Abstract
Although pain after craniotomy is a clinically significant problem that has a continuously expanding literature, it is still a source of concern and controversy. Postcraniotomy headache (PCH) has been neglected for years. It is assessed regularly by only a few neurosurgical centers, and its frequency and severity tend to be underestimated by medical staff; hence, PCH is often undertreated and poorly managed. Various patient and surgical factors have an impact on the severity and incidence of PCH; thus, effective analgesic protocols are hard to define, which could explain the absence of available therapeutic guidelines. According to recent studies, certain surgical measures and the use of local anesthetics are promising in the prevention of PCH. NSAIDs seem to have inadequate analgesic effects, whereas opioids have a wide range of drawbacks; nevertheless, both types of medicaments are regarded as cornerstones of a balanced and adequate multimodal therapy. The purpose of this review is to collect the currently available knowledge about the incidence, assessment, pathophysiological mechanism, and predictors of acute and chronic PCH. Therefore, a broad search of the literature has been carried out to collect evidence of potential prevention and treatment strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pain following intracranial surgery has been neglected for decades. Several studies have investigated its frequency and etiology and found that both acute and chronic postcraniotomy headaches (PCH) are common and clinically significant problems [1–3]. Furthermore, there are no available therapeutic guidelines on PCH because of the lack of well-designed studies, which makes pain management particularly difficult. Non-opioids and opioids are widely used to relieve pain, but often without any clinical evidence, and sometimes even without satisfactory results. Nevertheless, effective pain management and prevention are of great importance in avoiding postoperative complications and securing positive outcomes. Therefore, a literature search was performed to summarize current evidence on the incidence, etiology, risk factors, and available prevention and treatment methods of PCH.
Incidence
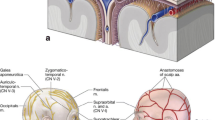
The International Headache Society defines PCH as a secondary headache developing within 7 days after craniotomy: the acute type lasts up to 3 months, and the chronic type persists more than 3 months [4]. An early study showed that almost 60 % of patients experienced pain following craniotomy, intensity being moderate or severe in two-thirds of the cases. The highest incidence was observed 12 h after operation [1]. Subsequent studies confirmed preliminary results: 69 % and 76 % of patients complained about pain after different types of craniotomies. The degree of pain, its onset, and its evolution were also comparable with previous studies; mainly, moderate and severe in nature with a maximum occurrence within 48 h postoperatively [2, 5]. Chronic postcraniotomy headache (CPCH) is often associated with acoustic neurinoma operations: 36.7–49.5 % of the patients still experienced pain after 12 weeks and 33.1–43 % beyond 1 year later [6, 7]. The incidence of chronic PCH was 28.4 % at 3 years postoperatively [7]. Although persistent headache has a smaller incidence than the acute type, because of its long-lasting nature the quality of life and ability to work is negatively influenced [8]. In a cohort group, 14.2 % of the responders were slightly and 18.5 % heavily affected in their everyday life after such interventions [7].
Pain assessment
Because it is subjective in nature, pain is hard to quantify and measure. Both pain assessment and management are essential and are practically useless without each other. Only 57 % of the neurosurgical centers assessed pain after craniotomy despite the fact that simple one-dimensional tools are widely available [9]. The most frequently used methods to measure acute pain are the discrete 0–10 numeric rating scale (NRS) and the visual analog scale (VAS): both have similar attributes and sensitivity. For the assessment of CPCH the most commonly used evaluation methods are the McGill Pain Questionnaire, which measures affective, sensory, and total pain index, and The Brief Pain Inventory, which focuses on seven aspects of life with which pain interferes [10]. Pain is often underestimated with direct observation, especially in severe cases; thus, the aforementioned methods prove to be essential [3].
Etiology
Numerous patient- and operation-related factors could influence PCH. Although several trials have investigated the possible correlations between PCH and various independent predictors, such as clinical diagnosis, surgical approach, age, sex, and mental status, only limited evidence was identified [1, 2, 6, 7, 11]. Age, sex, and surgical approach seem to have a significant influence on PCH [2, 7, 12].
The possible pathophysiological mechanisms are mechanical and chemical irritation of the muscles, periosteum, dura mater, and trigeminal nerves during surgery, whereas cerebrospinal fluid (CSF) leakage is also noticeable occasionally [1, 11, 12]. Dural tension, aseptic meningitis, and the adherence of cervical muscles to the dura could also contribute to PCH development, where the mass of the muscles being involved is also crucial [1, 6, 7, 13]. The development of CPCH is probably related to central sensitization, an increased ability to sense nociceptive signals that can be triggered by extensive and acute pain stimuli [14, 15].
Acute pain is reported to be pounding and pulsating with the highest intensity in the area of the craniotomy, but continuous and steady forms may also occur [1, 16]. CPCH is mainly presented as short attacks with occasional nausea and photophobia occurring approximately 20–30 times a month and is usually triggered by physical exercise, coughing, and bending [6].
Women and younger patients have worse outcomes with regard to incidence and severity [1, 2, 7]. A cohort study involving 1,375 patients showed that women experience headaches 40 % more often compared to men and were 60 % more likely to rate them severe [7]. Patients older than 75 years had less severe pain, required a smaller amount of narcotics, and were less likely to be affected by the headache [7]. Pain intensity and age showed an inverse correlation where VAS scores reduced by −0.18 U with every year of age [2]. CPCH sufferers 70 years or older had on average 1-h-long attacks, although such attacks lasted more than 4 h in patients 41–55 years of age [7].
The surgical approach, which can be either infratentorial or supratentorial [11], seems to have a great influence on the incidence, seriousness, and analgesic requirements of PCH [2, 5]. Infratentorial procedures, especially acoustic neuroma and posterior fossa surgeries, are associated with higher pain scores [2, 5, 7]. A study comparing supra- and infratentorial routes found that average pain intensity 24 h postoperatively was 4.5 ± 2.7 and 6.3 ± 2.6, with a total opioid consumption of 6.6 ± 12.6 mg versus 26.9 ± 88.9 mg, respectively [2]. Similar differences were noticed with a 1-year follow-up time: the prevalence of CPCH after supratentorial surgeries was 11.9 %, notably lower than the 60 % and 70 % experienced after retrosigmoid and translabyrinthine approaches, respectively [7, 13]. The relationship between pain intensity and the location of craniotomy could be explained by the difference in muscle mass underlying the surgical incision, and this also explains the higher pain scores after suboccipital and subtemporal interventions [1, 5, 17]. The surgical approach is crucial; therefore, to minimize complications, the possibly less invasive method should be chosen [2, 5, 7]. The aforementioned factors may also help to predict which patients are likely to develop PCH, thus enabling medical staff to give them special attention.
Potential prevention strategies
Scalp infiltration
The effects of bupivacaine and ropivacaine as intraoperatively used local anesthetics have been investigated recently [18–20]. The aim was to determine whether infiltration of the surgical wound during interventions could decrease postoperative pain or perhaps reduce the painkiller needs of patients (Table 1). With regard to their analgesic ability, a single dose of ropivacaine proved to be more potent than multiple bupivacaine injections [18, 19], whereas another study failed to detect any differences [20]. These local anesthetics showed no opioid-sparing effect, as it was previously suggested [18, 20]. Although bupivacaine and ropivacaine reduced morphine consumption in the first 2 h, after 16 h the total amount of administered morphine was similar in all groups [20]. This slight opioid-sparing effect could be potentially beneficial, as it might facilitate the recognition of neurosurgical complications in the early postoperative phase without additional risks.
Scalp block
The scalp is richly innervated with sensory nerves that are intensely stimulated during craniotomy [11]; this can lead to central sensitization, a process after which pain stimuli will be experienced with superior intensity. A way to prevent this phenomenon and to reduce the degree of acute PCH is the usage of scalp nerve blockade [21–24] (Table 1). A prospective study showed that patients who received infiltrative nerve block with 0.5 % bupivacaine had decreased pain scores on the first and second postoperative days [2]. Furthermore, bupivacaine successfully attenuated hemodynamic changes and reduced analgesic requirement during operations [24, 25]. Neither bupivacaine nor ropivacaine showed any postoperative opioid-sparing effect, as the cumulative dose of postoperatively given codeine and the first time of administration were similar in every case. Through unknown preemptive mechanisms, scalp block can decrease PCH, and its effect lasts longer than expected; therefore, it can be useful as an adjuvant analgesic [21–23].
Surgical measures
Besides the actual location of the surgery, numerous surgical factors may influence PCH [12, 26–29]. The retrosigmoid approach formerly included the removal of a bone flap (craniectomy) to gain appropriate access to the cerebropontine angle [7, 12]. Surgical techniques have been modified, and surgeons have concluded that bone flap replacement (craniotomy) not only serves cosmetic purposes but can also reduce the incidence of PCH. Schaller and co-workers [29] found a reduction from 94 to 27 % in CPCH frequency when the bone flap was replaced at the end of surgery. Patients who underwent craniotomy had a lower rate of headache at discharge (19 %) and in 1 year time (1 %) than patients with craniectomy, who had rates of 43 % and 10 %, respectively. The decrease of CSF leakage (6 % vs. 20 %) could be one explanation for this phenomenon [12]. The insertion of methyl methacrylate between the cervical muscles and dura, at the end of surgeries, is also capable of lessening the occurrence of PCH [26]. When an abdominal adipose graft was applied, chronic pain and its severity were reduced compared to standard wound closure techniques (11.9 vs. 30.3 %) [28]. A retrospective analysis showed that duraplasty instead of direct dural closure is an effective way to prevent postoperative consequences by avoiding the tension of the dura, because 100 % of patients with direct dura closure had PCH in contrast to the 0 % of the duraplasty group [29]. These techniques are primarily suitable to prevent CPCH and may include craniotomy instead of craniectomy, bone replacement with various filling materials, and duraplasty.
Medication
The ideal analgesic agent should provide adequate pain relief in both acute and chronic PCH without affecting consciousness and respiratory function or without increasing the prevalence of nausea, vomiting, and local bleeding. Nonopioid analgesics such as paracetamol, nonsteroidal antiinflammatory drugs (NSAIDs), cyclooxygenase-2 (COX-2) inhibitors, and opioid derivatives such as codeine, tramadol, and morphine are considered as treatment options. No clear consensus exists concerning the postoperative analgesic regime after craniotomies among neurosurgical centers within the UK. Only 23 % of these centers had standardized analgesic protocols and less than 65 % assessed PCH routinely in 2009 [30]. This field shows a surprisingly slow progress, as 4 years before the latter survey 54 % of the neurosurgical units had measured postoperative pain on a regular basis. However, the proportion of first-line medicaments has gone through remarkable changes during this period. Morphine has gained ground against codeine: codeine as the drug of first choice has decreased from 90 % to 70 % in UK neurosurgical centers, and 30 % of these used morphine as the first line compared to the previously observed 10 % [9, 30]. The most popular nonopioid painkiller was paracetamol, applied by 84 % of the centers, whereas 52 % used NSAIDs as second-line analgesics [30].
Nonopioid analgesics
Physicians are entitled to decide if nonopioid painkillers are administered alone [30], in combination with other opioids, or not at all after craniotomies. These medicaments usually work by reducing prostaglandin levels through COX-1 and COX-2 enzyme inhibition, but unfortunately they have no further effect on pain above a particular dosage [15]. Thus, nonopioid analgesics alone are reported to be insufficient after craniotomy [31–33], but according to a meta-analysis of randomized trials, they are capable of reducing postoperative opioid requirements as well as narcotic-related side effects such as nausea, vomiting, respiratory depression, sedation, and constipation [34].
Paracetamol is the most popularly used second-line analgesic after craniotomies in the UK [30]. Paracetamol is considered to be inferior to ketoprofen in terms of opioid-sparing effect and pain-relieving properties [35]; nevertheless, it does reduce the postoperative morphine need by 20 % during the first 24 h [36]. Based on these data, to achieve satisfying pain relief and to maximize its beneficial features, paracetamol should be accompanied by other more potent analgesics (Table 2).
Because there is only a small number of studies on NSAIDs (Table 2), there is no consensus regarding their usage after craniotomies. In 2009, 52 % of the neurosurgical centers in the UK used NSAIDs; 19 % prescribed these regularly and the first dose was administered on average 24 h after the operation [30]. The concern about NSAIDs is related to their antiplatelet function: they can increase the risk of bleeding and postoperative hematoma development, which could be fatal in neurosurgical patients [30, 37]. A study on postoperative intracranial hematoma occurrence and timing found that 2.2 % of the neurosurgical patients developed hematoma, 90 % of them within the first 6 h and the remaining 10 % 24 h after the operation [38]. These findings should lead to the restriction of NSAIDs in the first 6 h postoperatively or to their replacement with paracetamol or COX-2 inhibitors [30]. Neither lornoxicam nor ketoprofen caused postoperative hematoma, renal failure, or peptic ulcer after intracranial interventions; instead, ketoprofen even had an explicit opioid-sparing effect [35, 37]. Diclofenac as a preoperatively given, preemptive analgesic managed to reduce the incidence and severity of PCH, perhaps partly by anticipating the central sensitization of nociceptive nerves [14, 39]. It can be concluded that NSAIDs might be appropriate supplementary analgesic agents if used with caution regarding to the time of administration.
Cyclooxygenase-2 inhibitors such as parecoxib may increase cardiovascular events with long-term use [40], but there is no evidence of elevated risk when administered acutely [41]. Parecoxib has no antiplatelet effect, and does not increase bleeding risk, which could make it an ideal supplementary analgesic [15]. To date, only a few publications have been released on this topic, with the results stating that COX-2 inhibitors have only limited analgesic and opioid-sparing effects [31, 33], although contradictory data have also been published [42].
The treatment and prevention alternatives of CPCH have not yet been investigated in detail. The frequency and duration of the CPCH tends to decrease with time, but because of its long-lasting nature everyday life can be negatively influenced [8], and thus taking analgesics becomes necessary in some cases. The most widely used medicaments for this are nonprescription analgesics. In a study 61.3 % of the patients suffering from CPCH took NSAIDs, whereas another publication showed that simple painkillers, such as coxibs, paracetamol, and NSAIDs eased pain significantly in 79 % of people with CPCH, including 35 % whose headache resolved completely [6, 7].
Opioid drugs
Neurosurgeons have refused to use opioids for a long time because of the their potential adverse effects (sedation, nausea, vomiting, and miosis), and because, owing to these attributes, they may mask the signs of an intracranial event [2, 31]. Therefore, because neurological examinations still serve as primary evaluation methods of patients after intracranial procedures, opioids have been avoided as potential analgesic options. Another undesirable consequence is that opioids could reduce minute ventilation, even if administered in therapeutic doses, which causes carbon dioxide retention, hypercapnia, thereafter elevated cerebral blood flow, which could end in high intracranial pressure and cerebral edema [43]. In practice, hardly any studies in which opioids were administered in therapeutic dosage reported major side effects; furthermore, sedation scores and respiratory parameters were also unchanged [44–46].
Nowadays codeine is the first-line agent in postcraniotomy pain management, used by 71 % of the neurosurgical centers in the UK [9, 30]. Its widespread use can be explained by the limited respiratory-depressing effect, and it is considered not to affect neurological assessment [15]. Nevertheless, codeine has some drawbacks as well, such as suboptimal pain control [5, 17] (Table 3). With a 2-day follow-up time, 76 % of patients treated with intramuscular codeine had severe or moderate PCH [5]. According to the existing data codeine seems to have inadequate analgesic effect after craniotomy [5, 17]. The efficacy of codeine is also affected by individual differences: morphine, the active form of codeine, is responsible for the analgesic effect. Codeine is metabolized in the liver and it was found that almost 10 percent of the population is slow and another 10 % is fast metabolizer, which means that a codeine-based therapy could be inadequate for them [15, 47].
Tramadol, a synthetic analogue of codeine, modifies the reuptake of norepinephrine and 5-hydroxytryptamine and has only limited μ-opioid receptor affinity; thus, it can alter the pain sensation through multiple pathways [15]. Because tramadol is a weak opioid with limited sedative and respiratory effects, it is used in 42 % of the neurosurgical centers in the UK, mainly as a third- or fourth-line painkiller [30]. A reason why tramadol is less preferred is that it elevates postoperative nausea and vomiting (PONV) incidence: 75 mg intramuscular tramadol significantly increased the degree of sedation and the occurrence of PONV [48] (Table 3). This occurrence can cause serious complications after craniotomies, such as a sharp increase of intracranial pressure, which can lead to intracranial bleeding and hemorrhage [33, 49]. Tramadol has no clear benefit over codeine; it seems to be less effective with a dose-dependent impact on PONV [46, 49]. Nevertheless, an investigation by Rahimi et al. [50] pointed out the drug has a potentially beneficial effect as a supplemental pain medication after craniotomies (Table 3).
As mentioned earlier, a safe and more effective alternative to codeine is needed, because previous studies have found codeine ineffective in coping with this headache form [5, 17]. Morphine is a tenfold more potent opiate than codeine, which serves as the gold standard of moderate and severe pain management [15]. Nevertheless, only 30 % of the UK neurosurgical centers use it as a first-line analgesic after intracranial surgeries, presumably because of its feared sedative and respiratory effects [30]. However, 10 mg i.m. morphine postoperatively caused no sedation, pupillary contraction, or respiratory depression in neurosurgical patients [44] (Table 3). An alternative way to ease the pain is the use of the patient-controlled analgesia (PCA) technique, whereby patients titrate their own opioid boluses [46, 49] (Table 3). It is a common way to treat pain, although rarely used after craniotomies. According to an analysis, only 4 % of the centers in the UK used PCA regularly [30]. A prospective trial showed that the use of PCA-administered morphine does not associate with major adverse events; moreover, a nonsignificant pain reduction was observed compared to i.m. codeine [51]. Another study on either placebo PCA, morphine PCA, or morphine with ondansetron PCA showed that the morphine/ondansetron group was the most satisfied and had the lowest VAS scores after intracranial surgeries. Surprisingly, the incidence of PONV was the same among the three groups, which makes the administration of ondansetron after cranial interventions questionable; it elevates hospitalization cost without reducing PONV incidence, and therefore it is not recommended [49].
In summary, morphine provides better analgesia than codeine, without the additional risk of sedation or respiratory depression [46, 51]. Thus, morphine should be regarded as a potential alternative, and preferred over codeine in patients with moderate or severe acute PCH. The PCA administration route proved to be secure and effective when compared to other conventional analgesic therapies, with no further adverse events [46, 49].
Conclusion
Pain associated with intracranial surgery has been undertreated for decades, because the brain itself is insensitive to nociceptive stimuli and it was assumed that patients do not experience severe pain after the intervention [1, 17].
When patients with PCH are treated, it should be taken into account that different demographic and clinical factors may alter the characteristic and severity of pain; therefore, the optimal analgesic strategy could be different and should be personalized individually [1, 2, 6, 7, 11]. As PCH is the most severe during the first couple of hours after surgery [1], the preoperative administration of local anesthetics, such as bupivacaine and ropivacaine, in the form of local infiltration or scalp block seems to be a good initial approach to minimize postoperative complications [2, 18, 20, 22, 23]. Scalp infiltration seems to be inferior to scalp nerve block in relationship to effectiveness and duration [19, 22], but at the same time the incidence of CPCH has been significantly reduced with ropivacaine infiltration before the intervention [18].
Effective pain management and prevention is of great importance in avoiding postoperative complications (hypertension, agitation, and vomiting), which may lead to unfavorable outcome and extended hospital stay [52]. Some of these adverse events are caused by inadequate pain therapy, and others are side effects of the medication; therefore, it is important to find the proper balance. Unfortunately, presently available data are not satisfactory to define appropriate treatment guidelines, but the growing number of publications on this topic allows us to suggest some basic principles.
Studies suggest that non-opioids have proved to be insufficient when administered solely [22–24], but showed several advantages in combination with opioids, including more effective pain relief, decreased opioid need, shorter hospital stay, and lower expenses [33, 35, 36, 42, 50]. Non-opioids could be administered preoperatively to take their preemptive analgesic effect, and they are applicable in mild headaches solely or as supplementary agents, part of a combined, balanced therapy in moderate or severe cases [32, 35, 37, 39].
When adequate analgesia cannot be achieved with less potent agents, opioid analgesics may be unavoidable. Nowadays they are widely accepted and used after neurosurgical interventions [9, 30]. Various factors should be taken into account when deciding which opioid is suitable to be administered, such as pain intensity, drug interactions, hospital preferences, and the available routes. A multimodal approach is starting to gain ground: opioids and non-opioids are administered together to ease pain through various pathways [33, 35, 42]. The two types of drugs facilitate each other’s effects and decrease the incidence of side effects. In one study, 74 % of the patients reported to have excellent or very good pain relief after supratentorial craniotomy when their pain was prevented and treated with local scalp infiltration and the combination of PRN morphine and paracetamol [33]. An earlier study observed even better outcome with PCA-delivered oxycodone and oral ketoprofen, with 89 % of the patients considering analgesia excellent or good [35]. According to these data, future therapeutic guidelines should recommend combined treatments to provide adequate pain management, minimize the occurrence of side effects, and reduce hospital stay and hospitalization costs [7, 50].
References
De Benedittis G, Lorenzetti A, Migliore M, Spagnoli D, Tiberio F, Villani RM. Postoperative pain in neurosurgery: a pilot study in brain surgery. Neurosurgery. 1996;38(3):466–9 discussion 469–470.
Gottschalk A, Berkow LC, Stevens RD, Mirski M, Thompson RE, White ED, Weingart JD, Long DM, Yaster M. Prospective evaluation of pain and analgesic use following major elective intracranial surgery. J Neurosurg. 2007;106(2):210–6. doi:10.3171/jns.2007.106.2.210.
Grossman SA, Sheidler VR, Swedeen K, Mucenski J, Piantadosi S. Correlation of patient and caregiver ratings of cancer pain. J Pain Symptom Manag. 1991;6(2):53–7.
The International Classification of Headache Disorders: 2nd edition. Cephalalgia 2004;24(suppl 1):9–160
Thibault M, Girard F, Moumdjian R, Chouinard P, Boudreault D, Ruel M. Craniotomy site influences postoperative pain following neurosurgical procedures: a retrospective study. Can J Anaesth (J Can Anesth). 2007;54(7):544–8. doi:10.1007/BF03022318.
Rimaaja T, Haanpaa M, Blomstedt G, Farkkila M. Headaches after acoustic neuroma surgery. Cephalalgia. 2007;27(10):1128–35. doi:10.1111/j.1468-2982.2007.01410.x.
Ryzenman JM, Pensak ML, Tew JM Jr. Headache: a quality of life analysis in a cohort of 1,657 patients undergoing acoustic neuroma surgery; results from the acoustic neuroma association. Laryngoscope. 2005;115(4):703–11. doi:10.1097/01.mlg.0000161331.83224.c5.
de Gray LC, Matta BF. Acute and chronic pain following craniotomy: a review. Anaesthesia. 2005;60(7):693–704. doi:10.1111/j.1365-2044.2005.03997.x.
Roberts GC. Post-craniotomy analgesia: current practices in British neurosurgical centres—a survey of post-craniotomy analgesic practices. Eur J Anaesthesiol. 2005;22(5):328–32.
Breivik H, Borchgrevink PC, Allen SM, Rosseland LA, Romundstad L, Hals EK, Kvarstein G, Stubhaug A. Assessment of pain. Br J Anaesth. 2008;101(1):17–24. doi:10.1093/bja/aen103.
Rocha-Filho PA, Gherpelli JL, de Siqueira JT, Rabello GD. Post-craniotomy headache: characteristics, behaviour and effect on quality of life in patients operated for treatment of supratentorial intracranial aneurysms. Cephalalgia. 2008;28(1):41–8. doi:10.1111/j.1468-2982.2007.01465.x.
Teo MK, Eljamel. Role of craniotomy repair in reducing postoperative headaches after a retrosigmoid approach. Neurosurgery. 2010;67(5):1286–91. doi:10.1227/NEU.0b013e3181f0bbf1 discussion 1291–1292.
Kaur A, Selwa L, Fromes G, Ross DA. Persistent headache after supratentorial craniotomy. Neurosurgery. 2000;47(3):633–6.
Dirks J, Moiniche S, Hilsted KL, Dahl JB. Mechanisms of postoperative pain: clinical indications for a contribution of central neuronal sensitization. Anesthesiology. 2002;97(6):1591–6.
Gottschalk A, Yaster M. The perioperative management of pain from intracranial surgery. Neurocrit Care. 2009;10(3):387–402. doi:10.1007/s12028-008-9150-3.
Gee JR, Ishaq Y, Vijayan N. Postcraniotomy headache. Headache. 2003;43(3):276–8.
Quiney N, Cooper R, Stoneham M, Walters F. Pain after craniotomy. A time for reappraisal? Br J Neurosurg. 1996;10(3):295–9.
Batoz H, Verdonck O, Pellerin C, Roux G, Maurette P. The analgesic properties of scalp infiltrations with ropivacaine after intracranial tumoral resection. Anesth Analg. 2009;109(1):240–4. doi:10.1213/ane.0b013e3181a4928d.
Bloomfield EL, Schubert A, Secic M, Barnett G, Shutway F, Ebrahim ZY. The influence of scalp infiltration with bupivacaine on hemodynamics and postoperative pain in adult patients undergoing craniotomy. Anesth Analg. 1998;87(3):579–82.
Law-Koune JD, Szekely B, Fermanian C, Peuch C, Liu N, Fischler M. Scalp infiltration with bupivacaine plus epinephrine or plain ropivacaine reduces postoperative pain after supratentorial craniotomy. J Neurosurg Anesthesiol. 2005;17(3):139–43.
Girard F, Quentin C, Charbonneau S, Ayoub C, Boudreault D, Chouinard P, Ruel M, Moumdjian R. Superficial cervical plexus block for transitional analgesia in infratentorial and occipital craniotomy: a randomized trial. Can J Anaesth (J Can Anesth). 2010;57(12):1065–70. doi:10.1007/s12630-010-9392-3.
Nguyen A, Girard F, Boudreault D, Fugere F, Ruel M, Moumdjian R, Bouthilier A, Caron JL, Bojanowski MW, Girard DC. Scalp nerve blocks decrease the severity of pain after craniotomy. Anesth Analg. 2001;93(5):1272–6.
Ayoub C, Girard F, Boudreault D, Chouinard P, Ruel M, Moumdjian R. A comparison between scalp nerve block and morphine for transitional analgesia after remifentanil-based anesthesia in neurosurgery. Anesth Analg. 2006;103(5):1237–40. doi:10.1213/01.ane.0000244319.51957.9f.
Pinosky ML, Fishman RL, Reeves ST, Harvey SC, Patel S, Palesch Y, Dorman BH. The effect of bupivacaine skull block on the hemodynamic response to craniotomy. Anesth Analg. 1996;83(6):1256–61.
Lee EJ, Lee MY, Shyr MH, Cheng JT, Toung TJ, Mirski MA, Chen TY. Adjuvant bupivacaine scalp block facilitates stabilization of hemodynamics in patients undergoing craniotomy with general anesthesia: a preliminary report. J Clin Anesth. 2006;18(7):490–4. doi:10.1016/j.jclinane.2006.02.014.
Harner SG, Beatty CW, Ebersold MJ. Impact of cranioplasty on headache after acoustic neuroma removal. Neurosurgery. 1995;36(6):1097–9 discussion 1099–1100.
Koperer H, Deinsberger W, Jodicke A, Boker DK. Postoperative headache after the lateral suboccipital approach: craniotomy versus craniectomy. Minim Invasive Neurosurg (MIN). 1999;42(4):175–8. doi:10.1055/s-2008-1053393.
Porter RG Sr, Leonetti JP, Ksiazek J, Anderson D. Association between adipose graft usage and postoperative headache after retrosigmoid craniotomy. Otol Neurotol 2009;30(5):635–639 doi: 10.1097/MAO.0b013e3181ab3317
Schaller B, Baumann A. Headache after removal of vestibular schwannoma via the retrosigmoid approach: a long-term follow-up-study. Otolaryngol Head Neck Surg. 2003;128(3):387–95. doi:10.1067/mhn.2003.104.
Kotak D, Cheserem B, Solth A. A survey of post-craniotomy analgesia in British neurosurgical centres: time for perceptions and prescribing to change? Br J Neurosurg. 2009;23(5):538–42.
Jones SJ, Cormack J, Murphy MA, Scott DA. Parecoxib for analgesia after craniotomy. Br J Anaesth. 2009;102(1):76–9. doi:10.1093/bja/aen318.
Verchere E, Grenier B, Mesli A, Siao D, Sesay M, Maurette P. Postoperative pain management after supratentorial craniotomy. J Neurosurg Anesthesiol. 2002;14(2):96–101.
Williams DL, Pemberton E, Leslie K. Effect of intravenous parecoxib on post-craniotomy pain. Br J Anaesth. 2011;107(3):398–403. doi:10.1093/bja/aer223.
Elia N, Lysakowski C, Tramer MR. Does multimodal analgesia with acetaminophen, nonsteroidal antiinflammatory drugs, or selective cyclooxygenase-2 inhibitors and patient-controlled analgesia morphine offer advantages over morphine alone? Meta-analyses of randomized trials. Anesthesiology. 2005;103(6):1296–304.
Tanskanen P, Kytta J, Randell T. Patient-controlled analgesia with oxycodone in the treatment of postcraniotomy pain. Acta Anaesthesiol Scand. 1999;43(1):42–5.
Remy C, Marret E, Bonnet F. Effects of acetaminophen on morphine side-effects and consumption after major surgery: meta-analysis of randomized controlled trials. Br J Anaesth. 2005;94(4):505–13. doi:10.1093/bja/aei085.
Dolmatova EV, Imaev AA, Lubnin AY. “Scheduled” dosing of lornoxicam provides analgesia superior to that provided by “on request” dosing following craniotomy. Eur J Anaesthesiol. 2009;26(8):633–7.
Taylor WA, Thomas NW, Wellings JA, Bell BA. Timing of postoperative intracranial hematoma development and implications for the best use of neurosurgical intensive care. J Neurosurg. 1995;82(1):48–50. doi:10.3171/jns.1995.82.1.0048.
Simon E, Bank J, Gal J, Siro P, Novak L, Fulesdi B, Molnar C. Administration of preemptive analgesia by diclofenac to prevent acute postcraniotomy headache. Ideggyószaszati Szemle/Clin Neurosci. 2012;65(9–10):302–6.
Mukherjee D, Nissen SE, Topol EJ. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA. 2001;286(8):954–9.
White WB, Strand V, Roberts R, Whelton A. Effects of the cyclooxygenase-2 specific inhibitor valdecoxib versus nonsteroidal antiinflammatory agents and placebo on cardiovascular thrombotic events in patients with arthritis. Am J Ther. 2004;11(4):244–50.
Rahimi SY, Vender JR, Macomson SD, French A, Smith JR, Alleyne CH Jr. Postoperative pain management after craniotomy: evaluation and cost analysis. Neurosurgery. 2006;59(4):852–7. doi:10.1227/01.NEU.0000232646.35678.D8 discussion 857.
Cold GE, Felding M. Even small doses of morphine might provoke “luxury perfusion” in the postoperative period after craniotomy. Neurosurgery. 1993;32(2):327.
Goldsack C, Scuplak SM, Smith M. A double-blind comparison of codeine and morphine for postoperative analgesia following intracranial surgery. Anaesthesia. 1996;51(11):1029–32.
Morad AH, Winters BD, Yaster M, Stevens RD, White ED, Thompson RE, Weingart JD, Gottschalk A. Efficacy of intravenous patient-controlled analgesia after supratentorial intracranial surgery: a prospective randomized controlled trial. Clinical article. J Neurosurg. 2009;111(2):343–50. doi:10.3171/2008.11.jns08797.
Sudheer PS, Logan SW, Terblanche C, Ateleanu B, Hall JE. Comparison of the analgesic efficacy and respiratory effects of morphine, tramadol and codeine after craniotomy. Anaesthesia. 2007;62(6):555–60. doi:10.1111/j.1365-2044.2007.05038.x.
Cascorbi I. Pharmacogenetics of cytochrome p4502D6: genetic background and clinical implication. Eur J Clin Invest. 2003;33(suppl 2):17–22.
Jeffrey HM, Charlton P, Mellor DJ, Moss E, Vucevic M. Analgesia after intracranial surgery: a double-blind, prospective comparison of codeine and tramadol. Br J Anaesth. 1999;83(2):245–9.
Jellish WS, Leonetti JP, Sawicki K, Anderson D, Origitano TC. Morphine/ondansetron PCA for postoperative pain, nausea, and vomiting after skull base surgery. Otolaryngol Head Neck Surg. 2006;135(2):175–81. doi:10.1016/j.otohns.2006.02.027.
Rahimi SY, Alleyne CH, Vernier E, Witcher MR, Vender JR. Postoperative pain management with tramadol after craniotomy: evaluation and cost analysis. J Neurosurg. 2010;112(2):268–72. doi:10.3171/2008.9.17689.
Stoneham MD, Cooper R, Quiney NF, Walters FJ. Pain following craniotomy: a preliminary study comparing PCA morphine with intramuscular codeine phosphate. Anaesthesia 1996;51(12):1176–1178
Basali A, Mascha EJ, Kalfas I, Schubert A. Relation between perioperative hypertension and intracranial hemorrhage after craniotomy. Anesthesiology. 2000;93(1):48–54.
Acknowledgments
This work was not funded and had no financial support or sponsorship. None of the authors has any conflict of interest. The work of LM and RN was supported by the Junior Research fellow grant of the European Union and the Hungarian Government (National Excellency Programme), registration number: TÁMOP 4.2.4.A/2-11-1-2012-0001.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Molnár, L., Simon, É., Nemes, R. et al. Postcraniotomy headache. J Anesth 28, 102–111 (2014). https://doi.org/10.1007/s00540-013-1671-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-013-1671-z