Abstract
Perioperative ischemic stroke occurs in approximately 0.08–0.7% of patients after non-cardiovascular surgery and confers a significant risk of morbidity and mortality. The mortality rate of this major complication is similar in non-cardiovascular and cardiovascular surgery. Its incidence appears to be similar in Japan, Europe, and the United States. Perioperative physicians should be aware of the pathophysiology and predictors of ischemic stroke, and the anti-thrombotic strategies to prevent it. The main causes of perioperative ischemic stroke include cerebral atherothrombosis; lacuna stroke; cardiac thrombi due to atrial fibrillation; dehydration; hypotension; and perioperative systemic hypercoagulability. Perioperative management includes detailed informed consent regarding potential stroke risks, counseling, careful surgical treatment decisions, and identification of the high-risk patient for perioperative antithrombotic strategies. The 2009 Japanese guidelines for the management of stroke recommend using the appropriate intravenous infusions to avoid dehydration and consideration of anticoagulation in the patients who are at high risk for thrombosis and embolism while antithrombotic agents are discontinued. Understanding how to prevent perioperative ischemic stroke remains a challenge. In this article, we review the incidence, timing of the occurrence, mortality, risk factors, and pathophysiology of perioperative ischemic stroke in the non-cardiovascular surgery patient.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Perioperative ischemic stroke is one of the most debilitating and prognostically ominous complications of surgery and anesthesia. An accurate characterization of perioperative ischemic stroke can contribute to informed consent, patient counseling, and appropriate surgical treatment decisions. Cardiac and vascular surgeries are associated with an increased risk of stroke; the incidence rate ranges from 2.0 to 10.0% [1–3] and the mortality rate is 22.2% [4]. Non-cardiovascular surgery is associated with a lower risk of stroke, with an incidence rate that ranges from 0.08 to 0.7%. Perioperative mortality in non-cardiovascular surgery patients who suffer a perioperative ischemic stroke is remarkably high at 18–26% [5–7], and is comparable to the mortality rate in cardiovascular surgery patients [4]. However, there are few studies regarding the risk management of perioperative ischemic stroke in non-cardiovascular surgery.
Stroke is projected to remain the second leading cause of death worldwide until 2020, and is the main cause of long-term neurological disability in the adult [8]. A recent large comparative study documents a relatively high incidence of strokes in the Japanese population, compared with most other populations in the world [9]. Our recent study also documents a higher incidence of perioperative ischemic stroke than perioperative myocardial infarction [10]. This may indicate that Japanese patients are more vulnerable to perioperative ischemic stroke, compared with populations in other countries of the world.
In this article, we review the incidence, mortality, risk factors, and pathophysiology of symptomatic perioperative ischemic stroke in the non-cardiovascular surgery patient.
Incidence
Two recent large observational studies report that the incidence of symptomatic perioperative stroke is 0.3–0.4% in non-cardiovascular surgery patients [7, 10]. In these studies, perioperative new neurological deficits that persisted for more than 24 h were included. Silent or mild ischemic stroke may be overlooked and undiagnosed; therefore, the actual incidence of perioperative stroke, including transient or silent ischemic attacks, is probably higher than has been reported.
Age
Two recent observational studies demonstrate that the incidence of perioperative ischemic stroke increases with age (Fig. 1) [7, 10]. The incidence is 0.1–0.2% for people under 65 years old, increases to 0.5% in people 65–74 years old; and further increases to 1.0% in people over 75 years old. These two studies were based on different ethnic populations—one study was conducted in the United States [7] and the other in Japan [10]. Nevertheless, the incidence of perioperative ischemic stroke with aging was similar.
Operations
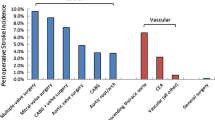
The incidence of perioperative ischemic stroke varies, depending on the type of operation (Table 1). High-risk surgery increases the risk of ischemic stroke by 1.5-fold [10]. The incidences of perioperative ischemic stroke reported in previous studies are 0.08–0.7% in general surgery [1, 7, 10–12]; 0.2–0.9% in orthopedic surgery [7, 10, 12]; 0.6–0.9% in lung operations [7, 10]; and 0.3–3.0% in peripheral vascular surgery [1, 10, 12].
Timing of the occurrence of an ischemic stroke
The time from the surgery to the occurrence of an ischemic stroke ranges from the day of surgery to postoperative day 30 (Fig. 2) [6, 10–13]. The median day for the occurrence of an ischemic stroke is postoperative days 2 through 9, while the mean day for the occurrence of an ischemic stroke is postoperative days 8 through 12. The peak incidence of perioperative stroke is early after the surgery, but the incidence declines thereafter [10, 12]. Our recent study shows the incidence is at a relatively constant rate during the postoperative period (Fig. 3) [10].
The daily distribution of postoperative stroke (n = 126) is shown, and a linear regression line best fitted the distribution of postoperative ischemic stroke (P = 0.0004). Dotted lines indicate 95% confidence intervals. From Kikura et al. [10], with permission
Risk factors
The main risk factors for perioperative ischemic stroke are an advanced age, high-risk surgery, a history of cerebrovascular disease, peripheral vascular disease, and chronic obstructive pulmonary disease [1]. Minor predictors are hypertension, atrial fibrillation, coronary or valvular heart disease, and congestive heart failure. Strong predisposing factors for postoperative stroke are prolonged hypotension, dehydration, hypoxemia, and prolonged ventilation.
Table 2 summarizes the odds ratios noted in previous reports concerning the risk factors of age, female sex, high-risk surgery, metabolic diseases, cardiac diseases, and cancer [7, 10, 14]. Since the number of surgery patients who have the risk factors associated with ischemic stroke has been increasing, the incidence of perioperative ischemic stroke is also projected to increase [14].
Pathophysiology: hypercoagulability
Anesthesia and surgery increase the risk of ischemic stroke by fourfold [12]. The mechanisms of perioperative ischemic stroke are cerebral atherothrombosis and embolism due to atherosclerosis; lacuna stroke; cardiac thrombi due to atrial fibrillation; and hypotension.
Perioperative systemic hypercoagulability also contributes to ischemic stroke. Surgery presents the opportunity for perioperative hemodynamic disturbance, bed rest, and a state described in the cell-based model of vascular thrombosis [15]. This state is characterized by hypercoagulability; reduced fibrinolysis; thrombin generation promoted by inflammatory cytokines and tissue factor; and thrombosis resulting from activated neutrophils and platelets adhering to activated endothelial cells [16–18]. All perioperative thromboembolic events such as myocardial infarction, vein thromboembolism, and ischemic stroke appear to share a common pathophysiology as a syndrome [19].
Hoffman and Monroe [15] proposed a cell-based model of blood coagulation (Fig. 4). In this model, tissue factor and factor VIIa initially activate tissue factor-bearing cells. These cells activate the conversion of prothrombin to thrombin (Initiation). The thrombin activates platelets; the platelet activation amplifies, thereby activating more platelets and generating more thrombin (Amplification). The thrombin propagates the activation of platelets, which generates more thrombin (Propagation). This cell-based model clearly explains the upregulation of coagulability under the generation of tissue factor and thrombin. Figure 5 illustrates the mechanism of arterial thrombosis formation by the tissue factor-bearing cells (Fig. 5) [20]. In the artery, tissue factor-bearing cells activate cytokines and thrombins on the surface of the injured and dysfunctional endothelium, thereby leading to platelet activation and vasoconstriction. These inflammatory responses and platelet activation are gradually amplified and propagated to cause arterial thrombosis.
Tissue factor (TF) and factor VIIa (VIIa) initially activate tissue factor-bearing cells, and these cells activate the conversion of prothrombin to thrombin (Initiation). The thrombin activates platelets; the platelet activation amplifies, thereby activating more platelets and generating more thrombin (Amplification). The thrombin propagates the activation of platelets, which generates more thrombin (Propagation). vWF, von Willebrand factor. From Hoffman and Monroe [15], with permission
The mechanism of arterial thrombosis formation by tissue factor-bearing cells. TNF-α, tumor necrosis factor alpha; ADP, adenosine diphosphate; TAF-I, thrombin activatable fibrinolysis inhibitor; PAI-1, plasminogen activator inhibitor-1. From Kikura et al. [20], with permission
In Fig. 6, we illustrate a conceptualization of how the timing of perioperative ischemic stroke may be related to the time course of perioperative inflammatory and hypercoagulable responses. The perioperative inflammatory response and hypercoagulable responses peak following surgery, while the incidence of ischemic stroke declines linearly. Pre-existing risk factors shift the incidence regression curve upward. The speculated responses of C-reactive protein and tissue factor-bearing cells following surgery are shown as a dotted line in this figure. These inflammatory and hypercoagulable responses have important roles for the development of perioperative ischemic stroke (Fig. 6).
Perioperative considerations
Recognition of the incidence, risks, and pathophysiology are necessary for informed consent, patient counseling, and surgical treatment decisions; and for identifying high-risk patients for prophylactic antithromboembolic treatment and identifying the critical postoperative period. In high-risk patients, preoperative consultations are necessary for assessing their past history, preoperative comorbidities, systemic atherosclerosis (especially in the cerebral and carotid arteries), performing echocardiography, controlling atrial fibrillation, and determining the risk–benefit balance in preoperatively discontinuing antiplatelet and anticoagulant agents.
The 2009 Japanese guidelines for the management of stroke recommend using the appropriate infusion to avoid dehydration and discontinuing the following antiplatelet agents prior to general surgery: aspirin (7 days prior); clopidogrel (14 days prior); ticlopidine (10–14 days prior); and cilostazol (3 days prior) and recommend heparinization should be appropriately considered in the patients who are at high risk for thrombosis and embolism while anticoagulants are discontinued [21]. The guidelines for the perioperative management of antiplatelet therapy published in Chest in 2008 suggest that for patients undergoing a major surgical or invasive procedure, if the intent is to eliminate any effect of antithrombotic therapy, such therapy should be stopped at a time before the procedure (e.g., 7–10 days in patients receiving aspirin and clopidogrel) such that there will be minimal or no residual antithrombotic effect at time of the procedure and in doing so will minimize the risk for intraprocedural bleeding [22]. However, it should be noted that the withdrawal of antiplatelet therapy is a risk factor for ischemic stroke [23], and in some instances, when bleeding risks are acceptable, continuation of antiplatelet therapy may be warranted [24]. Further, the American College of Chest Physicians evidence-based clinical practice guidelines (8th edition), published in Chest in 2008, suggest that, in patients who are at high risk for cardiac events, consideration be given to continuation of aspirin in the perioperative period [22]. Whether this recommendation should be applied to patients at high risk for stroke, and aspirin continued in the perioperative period, is a question that merits investigation, and balancing thromboembolic risk and bleeding risk could be warranted.
Perioperative hypotension and dehydration should be avoided. The early neurological symptoms of ischemic stroke (e.g., hemiplegia and aphasia) should be carefully noted following recovery from general and/or regional anesthesia. Variation in the risks of cerebrovascular accident obtained by an anesthetic technique is controversial [25, 26]. Epidural and/or lumbar spinal subarachnoid anesthesia is less likely than general anesthesia to result in perioperative complications [26]. However, patients who receive antiplatelet and anticoagulant drugs have a risk of developing an epidural hematoma when undergoing epidural and spinal subdural arachnoid anesthesia. In patients who should receive or restart antithrombotic drugs after surgery, intravenous patient-controlled analgesia (i.v. PCA) provides a safe approach for perioperative analgesia. Regional neural block techniques also provide an alternative method for perioperative analgesia.
In elderly patients, the cerebral vascular function that maintains brain regional oxygen saturation may deteriorate under general anesthesia. As illustrated in Fig. 7, when arterial blood pressure was appropriately maintained, we observed a dissociation in the brain regional oxygen saturation after the induction of general anesthesia in patients under 70 and in patients over 70 years old (Fig. 7) [27]. In patients under 70 years old, the brain regional oxygen saturation was maintained above the baseline before the induction of general anesthesia and during the following 60 min after anesthetic induction, whereas brain regional oxygen saturation significantly deteriorated by approximately 5% from the baseline after anesthetic induction in patients over 70 years old. Administration of more than 60% oxygen increased brain regional oxygen saturation in the elderly patient group, to almost the same extent as in the younger patient group, but the increase was not complete [28]. In elderly patients, peripheral cerebral vascular circulation might be decreased. The extent to which this predisposes to watershed infarct, or other stroke types, remains to be determined.
In mechanically ventilated patients, sedatives and narcotics will make neurological assessment difficult after surgery and mask the occurrence of postoperative ischemic stroke. Dexmedetomidine—an alpha II agonist that reduces delirium, hypertension, and tachycardia—provides better sedation than the benzodiazepines (e.g., midazolam) [29, 30], and may help in neurological assessment in patients under sedation.
Perspectives
Our understanding of the prophylaxis, monitoring, and prevention of perioperative ischemic stroke continues to evolve for non-cardiovascular surgery patients. Ischemic stroke in high-risk patients is relatively common and mortality is high. Further research is needed to better understand how to prevent this devastating complication.
References
Selim M. Perioperative stroke. N Engl J Med. 2007;356:706–13.
Grocott HP, Yoshitani K. Neuroprotection during cardiac surgery. J Anesth. 2007;21:367–77.
Baba T, Goto T, Maekawa K, Ito A, Yoshitake A, Koshiji T. Early neuropsychological dysfunction in elderly high-risk patients after on-pump and off-pump coronary bypass surgery. J Anesth. 2007;21:452–8.
Bucerius J, Gummert JF, Borger MA, Walther T, Doll N, Onnasch JF, Metz S, Falk V, Mohr FW. Stroke after cardiac surgery: a risk factor analysis of 16,184 consecutive adult patients. Ann Thorac Surg. 2003;75:472–8.
Parikh S, Cohen JR. Perioperative stroke after general surgical procedures. N Y State J Med. 1993;93:162–5.
Limburg M, Wijdicks EF, Li H. Ischemic stroke after surgical procedures: clinical features, neuroimaging, and risk factors. Neurology. 1998;50:895–901.
Bateman BT, Schumacher HC, Wang S, Shaefi S, Berman MF. Perioperative acute ischemic stroke in noncardiac and nonvascular surgery: incidence, risk factors, and outcomes. Anesthesiology. 2009;110:231–8.
Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet. 1997;349:1498–504.
Steg PG, Bhatt DL, Wilson PW, D’Agostino R Sr, Ohman EM, Rother J, Liau CS, Hirsch AT, Mas JL, Ikeda Y, Pencina MJ, Goto S. One-year cardiovascular event rates in outpatients with atherothrombosis. JAMA. 2007;297:1197–206.
Kikura M, Oikawa F, Yamamoto K, Iwamoto T, Tanaka KA, Sato S, Landesberg G. Myocardial infarction and cerebrovascular accident following non-cardiac surgery: differences in postoperative temporal distribution and risk factors. J Thromb Haemost. 2008;6:742–8.
Larsen SF, Zaric D, Boysen G. Postoperative cerebrovascular accidents in general surgery. Acta Anaesthesiol Scand. 1988;32:698–701.
Wong GY, Warner DO, Schroeder DR, Offord KP, Warner MA, Maxson PM, Whisnant JP. Risk of surgery and anesthesia for ischemic stroke. Anesthesiology. 2000;92:425–32.
Landercasper J, Merz BJ, Cogbill TH, Strutt PJ, Cochrane RH, Olson RA, Hutter RD. Perioperative stroke risk in 173 consecutive patients with a past history of stroke. Arch Surg. 1990;125:986–9.
Kikura M, Takada T, Sato S. Preexisting morbidity as an independent risk factor for perioperative acute thromboembolism syndrome. Arch Surg. 2005;140:1210–7 (discussion 8).
Hoffman M, Monroe DM 3rd. A cell-based model of hemostasis. Thromb Haemost. 2001;85:958–65.
Dahl OE. Mechanisms of hypercoagulability. Thromb Haemost. 1999;82:902–6.
McEver RP. Adhesive interactions of leukocytes, platelets, and the vessel wall during hemostasis and inflammation. Thromb Haemost. 2001;86:746–56.
Goel MS, Diamond SL. Neutrophil enhancement of fibrin deposition under flow through platelet-dependent and -independent mechanisms. Arterioscler Thromb Vasc Biol. 2001;21:2093–8.
Kikura M, Takada T, Sato S. Age- and sex-specific incidence, risk, and latency period of a perioperative acute thromboembolism syndrome (PATS). Thromb Haemost. 2004;91:725–32.
Kikura M, Ishiguro Y. Perioperative management of hemostasis and thrombosis in cardiovascular anesthesia (in Japanese with English Abstract). Nihon Rinsho Masui Gakkaishi (J Jpn Soc Clin Anesth). 2009;29:809–14.
Shinohara Y, The Joint Committee on Guidelines for the Management of Stroke. Japanese guidelines for the management of stroke 2009. Tokyo: Kyowa Kikaku (Corp.); 2009. p. 103–9.
Douketis JD, Berger PB, Dunn AS, Jaffer AK, Spyropoulos AC, Becker RC, Ansell J. The perioperative management of antithrombotic therapy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133:299S–339S.
Sibon I, Orgogozo JM. Antiplatelet drug discontinuation is a risk factor for ischemic stroke. Neurology. 2004;62:1187–9.
Neilipovitz DT, Bryson GL, Nichol G. The effect of perioperative aspirin therapy in peripheral vascular surgery: a decision analysis. Anesth Analg. 2001;93:573–80.
Bode RH Jr, Lewis KP, Zarich SW, Pierce ET, Roberts M, Kowalchuk GJ, Satwicz PR, Gibbons GW, Hunter JA, Espanola CC. Cardiac outcome after peripheral vascular surgery. Comparison of general and regional anesthesia. Anesthesiology. 1996;84:3–13.
Rodgers A, Walker N, Schug S, McKee A, Kehlet H, van Zundert A, Sage D, Futter M, Saville G, Clark T, MacMahon S. Reduction of postoperative mortality and morbidity with epidural or spinal anaesthesia: results from overview of randomised trials. BMJ. 2000;321:1493.
Iwamoto T, Itagaki T, Oikawa F, Kikura M, Hirano K, Sato S. The effect of general anesthesia on regional cerebral oxygen saturation and volume of hemoglobin in elderly patients. J Anesth. 2007;21(suppl):O57-07.
Iwamoto T, Oikawa H, Kume Y, Ishida C, Kikura M. Changes under anesthesia in cerebral hemoglobin density and oxygen saturation in elderly patients. Anesthesiology. 2008;109:A241.
Pandharipande PP, Pun BT, Herr DL, Maze M, Girard TD, Miller RR, Shintani AK, Thompson JL, Jackson JC, Deppen SA, Stiles RA, Dittus RS, Bernard GR, Ely EW. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298:2644–53.
Riker RR, Shehabi Y, Bokesch PM, Ceraso D, Wisemandle W, Koura F, Whitten P, Margolis BD, Byrne DW, Ely EW, Rocha MG. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301:489–99.
Acknowledgments
The authors are thankful to Dr. Kuniyoshi Tanaka (Department of Cardiothoracic and Vascular surgery, Hamamatsu Medical Center, Hamamatsu, Japan) for his thoughtful critiques and comments on this review article.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Kikura, M., Bateman, B.T. & Tanaka, K.A. Perioperative ischemic stroke in non-cardiovascular surgery patients. J Anesth 24, 733–738 (2010). https://doi.org/10.1007/s00540-010-0969-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-010-0969-3