Abstract
Purpose
We examined the effects of dexamethasone, droperidol, naloxone, and a combination of these three agents on postoperative nausea and vomiting (PONV) in female patients.
Methods
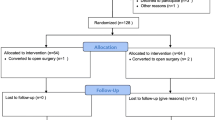
In this randomized, controlled study, 120 female patients with ASA PS I or II undergoing laparoscopic gynecological surgery were randomly allocated into four groups. Patients received dexamethasone 8 mg (Dx group) or droperidol 1 mg (Dr group) before induction of general anesthesia. Anesthesia was induced and maintained with propofol and remifentanil. Postoperative analgesia was provided by intravenous patient-controlled analgesia using a disposable infusion pump filled with fentanyl 20 μg/kg alone (Dx group), fentanyl 20 μg/kg with droperidol 2 mg (Dr group), fentanyl 20 μg/kg with naloxone 0.1 mg (Nx group), or fentanyl 20 μg/kg with droperidol 2 mg and naloxone 0.1 mg (Cm group) in a total volume of 80 ml, with a constant infusion rate of 4 ml/h and a bolus dose 2 ml with a 30-min lockout time.
Results
The number of patients who developed PONV and required a rescue antiemetic was significantly less in the Cm group than in the Nx group (p < 0.001 for all). The incidence of PONV was 43, 43, 70, and 17 % in the Dx, Dr, Nx, and Cm groups, respectively.
Conclusion
A combination of naloxone, droperidol, and dexamethasone was effective for preventing PONV in patients receiving fentanyl for postoperative analgesia after laparoscopic gynecological surgery, although further investigations are required to examine the effect of adding naloxone to other antiemetics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidence of postoperative nausea and vomiting (PONV) is significant after laparoscopic gynecological surgery, affecting nearly 80 % of patients [1]. Such a high incidence is attributable to multiple risk factors for PONV related to patients undergoing this surgery, including female gender, young age, nonsmoking status, type of surgery (laparoscopic, gynecological, abdominal), use of pneumoperitoneum, and intraoperative and postoperative use of opioids [2, 3]. For improving patient satisfaction and enhancing patient recovery, antiemetic therapy for PONV prophylaxis is essential [3].
Despite extensive use of 5-hydroxytryptamine type 3 receptor (5-HT3) antagonists as a first-choice agent for preventing PONV, no single drug therapy is completely effective [3]. Therefore, use of combination therapy, such as dexamethasone or droperidol combined with 5-HT3 antagonists, is recommended for patients with high risk of PONV [3, 4]. However, the higher cost of 5-HT3 antagonists compared with other agents, such as dexamethasone and droperidol, remains a major problem in clinical practice [5].
Reportedly, low-dose naloxone added to intravenous morphine effectively reduces the incidence of nausea without affecting the analgesic effect of morphine [6, 7]. Despite being one of the predominant risk factors for PONV [2–4], fentanyl is still one of the most commonly used analgesics after surgery, and naloxone is expected to exert an antiemetic effect when used with fentanyl. However, the effect of naloxone, with a lower cost than 5-HT3 antagonists, on PONV in patients receiving fentanyl for postoperative analgesia has not been examined.
The present prospective, randomized, controlled study was designed to evaluate the effect of intravenous naloxone, used alone and in combination with other antiemetics, on the incidence of PONV after laparoscopic gynecological surgery.
Patients and methods
Patients
After obtaining approval from the Institutional Review Board of Juntendo University Hospital (Tokyo, Japan) and written informed patient consent, 120 female patients, aged 20–50 years, with American Society of Anesthesiologists (ASA) physical status I or II undergoing gynecological laparoscopic surgery for benign diseases during the period from November 2011 through March 2012, were included. Patients chronically taking psychoneurotic agents, anticonvulsants, or analgesics, patients with infectious diseases, diabetes mellitus, or an allergic history to the study drugs, and obese patients with a body mass index of 28 kg/m2 or more were excluded. Patients were randomly allocated to the dexamethasone (Dx), droperidol (Dr), naloxone (Nx), and combination (Cm) groups by an opaque envelope method.
Management of anesthesia and postoperative analgesia
No premedication was given. With standard monitoring including the bispectral index, general anesthesia was induced with propofol following a target-controlled manner with an effect site concentration 3.5 µg/ml and remifentanil (0.5 µg/kg/min for 2 min followed by 0.2 µg/kg/min). Tracheal intubation was done after rocuronium 1 mg/kg IV. General anesthesia was maintained with propofol with target concentrations between 2 and 5 µg/ml for maintaining the bispectral index between 40 and 60, and remifentanil 0.1–0.5 µg/kg/min for maintaining systolic blood pressure between 90 and 120 mmHg and heart rate of 75 bpm or less. Fentanyl 100 µg was administered immediately before and at the end of pneumoperitoneum. Flurbiprofen 50 mg was administered at the end of pneumoperitoneum. Residual neuromuscular blockade was reversed with sugammadex 0.3 mg/kg on completion of surgery, and the tracheal tube was removed. Pain was assessed by verbal pain ratings (no/mild/moderate/severe) after extubation. If a patient complained of moderate or severe pain, fentanyl 50 µg IV was repeatedly administered until the level of pain decreased to mild or none in the operating room as reported previously [1].
Postoperative analgesia was provided with intravenous fentanyl patient-controlled analgesia (IV-PCA) using a disposable infusion pump (Baxter Infusor, BB30LV4; Baxter Limited, Tokyo, Japan), filled with fentanyl 20 µg/kg and the study drug(s), if any, in a total volume of 80 ml. A constant infusion rate was 4 ml/h (fentanyl 1 µg/kg/h) and a bolus dose was 2 ml (fentanyl 0.5 µg/kg) with a 30-min lockout time. Postoperatively, diclofenac suppository 50 mg or flurbiprofen 50 mg IV was administered as a rescue analgesic. Metoclopramide 10 mg IV was used as a rescue antiemetic. Continuous infusion was terminated after infusing 80 ml of the test solution, irrespective of the volume of bolus doses.
Study drugs
In the Dx group, dexamethasone 8 mg was administered IV before inducing anesthesia. In the Dr group, droperidol 1 mg was administered IV before inducing anesthesia and 2 mg was added to the PCA pump. In the Nx group, naloxone 0.1 mg was added to the PCA pump. In the Cm group, dexamethasone 8 mg and droperidol 1 mg were administered IV before inducing anesthesia and droperidol 2 mg and naloxone 0.1 mg were added to the PCA pump. Patients were blinded to the group allocation. Anesthetists responsible for intraoperative management were aware of the group allocation but were not involved in evaluation of outcome measures associated with PONV.
The dose of dexamethasone was determined based on previous reports [3, 4, 8]. The total dose of droperidol (3 mg) was determined based on a study [9] showing that the total dose of droperidol of 4 mg/day or more increases the incidence of side effects such as sedation, dizziness, and restlessness, although it does not enhance the antiemetic effect. We adjusted the total dose to the body weight of our subjects. The dose of naloxone was determined based on a previous study [7], showing that naloxone given at a rate of approximately 0.05 μg/kg/h with intravenous morphine exerted the antiemetic effect without antagonizing morphine analgesia.
Outcome measures
Postoperatively, electrocardiogram, heart rate, respiratory rate by transthoracic impedance pneumography, and percutaneous oxygen saturation (SpO2) by pulse oximetry were continuously monitored. IV fentanyl PCA would be discontinued whenever the respiratory rate remained <8 bpm, SpO2 remained <95 % without supplemental oxygen, or a patient remained unresponsive to a call or was intolerant of PONV.
Pain at rest, nausea, and drowsiness were evaluated using an 11-point numeric rating score (0 = none, 10 = worst imaginable) as reported previously [10]; 3 and 6 h after surgery, at the time of the patient’s awakening in the next morning, and 20 h after surgery. Patients with nausea score 0–2 without developing vomiting nor requiring metoclopramide during the study period were assumed to be no PONV. The amount of breakfast consumed the next morning was also recorded.
Statistical analysis
Based on a previous study in which the incidence of PONV in patients undergoing laparoscopic surgery was 65 %, required sample size was 30 per group to detect a 30 % reduction in the incidence of PONV with α = 0.05 and β = 0.8 [11]. Results were expressed as mean ± SD, median (range), or median and 10th, 25th, 75th, and 90th percentiles. Patient characteristics, duration of anesthesia, surgery, and pneumoperitoneum, and dose of fentanyl in the operating room were compared among the four groups by one-way analysis of variance. Numbers of patients with motion sickness and smoking history, numbers of patients who developed PONV and vomiting and who required metoclopramide and rescue analgesics, and types of surgery were compared by chi square test followed by Tukey’s multiple comparisons. Pain, nausea, and drowsiness scores, frequency of vomiting, use of analgesics, dose of metoclopramide required, and amount of breakfast consumed the next morning were compared among the four groups by Kruskal–Wallis test, followed by the Steel–Dwass test for multiple comparisons. p < 0.05 was considered statistically significant.
Results
All patients completed the study with no clinically significant adverse effects and without discontinuation of IV fentanyl PCA. There were no differences among the four groups with respect to patient characteristics, number of patients with PONV risk factors, types of surgery, or total dose of fentanyl administered in the operating room (Table 1).
The numbers of patients who developed PONV and vomiting, and who required metoclopramide, the frequency of vomiting, and the dose of metoclopramide were significantly less in the Cm group than in the Nx group (p < 0.001 for all; Table 2). There were no differences in the nausea score among the four groups 3 h after surgery (Fig. 1). However, the score was lower in the Cm group than in the Nx group 6 h after surgery (p = 0.042), and lower in the Dr and Cm groups than in the Nx group the next morning (p = 0.023 and 0.005, respectively) and 20 h after surgery (p = 0.026 and 0.002, respectively) (Fig. 1). The amount of breakfast consumed in the next morning (% ratio of consumed to served amount) was larger in the Dx and Cm groups than in the Nx group (p = 0.042 and 0.019, respectively) (Table 2).
Nausea score during the first 20 postoperative hours. Postoperative nausea was evaluated using an 11-point numeric rating score (0 = none, 10 = worst imaginable). The bold horizontal bars, box boundaries, and whiskers represent the median values, interquartile ranges, and 10th to 90th percentiles, respectively. The Dx, Dr, Nx, and Cm groups indicate groups of patients treated with dexamethasone, droperidol, naloxone, and a combination of these three drugs, respectively. p values depicted above the nadir were calculated by comparing the four groups. *p < 0.05, **p < 0.01 between the indicated two groups
There were no differences in pain score, the number of patients who required rescue analgesics, or the frequency of its use among the four groups during the study period (Table 2, Fig. 2). Drowsiness score was higher in the Dr group than in the Dx and Nx groups (p = 0.001 and 0.008, respectively) 3 h after surgery, but with no significant differences among the four groups 6 h after surgery or the next day (Fig. 3).
Pain score at rest during the first 20 postoperative hours. Postoperative pain was evaluated using an 11-point numeric rating score (0 = none, 10 = worst imaginable). The bold horizontal bars, box boundaries, and whiskers represent the median values, interquartile ranges, and 10th to 90th percentiles, respectively. The Dx, Dr, Nx, and Cm groups indicate groups of patients treated with dexamethasone, droperidol, naloxone, and a combination of these three drugs, respectively. p values depicted above the nadir were calculated by comparing the four groups. There were no differences among the four groups at any time point
Drowsiness score during first 20 h postoperatively. Drowsiness was evaluated using an 11-point numeric rating score (0 = none, 10 = worst imaginable). The bold horizontal bars, box boundaries, and whiskers represent the median values, interquartile ranges, and 10th to 90th percentiles, respectively. The Dx, Dr, Nx, and Cm groups indicate groups of patients treated with dexamethasone, droperidol, naloxone, and a combination of these three agents, respectively. p values depicted above the nadir were calculated by comparing the four groups. **p < 0.01 between the indicated two groups
Discussion
In the present study, incidences of PONV were 43, 43, 70, and 17 % in patients receiving dexamethasone, droperidol, naloxone, and a combination of these three drugs, respectively. Including this, all variables related to PONV, i.e., incidence of PONV, nausea score, number of patients with vomiting and its frequency, and the number of patients requiring metoclopramide and its dose, were significantly less in patients receiving a combination of dexamethasone, droperidol, and naloxone than those receiving naloxone alone. Notably, there were no differences in the pain score or the number of doses of rescue analgesics among the four groups, suggesting that adding naloxone would not have antagonized the analgesic effect of fentanyl.
Incidences of PONV and vomiting were 70 % and 57 %, respectively, in patients receiving only naloxone, comparable with the previously reported data in patients undergoing laparoscopic gynecological surgery without receiving prophylactic antiemetics [1]. Although there was not a control group without any antiemetics in our study, these results suggest that naloxone alone unlikely exerted an antiemetic effect, in contrast with the results reported by Cepeda et al. [7], showing that low-dose naloxone (0.05 μg/kg/h for 2 h followed by 0.009 μg/kg/h) added to morphine in postoperative IV-PCA reduced the number of patients who complained of nausea by approximately 20 %. Gan et al. [6] also reported that naloxone 0.25 μg/kg/h added to morphine also decreased the incidence of PONV. Although the reason for these differences is unclear, a dose of naloxone required to exert an antiemetic effect when used with fentanyl might be different from that used with morphine, because these effects may result from blockade of presynaptic opioid receptors [12]. Different profiles of the study subjects might also be one of the reasons. In contrast to female patients undergoing gynecological laparoscopic surgery associated with high risk of PONV in our study [3], patients receiving various types of surgery without pneumoperitoneum were included in the study by Cepeda et al. [7]. On the other hand, Gan et al. [6] used 500–600 μg fentanyl during anesthesia, which may account for the higher dose of naloxone required for preventing PONV compared with the study by Cepeda et al. Despite controversies about the relationships between laparoscopic surgery and an increased risk of PONV [2, 3], pneumoperitoneum with carbon dioxide might contribute to the increased incidence of PONV [13].
Previous studies have shown that both dexamethasone and droperidol are effective for preventing PONV in high-risk patients, to some extent [3, 4]. In the present study, although the absolute numbers of patients receiving dexamethasone alone or droperidol alone who developed PONV and required metoclopramide were smaller than those receiving naloxone alone, there were no statistical differences in the incidence of PONV among those groups, probably because of the decreased power of detection of the differences resulting from multiple comparisons and a relatively small number of patients in each group.
Besides decreased incidence of PONV and nausea score, we found that patients receiving a combination of these three agents consumed a larger amount of food than those receiving naloxone alone, probably resulting from decreased level of nausea. An increase in the food intake was also noted in patients receiving dexamethasone, consistent with the results reported previously [14]. This effect seems to be beneficial for enhancing recovery after surgery, targeting reduction of the hospitalization period by making early oral food intake possible. Of importance, side effects of dexamethasone such as infection and hyperglycemia were not detected, consistent with previous studies [8, 15].
Effectiveness of a small dose of droperidol for preventing PONV has been extensively reported [4, 16]. In addition to 1 mg before induction of anesthesia, we continuously administered droperidol after surgery, based on a report showing that addition of droperidol after surgery further reduces the severity of nausea and the requirement of rescue antiemetics [16]. In the present study, the nausea score the next morning and at 20 h after surgery in patients receiving droperidol was significantly lower than those receiving only naloxone, suggesting that droperidol also exhibited an antiemetic effect, although the drowsiness score was significantly higher than in those receiving only dexamethasone or naloxone 3 h after surgery. Drowsiness seems to be a minor and transient side effect of droperidol [16], which was counteracted by concomitantly administered dexamethasone. Improvement of quality of recovery score, including emotional state and physical comfort, after surgery by small-dose dexamethasone was also reported previously [17].
Combinations of agents, particularly adding droperidol or corticosteroids to 5-HT3 antagonists, are effective for preventing PONV, and have been studied extensively [3, 4]. However, the high cost of 5-HT3 antagonists [ondansetron, 4 mg 4,749 Japanese yen (JPY), granisetron, 3 mg 4,731 JPY, and ramosetron 0.3 mg, 5,149 JPY] as well as regulation by the national health insurance system in Japan prohibits its use for preventing PONV. Agents in the present study were safely used with no significant side effects, and the total combined cost of dexamethasone (8 mg, 367 JPY), droperidol (3 mg, 150 JPY), and naloxone (0.2 mg, 944 JPY) is still much lower than that of a single 5-HT3 antagonist. As naloxone alone at a dose used in our study was unlikely to exert an antiemetic effect, the effect of its addition to the combination of droperidol and dexamethasone, together with its effective dose range, should be examined in further studies.
There were several limitations of the present study. First, we did not set up a control group with no antiemetics. However, we found a remarkable difference in the incidence and degree of PONV between patients receiving a combination of the three agents and naloxone alone, suggesting that the concomitant administration of these three agents exhibited an excellent antiemetic effect. Second, we used a disposable infusion pump instead of an electric infusion device. Despite its reliable performance and easier handling compared with an electric infusion device [18], the number of bolus injections of PCA was unknown. However, there were no differences in the total dose of fentanyl or rescue analgesics or pain score among the four groups, indicating that similar levels of analgesia could be achieved with PCA in these groups.
In conclusion, a combination of dexamethasone, droperidol, and naloxone was effective for preventing PONV in patients receiving fentanyl following gynecological laparoscopic surgery; however, naloxone alone was unlikely to have prevented PONV. Further investigations are required for examining the antiemetic effect of adding naloxone to other antiemetics.
References
Eriksson H, Korttila K. Recovery profile after desflurane with or without ondansetron compared with propofol in patients undergoing outpatient gynecological laparoscopy. Anesth Analg. 1996;82:533–8.
Apfel CC, Laara E, Koivuranta M, Greim CA, Roewer N. A simplified risk score for predicting postoperative nausea and vomiting: conclusions from cross-validations between two centers. Anesthesiology. 1999;91:693–700.
Gan TJ, Meyer TA, Apfel CC, Chung F, Davis PJ, Habib AS, Hooper VD, Kovac AL, Kranke P, Myles P, Philip BK, Samsa G, Sessler DI, Temo J, Tramer MR, Vander Kolk C, Watcha M, Society for Ambulatory Anesthesia. Society for Ambulatory Anesthesia guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2007;105:1615–28.
Apfel CC, Korttila K, Abdalla M, Kerger H, Turan A, Vedder I, Zernak C, Danner K, Jokela R, Pocock SJ, Trenkler S, Kredel M, Biedler A, Sessler DI, Roewer N. A factorial trial of six interventions for the prevention of postoperative nausea and vomiting. N Engl J Med. 2004;350:2441–51.
Lachaine J. Therapeutic options for the prevention and treatment of postoperative nausea and vomiting: a pharmacoeconomic review. Pharmacoeconomics. 2006;24:955–70.
Gan TJ, Ginsberg B, Glass PS, Fortney J, Jhaveri R, Perno R. Opioid-sparing effects of a low-dose infusion of naloxone in patient-administered morphine sulfate. Anesthesiology. 1997;87:1075–81.
Cepeda MS, Alvarez H, Morales O, Carr DB. Addition of ultralow dose naloxone to postoperative morphine PCA: unchanged analgesia and opioid requirement but decreased incidence of opioid side effects. Pain. 2004;107:41–6.
Thangaswamy CR, Rewari V, Trikha A, Dehran M, Chandralekha. Dexamethasone before total laparoscopic hysterectomy: a randomized controlled dose-response study. J Anesth. 2010;24:24–30.
Tramer MR, Walder B. Efficacy and adverse effects of prophylactic antiemetics during patient-controlled analgesia therapy: a quantitative systematic review. Anesth Analg. 1999;88:1354–61.
Song D, Chung F, Yogendran S, Wong J. Evaluation of postural stability after low-dose droperidol in outpatients undergoing gynaecological dilatation and curettage procedure. Br J Anaesth. 2002;88:819–23.
Habib AS, White WD, Eubanks S, Pappas TN, Gan TJ. A randomized comparison of a multimodal management strategy versus combination antiemetics for the prevention of postoperative nausea and vomiting. Anesth Analg. 2004;99:77–81.
Crain SM, Shen KF. Antagonists of excitatory opioid receptor functions enhance morphine’s analgesic potency and attenuate opioid tolerance/dependence liability. Pain. 2000;84:121–31.
Koivusalo AM, Kellokumpu I, Lindgren L. Postoperative drowsiness and emetic sequelae correlate to total amount of carbon dioxide used during laparoscopic cholecystectomy. Surg Endosc. 1997;11:42–4.
Halvorsen P, Raeder J, White PF, Almdahl SM, Nordstrand K, Saatvedt K, Veel T. The effect of dexamethasone on side effects after coronary revascularization procedures. Anesth Analg. 2003;96:1578–83.
Wang JJ, Ho ST, Liu HS, Ho CM. Prophylactic antiemetic effect of dexamethasone in women undergoing ambulatory laparoscopic surgery. Br J Anaesth. 2000;84:459–62.
Klahsen AJ, O’Reilly D, McBride J, Ballantyne M, Parlow JL. Reduction of post-operative nausea and vomiting with the combination of morphine and droperidol in patient-controlled analgesia. Can J Anaesth. 1996;43:1100–7.
Murphy GS, Sherwani SS, Szokol JW, Avram MJ, Greenberg SB, Patel KM, Wade LD, Vaughn J, Gray J. Small-dose dexamethasone improves quality of recovery scores after elective cardiac surgery: a randomized, double-blind, placebo-controlled study. J Cardiothorac Vasc Anesth. 2011;25:950–60.
Sumikura H, van de Velde M, Tateda T. Comparison between a disposable and an electronic PCA device for labor epidural analgesia. J Anesth. 2004;18:262–6.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Kasagi, Y., Hayashida, M., Sugasawa, Y. et al. Antiemetic effect of naloxone in combination with dexamethasone and droperidol in patients undergoing laparoscopic gynecological surgery. J Anesth 27, 879–884 (2013). https://doi.org/10.1007/s00540-013-1630-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-013-1630-8