Abstract
Purpose
We investigated the effect of low-dose droperidol on heart rate-corrected QT (QTc) interval and interaction with propofol.
Methods
Seventy-two patients undergoing upper limb surgery were included in this study. Patients were randomly allocated to one of three groups: group S (n = 24), which received 1 ml saline; group D1 (n = 24), which received 1.25 mg droperidol; or group D2 (n = 24), which received 2.5 mg droperidol. One minute later, fentanyl (3 μg/kg) was administered. Two minutes after fentanyl administration, anesthesia was induced using propofol (1.5 mg/kg) and vecronium. Tracheal intubation was performed 3 min after the administration of propofol. Heart rate, mean arterial pressure, bispectral index, and QTc interval were recorded at the following time points: immediately before the droperidol injection (baseline); 3 min after the saline or droperidol injection; 3 min after the propofol injection; and 2 min after tracheal intubation.
Results
Compared to baseline, the QTc interval in group S and group D1 was significantly shorter after propofol injection, but recovered after tracheal intubation. In group D2, the QTc interval was significantly prolonged after droperidol injection, but recovered after propofol injection, and was significantly prolonged after tracheal intubation.
Conclusions
We found that saline or 1.25 mg droperidol did not prolong QTc interval, whereas 2.5 mg droperidol prolonged the QTc interval significantly, and that propofol injection counteracted the prolongation of the QTc interval induced by 2.5 mg droperidol.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Droperidol, an anesthetic agent, has a low cost and is a very effective antiemetic when administered at a low dose. The maximum recommended initial dose is 2.5 mg by intravenous (IV) or intramuscular administration (IM) to prevent or treat postoperative nausea and vomiting (PONV) [1]. Additional 1.25-mg doses of droperidol may be administered to achieve the desired antiemetic effect [1]. Moreover, 0.625–1.25 mg IV doses of droperidol have been widely accepted as the first-line therapy for the prophylaxis and treatment of PONV [2]. However, the U.S. Food and Drug Administration (FDA) mandated a black-box warning concerning the risk of critical arrhythmia because of the drug’s QT prolongation effect [1]. The prolongation of the QTc interval causes serious arrhythmias such as torsade de pointes (TdP) and it is associated with sudden death. However, reports of critical arrhythmias caused by droperidol are uncommon [3], and there are conflicting reports on whether low-dose droperidol has a QTc prolongation effect [3, 4]. Whether the effect of low-dose droperidol on the QTc interval prolongation is dose dependent remains unknown.
We have reported that intravenous anesthetic propofol shortens the QTc interval during anesthetic induction [5, 6]. Kleinsasser et al. [7] further reported that sevoflurane-associated QTc interval prolongation reverses within 15 min after substituting sevoflurane with propofol. The counteracting effect of propofol on droperidol-induced QTc prolongation appears not to have been previously examined.
In this prospective randomized controlled clinical study, we investigated the dose dependency of low-dose droperidol on the QTc interval and the interaction between low-dose droperidol and propofol during anesthetic induction.
Patients, materials, and methods
The study protocol was approved by the Institutional Research and Ethic Committee, and a written informed consent was obtained from each participant. We studied patients 20 to 80 years old, with an American Society of Anesthesiologists (ASA) status of 1 or 2, who underwent elective upper limb surgery under general anesthesia. We excluded patients who had preoperative electrocardiogram (ECG) abnormalities or a medical history of ischemic heart disease and diabetes, or had received preoperative medications such as β-blockers or antiarrhythmic drugs.
In the operating room, the following monitors were applied to the patient: pulse oximetry, 12-lead ECG (FX-7432; Fukuda Denshi, Tokyo, Japan), noninvasive blood pressure, and bispectral index (BIS) monitor (A2000 BIS Monitoring System; Aspect Medical System, Norwood, MA, USA). Lead II of the ECG was used to analyze arrhythmias. The QT intervals were determined by using newly developed software (QTD-1; Fukuda Denshi) that detects the onset of the QRS complex and the end of the T wave [8].
The QT intervals were corrected for heart rate by using the Fridericia formula [9]: \( {\text{QTc}} = {\text{QT}}/\root{3}\of{\text{RR}} \). The QTcD was defined as the difference between the maximum and minimum QTc values in all leads. A mean QTc interval value was calculated from all available QTc intervals, which were averaged from three consecutive cycles in all leads during the measurement time. The investigator, who was blinded to the dose of droperidol, examined and analyzed the ECGs.
Patients were randomly allocated to one of three groups by sealed-envelope assignments to receive either 1 ml saline (group S, n = 24); 1.25 mg droperidol (group D1, n = 24); or 2.5 mg droperidol (group D2, n = 24). For 1 min, the patients breathed 100 % oxygen at a flow rate of 5 l/min via a facemask and received a bolus injection of one of the study drugs (total amount of 1 ml for each). One minute later, fentanyl (3 μg/kg) was administered. Two minutes after the fentanyl administration, anesthesia was induced using propofol (1.5 mg/kg). Immediately after the loss of consciousness, a bolus of vecuronium (0.15 mg/kg) was administered to facilitate tracheal intubation and manually controlled ventilation was initiated via a facemask. Tracheal intubation was performed 3 min after the administration of propofol. If the BIS was greater than 50, additional boluses of propofol (0.5 mg/kg) were administered.
Heart rate, mean arterial pressure, BIS, and 12-lead ECG were recorded at the following time points: T1, immediately before the injection of the study drugs (baseline); T2, 3 min after the injection of the study drugs; T3, 3 min after propofol injection; and T4, 2 min after tracheal intubation.
A factorial analysis of variance (ANOVA) with repeated measures was used for analyzing the differences in data among the time points and among the groups and for analyzing the interaction between time and the groups. Post hoc comparisons between the groups at each time point and among the repeated measures in each group were performed by using the Bonferroni–Dunn procedure, if appropriate. Dichotomous variables were analyzed with Fisher’s exact probability test or the chi square test. Values are expressed as the median (interquartile range). Statistical significance was defined as a p value < 0.05. Sample size was determined on the basis of our previous study with a standard deviation of 19 ms [5], which indicated that a power of 90 % would be required to detect a difference of 19 ms in the mean QTc interval between the two groups with 22 patients in each group at a significance level of 5 %.
Results
Of the 75 enrolled patients, 3 were excluded from the analysis because their ECG data were erroneously entered into the software. Thus, 24 patients in each group completed the study. The patients’ demographics and the number of additional boluses of propofol showed no differences among the groups (Table 1). There were no significant differences among the groups in the incidence of arrhythmias (Table 2). In all groups, the BIS values, heart rate, and mean arterial pressure significantly decreased after anesthetic induction (Table 3). Heart rate recovered after tracheal intubation. There were no significant differences among the groups at any time point, and the QTcD did not change at any time point, compared to the QTcD at T1 in any group (Table 3).
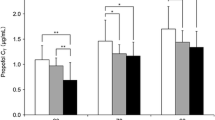
Figure 1 shows the time course of the QTc interval. ANOVA indicated that QTc changes with dose and time since dose (p = 0.028) and time (p = 0.0005) had significant effects on the QTc interval. Furthermore, the interaction between dose and time was significant (p = 0.002), suggesting that the effect of time varied with the dose. In group S and group D1, the QTc interval was significantly shorter after the propofol injection compared to the QTc interval at baseline, but the QTc interval recovered after tracheal intubation. In group D2, the QTc interval was significantly prolonged after droperidol injection, but recovered after propofol injection, and the QTc interval was significantly prolonged after tracheal intubation (Fig. 1).
Time course in heart rate-corrected QT (QTc) interval (ms). Values are median (line inside box) with interquartile range. Capped lines indicate the 10th and 90th percentiles of the data; closed circles indicate individual values. 0, 0 mg droperidol group; 1.25, 1.25 mg droperidol group; 2.50, 2.50 mg droperidol group; T 1 , baseline; T 2 , 3 min after droperidol injection; T 3 , 3 min after anesthetic injection; T 4 , 2 min after tracheal intubation; *p < 0.05 vs. T1 value; # p < 0.05 vs. 0 mg droperidol group
Discussion
The present results showed that 1.25 mg droperidol does not prolong the QTc interval, whereas 2.5 mg droperidol significantly prolongs it, and that the effect of droperidol at an antiemetic dose on the QTc interval is dose dependent. The results also showed that an injection of propofol counteracts the QTc interval prolongation induced by 2.5 mg droperidol.
High-dose droperidol (>0.1 mg/kg) prolonged the QTc interval in a dose-dependent manner [10]; however, there are conflicting reports on whether low-dose droperidol has a QTc prolongation effect. Previous studies reported that 0.625 and 1.25 mg droperidol does not prolong the QTc interval [3, 11]. On the other hand, Charbit et al. [4, 12] reported that 0.75- and 1-mg doses of droperidol induce QTc interval prolongation. The discrepancy may be attributed to the ECG lead used in the measurement (a single lead vs. the average from all leads) or the type of heart rate correction formula used (Bazett formula vs. Fridericia formula). The average value from 12 leads was selected because of the interlead differences in the QT interval [13]. Bazett’s formula is most widely used for heart rate correction, but this formula is known to overcorrect the QT interval and can lead to a false diagnosis of prolonged QTc interval [9]. In the present study, the QT interval was corrected for the heart rate by using the Fridericia correction in accordance with the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use E14 guidance [14].
The consensus guidelines indicate that 0.625 to 1.25 mg droperidol effectively prevents PONV [15]. When PONV is severe, 2.5 mg droperidol is the maximum recommended dose that can be used for its prevention and treatment [1]. The present results showed that 1.25 mg droperidol did not prolong the QTc interval, whereas 2.5 mg droperidol did prolong the QTc interval. Antiemetic doses of droperidol are most effective when administered at the end of surgery, according to the consensus guidelines [15]. However, we administered droperidol 3 min before inducing anesthesia to simplify the blinding procedure and to avoid confounding the changes that occur in the QTc interval with anesthetics [16]. Moreover, when droperidol is administered for the prophylaxis of PONV, it is typically given immediately before and after the induction of anesthesia [2]. The change in the QTc interval occurs typically within 2 min after the injection of droperidol and can be maintained for not more than 5 min [12]. Fentanyl at 2 μg/kg attenuates QTc interval prolongation because of tracheal intubation, but the drug itself does not prolong the QTc interval [17].
The possible mechanisms by which droperidol causes QTc interval prolongation have been attributed to its ability to inhibit the cardiac rapidly activating delayed rectifier K+ current (I Kr), which contributes significantly to a prolonged action potential duration (APD) [18]. Propofol predominantly suppresses L-type calcium currents (I Ca) in a concentration-dependent manner in clinical dose, rather than the cardiac slowly activating delayed rectifier K+ current (I Ks) and I Kr [19]. The propofol-induced shortening of the APD mainly results from its effect on I Ca. The QTc prolongation caused by droperidol’s inhibition of I Kr channels may be counteracted with propofol’s suppression of I Ca.
There is a limitation in the present study. Although manual measurement using a digitizer is a standard method to assess QT interval, we used QT automatic analysis software. This software is superior in reproducibility and has few differences with manual measurements [20].
In conclusion, the present results showed that 1.25 mg droperidol does not prolong the QTc interval, whereas 2.5 mg droperidol prolongs it significantly. This finding suggests that at antiemetic doses the effect of droperidol on the QTc interval is dose dependent. Propofol furthermore counteracted the QTc interval that had been prolonged by 2.5 mg droperidol. Propofol would be suitable for inducing anesthesia in patients needing droperidol for preventing PONV.
References
Nuttall GA, Eckerman KM, Jacob KA, Pawlaski EM, Wigersma SK, Shirk Marienau ME, Oliver WC, Narr BJ, Ackerman MJ. Does low-dose droperidol administration increase the risk of drug-induced QT prolongation and torsade de pointes in the general surgical population? Anesthesiology. 2007;107:531–6.
White PF. Droperidol: a cost-effective antiemetic for over thirty years. Anesth Analg. 2002;95:789–90.
White PF, Song D, Abrao J, Klein KW, Navarette B. Effect of low-dose droperidol on the QT interval during after general anesthesia: a placebo-controlled study. Anesthesiology. 2005;102:1101–5.
Charbit B, Alvarez JC, Dasque E, Abe E, Demolis JL, Funck-Brentano C. Droperidol and ondansetron-induced QT interval prolongation. Anesthesiology. 2008;109:206–12.
Higashijima U, Terao Y, Ichinomiya T, Miura K, Fukusaki M, Sumikawa K. A comparison of the effect on QT interval between thiamylal and propofol during anaesthetic induction. Anaesthesia. 2010;65:679–83.
Oji M, Terao Y, Toyoda T, Kuriyama T, Miura K, Fukusaki M, Sumikawa K. Differential effects of propofol and sevoflurane on QT interval during anesthetic induction. J Clin Monit Comput 2013 (in press).
Kleinsasser A, Loeckinger A, Lindner KH, Keller C, Boehler M, Puehringer F. Reversing sevoflurane-associated Q-Tc prolongation by changing to propofol. Anaesthesia. 2001;56:248–50.
Ichinomiya T, Terao Y, Miura K, Higashijima U, Tanise T, Fukusaki M, Sumikawa K. QTc interval and neurological outcomes in aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2010;13:347–54.
Charbit B, Samain E, Merckx P, Funck-Brentano C. QT interval measurement. Evaluation of automatic QTc measurement and new simple method to calculate and interpret corrected QT interval. Anesthesiology. 2006;104:255–60.
Lischke V, Behne M, Doelken P, Schledt U, Probst S, Vettermann J. Droperidol causes a dose-dependent prolongation of the QT interval. Anesth Analg. 1994;79:983–6.
Chu CC, Shieh JP, Tzeng JI, Chen JY, Lee Y, Ho ST, Wang JJ. The prophylactic effect of haloperidol plus dexamethasone on postoperative nausea and vomiting in patients undergoing laparoscopically assisted vaginal hysterectomy. Anesth Analg. 2008;106:1402–6.
Charbit B, Albaladejo P, Funck-Brentano C, Legrand M, Samain E, Marty J. Prolongation of QTc interval after postoperative nausea and vomiting treatment by droperidol or ondansetron. Anesthesiology. 2005;102:1094–100.
Cowan JC, Yusoff K, Moore M, Amos PA, Gold AE, Bourke JP, Tansuphaswadikul S, Campbell RWF. Importance of lead selection in QT interval measurement. Am J Cardiol. 1998;61:83–7.
Darpo B, Nebout T, Sager PT. Clinical evaluation of QT/QTc prolongation and proarrhythmic potential for nonantiarrhythmic drugs: the international conference on harmonization of technical requirements for registration of pharmaceuticals for human use E14 guideline. J Clin Pharmacol. 2006;46:498–507.
Gan TJ, Meyer T, Apfel CC, Chung F, Davis PJ, Eubanks S, Kovac A, Philip BK, Sessler DI, Temo J, Tramer MR, Watcha M. Consensus guidelines for managing postoperative nausea and vomiting. Anesth Analg. 2003;97:62–71.
Chan MTV, Choi KC, Gin T, Chui PT, Short TG, Yuen PM, Poon AHY, Apfel CC, Gan TJ. The additive interactions between ondansetron and droperidol for preventing postoperative nausea and vomiting. Anesth Analg. 2006;103:1155–62.
Chang DJ, Kweon TD, Nam SB, Lee JS, Shin CS, Park CH, Han DW. Effects of fentanyl pretreatment on the QTc interval during propofol induction. Anaesthesia. 2008;63:1056–60.
Luo T, Luo A, Liu M, Liu X. Inhibition of the HERG channel by droperidol depends on channel gating and involves the S6 residue F656. Anesth Analg. 2008;106:1161–70.
Hatakeyama N, Sakuraya F, Matsuda N, Kimura J, Kinoshita H, Kemmotsu O, Yamazaki M, Hattori Y. Pharmacological significance of the blocking action of the intravenous general anesthetic propofol on the slow component of cardiac delayed rectifier K+ current. J Pharmacol Sci. 2009;110:334–43.
Miyauchi Y, Katoh T, Iwasaki Y, Hayashi M, Mizuno K. Comparison and problems of manual and automated methods for detailed measurement of QT interval. Jpn J Electrocardiol. 2008;28:210–5.
Acknowledgments
This research was supported, in part, by research funds to promote hospital functions of the Japan Labor Health and Welfare Organization.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Toyoda, T., Terao, Y., Oji, M. et al. The interaction of antiemetic dose of droperidol with propofol on QT interval during anesthetic induction. J Anesth 27, 885–889 (2013). https://doi.org/10.1007/s00540-013-1625-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-013-1625-5