and propofol have not been reported in recent literature, our study may be the first to report that the time to awakening and extubation in the combined group is shorter than that of the sevoflurane group, possibly because the low administered amounts of each anesthetic provide a relatively fast drug elimination. In outpatient anesthesia, it has been reported that the time to verbal commands and extubation in the sevoflurane maintenance group are 7.8 ± 3.8 and 8.2 ± 4.2 min, respectively, both of which are shorter than that of our study of sevoflurane alone. The discrepancy may be attributed to the type and short duration of operation in their study, and the flow rate, which was adjusted to 10 l/min (8 l/min in our group) at the end of surgery, may also accelerate the sevoflurane washout [19].
The airway protective effects of propofol have been reported in many studies [20–22]. In our study, the incidence of serious coughing in group SP (30 %) was lower than that of group S (68 %), which indicates the anti-coughing effect of propofol is better than that of sevoflurane. The deeper residual anesthetic effect of propofol at the time of extubation could be responsible for the low incidence of coughing in group SP, because the anti-coughing effect of propofol is related to its high residual concentration [13]. In other words, during emergence, a certain residual concentration of propofol effectively retrieves the poor anti-coughing effects of sevoflurane, resulting in a low coughing rate in group SP. Furthermore, by using the Ricker sedation–agitation scale, our study also demonstrated the combination of propofol and sevoflurane decreased the agitation induced by sevoflurane, which is consistent with the results in children [6–8]. Another consideration for coughing is the patient’s smoking habit, because the incidence of serious coughing during emergence is significantly higher in smokers than in nonsmokers [19]. However, the ratio of smokers to nonsmokers was comparable among the two groups in the present study.
For the purpose of investigating the pure effects of combined sevoflurane/propofol maintenance on the patient recovery profiles, we did not use lidocaine [24], short-acting opioids [23], or dexmedetomidine [25] to prevent emergence coughing or agitation. On the other hand, these drugs may also delay the emergence from general anesthesia. BIS seems to perform quite well in deep, steady-state anesthesia with propofol [26] and sevoflurane [27]. However, it has been reported that BIS values at the time of loss of consciousness in patients anesthetized with sevoflurane were significantly higher than those in patients anesthetized with propofol [28]. Consistently, the average MAC/BIS values during maintenance in group S and group SP were 0.98/48 and 0.57/47, respectively, which indicates that the influence of propofol on BIS values is greater than that of sevoflurane.
There are several limitations to this study. First, we only used one constant concentration of propofol (1.2 μg/ml) and controlled the depth of anesthesia by adjusting the concentration of sevoflurane, so it is unclear what is the best combination regimen of these two drugs, and whether the different “turn off” regimen of these two drugs can also influence patient recovery profiles. Second, as the results from our study are mainly related to patients undergoing combined epidural/general anesthesia, the effects of coadministration on the recovery profiles in patients undergoing common general anesthesia still need to be studied. Third, as sample size calculation was based on the incidence of emergence coughing and the time to extubation, the sample size may not be sufficient to check the comprehensive quality of recovery.
In conclusion, compared to the sevoflurane maintenance regimen, the coadministration of propofol/sevoflurane provides faster recovery with a low incidence of emergence coughing and agitation. These results may support the coadministration of propofol/sevoflurane for anesthesia maintenance in patients under combined general/epidural anesthesia.
References
Schumacher PM, Dossche J, Mortier EP, Luginbuehl M, Bouillon TW, Struys MM. Response surface modeling of the interaction between propofol and sevoflurane. Anaesthesiology. 2009;111:790–804.
Diz JC, Del Rio R, Lamas A, Mendoza M, Duran M, Ferreira LM. Analysis of pharmacodynamic interaction of sevoflurane and propofol on bispectral index during general anaesthesia using a response surface model. Br J Anaesth. 2010;104:733–9.
Tramer M, Moore A, McQuay H. Meta-analytic comparison of prophylactic antiemetic efficacy for postoperative nausea and vomiting: propofol anaesthesia vs omitting nitrous oxide vs. total i.v. anaesthesia with propofol. Br J Anaesth. 1997;78:256–9.
Djalali AG, Sadovnikoff N. Cardioprotective properties of sevoflurane in patients undergoing coronary surgery with cardiopulmonary bypass are related to the modalities of its administration. Anaesthesiology. 2005;102:699–700 author reply.
Guarracino F, Landoni G, Tritapepe L, Pompei F, Leoni A, Aletti G, Scandroglio AM, Maselli D, De Luca M, Marchetti C, Crescenzi G, Zangrillo A. Myocardial damage prevented by volatile anesthetics: a multicenter randomized controlled study. J Cardiothorac Vasc Anesth. 2006;20:477–83.
Kim MS, Moon BE, Kim H, Lee JR. Comparison of propofol and fentanyl administered at the end of anaesthesia for prevention of emergence agitation after sevoflurane anaesthesia in children. Br J Anaesth. 2013;110:274–80.
Abu-Shahwan I. Effect of propofol on emergence behavior in children after sevoflurane general anaesthesia. Paediatr Anaesth. 2008;18:55–9.
Aouad MT, Yazbeck-Karam VG, Nasr VG, El-Khatib MF, Kanazi GE, Bleik JH. A single dose of propofol at the end of surgery for the prevention of emergence agitation in children undergoing strabismus surgery during sevoflurane anaesthesia. Anaesthesiology. 2007;107:733–8.
Sebel LE, Richardson JE, Singh SP, Bell SV, Jenkins A. Additive effects of sevoflurane and propofol on gamma-aminobutyric acid receptor function. Anaesthesiology. 2006;104:1176–83.
Schnider TW, Minto CF, Gambus PL, Andresen C, Goodale DB, Shafer SL, Youngs EJ. The influence of method of administration and covariates on the pharmacokinetics of propofol in adult volunteers. Anaesthesiology. 1998;88:1170–82.
Kim SY, Kim JM, Lee JH, Song BM, Koo BN. Efficacy of intraoperative dexmedetomidine infusion on emergence agitation and quality of recovery after nasal surgery. Br J Anaesth. 2013;111:222–8.
Minogue SC, Ralph J, Lampa MJ. Laryngotracheal topicalization with lidocaine before intubation decreases the incidence of coughing on emergence from general anaesthesia. Anesth Analg. 2004;99:1253–7 table of contents.
Hans P, Marechal H, Bonhomme V. Effect of propofol and sevoflurane on coughing in smokers and non-smokers awakening from general anaesthesia at the end of a cervical spine surgery. Br J Anaesth. 2008;101:731–7.
Agarwal A, Pandey R, Dhiraaj S, Singh PK, Raza M, Pandey CK, Gupta D, Choudhury A, Singh U. The effect of epidural bupivacaine on induction and maintenance doses of propofol (evaluated by bispectral index) and maintenance doses of fentanyl and vecuronium. Anesth Analg. 2004;99:1684–8 table of contents.
Hendrickx JF, Eger EI 2nd, Sonner JM, Shafer SL. Is synergy the rule? A review of anesthetic interactions producing hypnosis and immobility. Anesth Analg. 2008;107:494–506.
Gauthier A, Girard F, Boudreault D, Ruel M, Todorov A. Sevoflurane provides faster recovery and postoperative neurological assessment than isoflurane in long-duration neurosurgical cases. Anesth Analg. 2002;95:1384–8 table of contents.
Hughes MA, Glass PS, Jacobs JR. Context-sensitive half-time in multicompartment pharmacokinetic models for intravenous anesthetic drugs. Anaesthesiology. 1992;76:334–41.
Hocker J, Tonner PH, Bollert P, Paris A, Scholz J, Meier-Paika C, Bein B. Propofol/remifentanil vs. sevoflurane/remifentanil for long lasting surgical procedures: a randomised controlled trial. Anaesthesia. 2006;61:752–7.
Nathanson MH, Fredman B, Smith I, White PF. Sevoflurane versus desflurane for outpatient anaesthesia: a comparison of maintenance and recovery profiles. Anesth Analg. 1995;81:1186–90.
Sundman E, Witt H, Sandin R, Kuylenstierna R, Boden K, Ekberg O, Eriksson LI. Pharyngeal function and airway protection during subhypnotic concentrations of propofol, isoflurane, and sevoflurane: volunteers examined by pharyngeal videoradiography and simultaneous manometry. Anaesthesiology. 2001;95:1125–32.
Batra YK, Ivanova M, Ali SS, Shamsah M, Al Qattan AR, Belani KG. The efficacy of a subhypnotic dose of propofol in preventing laryngospasm following tonsillectomy and adenoidectomy in children. Paediatr Anaesth. 2005;15:1094–7.
Guglielminotti J, Rackelboom T, Tesniere A, Panhard X, Mentre F, Bonay M, Mantz J, Desmonts JM. Assessment of the cough reflex after propofol anaesthesia for colonoscopy. Br J Anaesth. 2005;95:406–9.
Shajar MA, Thompson JP, Hall AP, Leslie NA, Fox AJ. Effect of a remifentanil bolus dose on the cardiovascular response to emergence from anaesthesia and tracheal extubation. Br J Anaesth. 1999;83:654–6.
Saghaei M, Reisinejad A, Soltani H. Prophylactic versus therapeutic administration of intravenous lidocaine for suppression of post-extubation cough following cataract surgery: a randomized double blind placebo controlled clinical trial. Acta Anaesthesiol Taiwan. 2005;43:205–9.
Guler G, Akin A, Tosun Z, Eskitascoglu E, Mizrak A, Boyaci A. Single-dose dexmedetomidine attenuates airway and circulatory reflexes during extubation. Acta Anaesthesiol Scand. 2005;49:1088–91.
Bonhomme V, Deflandre E, Hans P. Correlation and agreement between bispectral index and state entropy of the electroencephalogram during propofol anaesthesia. Br J Anaesth. 2006;97:340–6.
Revuelta M, Paniagua P, Campos JM, Fernandez JA, Martinez A, Jospin M, Litvan H. Validation of the index of consciousness during sevoflurane and remifentanil anaesthesia: a comparison with the bispectral index and the cerebral state index. Br J Anaesth. 2008;101:653–8.
Kodaka M, Johansen JW, Sebel PS. The influence of gender on loss of consciousness with sevoflurane or propofol. Anesth Analg. 2005;101:377–81 table of contents.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Liang, C., Ding, M., Du, F. et al. Sevoflurane/propofol coadministration provides better recovery than sevoflurane in combined general/epidural anesthesia: a randomized clinical trial. J Anesth 28, 721–726 (2014). https://doi.org/10.1007/s00540-014-1803-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-014-1803-0