Abstract
Background
The prognostic importance of the neuroendocrine (NE) markers involving neural cell adhesion molecule (NCAM) has been unclear enough to be adopted for WHO classification in patients with pancreatic neuroendocrine neoplasms (Pan-NENs). This study aimed to elucidate whether the three NE markers such as chromogranin A, synaptophysin, and NCAM decide prognoses for patients with well-differentiated tumors.
Methods
Between April 2002 and October 2018, 217 patients were included in this study. Tissue samples from tumors of Pan-NENs were immunochemically stained for the aforementioned NE markers. Diffuse and intense staining was defined as positive, while faint or focal staining and non-staining were considered negative.
Results
The median age of patients was 55 years. The median observation period was 1415 days. In multivariate analysis of progression-free survival (PFS), liver metastasis, Ki-67 index, and triple-positive staining of NE markers were risk factors. The 5-year PFS rate of patients with and without triple-positive NE markers was 56.3% and 23.8%, respectively (P < 0.0001). In multivariate analysis of overall survival (OS), R0 resection, Ki-67 index and triple-positive NE markers (hazard ratio 0.4, P = 0.02) were the risk factors. The 5-year OS rate of patients with and without triple-positive NE markers was 88.8% and 66.4%, respectively (P = 0.014). The tumors of patients without triple-positive NE markers were associated with large tumor size, a high mitotic rate and high Ki-67 index.
Conclusions
Triple-positive NE marker staining was a simple and practical indicator of prognoses in patients with well-differentiated Pan-NETs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreatic neuroendocrine neoplasm (Pan-NEN) is a rare disease [1, 2], although autopsy reports estimate its prevalence to be much higher [3]. Recent advances in medical technology have resulted in an increased incidence of Pan-NENs [4]. Pan-NENs represent approximately 3% of pancreatic malignancies [5]. Distant metastases at diagnosis were identified in about 42% of Pan-NENs, with a 5-year overall survival (OS) of about 40% [6]. In a nationwide study, 33% of patients with nonfunctioning Pan-NENs < 5 mm presented with regional lymph node metastases and 11% with distant metastasis [7]. Surgical resection has been regarded as the primary treatment in European Neuroendocrine Tumor Society (ENETS) and National Comprehensive Cancer Network (NCCN) guidelines [8, 9]. North American Neuroendocrine Tumor Society (NANETS) guideline suggested that primary tumor resection and aggressive surgical approach had improved the long-term survival in patients with advanced Pan-NENs [10, 11]. The 5-year survival rate was 65–86% after surgical resection of nonfunctioning Pan-NENs [12,13,14]. The American Joint Committee on Cancer (AJCC) staging classification is a good indicator of relapse-free survival in patients with surgically resected non-metastatic Pan-NENs, but it is not good indicator of OS [15].
Various factors have been used to estimate the malignant potential in the World Health Organization (WHO) classifications. The key factors of the 2004 WHO classification were distant metastases, gross local invasion, and high mitotic rate. The Ki-67 index was not adopted to judge the well-differentiated and poorly differentiated neuroendocrine carcinoma (NEC), and it was suggested only to evaluate uncertain behavior of well-differentiated tumors. The 2010 WHO classification divided Pan-NENs into neuroendocrine tumor-grade 1 (NET-G1), NET-G2, and NEC based on both mitotic rate and the Ki-67 index. In the WHO 2017 classification, tumor differentiation, Ki-67 index, and mitotic rate were key factors to decide the malignant characteristics of tumors [16]. Well-differentiated tumors were defined as Pan-NETs and categorized as NET-G1, -G2, or -G3 based on the Ki-67 index or mitotic rate, while all poorly differentiated tumors were defined as NEC-G3. NEC-G3 defined by the WHO 2010 classification was divided into NET-G3 (well differentiated) and NEC-G3 (poorly differentiated). Historically, the aforementioned proliferative capacity and morphological factors played a pivotal role in estimating the malignant potential, although discordance of these factors and the optimal cutoff points to stratify outcome remain controversial [17, 18]. It is difficult to objectively distinguish well from poor differentiation. Many indices such as KRAS and Rb expression have been advocated only to distinguish tumors with high and low Ki-67 values because it is unknown whether such indices properly classify tumor differentiation [19]. A high Ki-67 value is often associated with poorly differentiated tumors to predict poor patient prognosis. Therefore, the Ki-67 index has been the primary indicator used to estimate the malignant potential of Pan-NENs. However, to our knowledge, no report has assessed whether neuroendocrine markers (NE markers) rather than Ki-67 or mitosis could decide the malignant potential of tumors or predict poor prognosis of Pan-NEN patients. There are few studies that illustrated the importance of mitosis predicting prognoses. In this line, the present study excluded poor differentiation to simplify discussion.
The NANETS guideline recommends the use of chromogranin A, synaptophysin, and neural cell adhesion molecule (NCAM) as NE markers of Pan-NEN phenotype [10]. Chromogranin A, also known as parathyroid secretory protein 1, is found in the neurosecretory vesicles of neuroendocrine cells such as the pancreatic islet beta cells and enterochromaffin-like cells. Synaptophysin is extensively detected in a variety of neuroendocrine cells and plays a key role in synaptic transmission. A diffuse unequivocal labeling for synaptophysin indicates neuroendocrine differentiation. Positive staining for chromogranin A and/or synaptophysin has been used for the diagnosis of Pan-NETs and many NEN tumors, including well-differentiated and poorly differentiated tumors, which also express chromogranin A and/or synaptophysin [16, 20]. NCAM is similarly a useful immunoperoxidase stain for neuroendocrine tumors [21, 22]. NCAM is also expressed in Pan-NETs, and membranous cell labeling in conjunction with synaptophysin positivity contributes to the diagnosis of Pan-NECs [16]. NE marker staining is classified into diffuse and intense staining, faint or focal staining, and no staining subtype. The present study defined the diffuse and intense staining as positive and the other staining as negative to retrospectively examine the prognoses of Pan-NET patients. To this end, we found that one of the most important predictors of prognoses was triple-positive staining for these NE markers in well-differentiated tumors.
Methods
Between April 2002 and October 2018, 243 patients were pathologically diagnosed with pancreatic NEN and received treatment at Tokyo Medical and Dental University Hospital (Tokyo, Japan). In 230 patients, all three NE markers (chromogranin A [DAK-A3, Agilent DAKO, Santa Clara, CA], synaptophysin [RMAB018, Diagnostic BioSystems, Pleasanton, CA], and NCAM [NCL-CD56-504, Novacastra, Leica Biosystems Newcastle Ltd, UK]) were stained. Thirteen patients had poor-differentiated tumors who were excluded from this study. Diffuse and intense staining in tumors was defined as positive staining, while faint or focal staining and non-staining were defined as negative staining (Fig. 1). We defined diffuse staining when more than 70% of the tumor cells were stained. The expression levels of each NE markers were evaluated based on the degree of staining of the pancreatic islet of Langerhans. A case where tumor cells were stained faintly than islets of Langerhans was defined as “Faint”. The levels were determined independently by three pathologists in a blinded manner. In case of disagreement, a consensus was reached by joint review.
Immunohistochemical staining of a chromogranin A, b synaptophysin, and c NCAM. The left column, showing diffuse and intense staining, was defined as positive staining. The middle and right columns were defined as negative staining. Note the focal and faint staining of the middle column and the lack of staining in the right column. All judgments were made based on the degree of dyeing on the islet of Langerhans
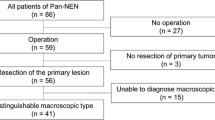
As shown in Fig. 2, triple-positive staining was observed in 172 cases (triple-positive group). Non-triple-positive staining was observed in 45 patients (non-triple-positive group). Background characteristics included patient age, gender, hereditary syndromes such as multiple endocrine neoplasia type 1 or von Hippel–Lindau disease, hormonal symptoms (insulinoma, gastrinoma, glucagonoma, VIPoma, and nonfunctioning), location of the primary tumor, primary tumor size and number, metastasis of the lymph nodes and liver, macroscopic invasion to adjacent organs, vascular invasion, and pathological factors (Table 1). The pathological factors included the Ki-67 index, mitotic rate, and NE markers (chromogranin A, synaptophysin, and NCAM). Tumor grades were defined according to the 2017 WHO classification. The Ki-67 proliferative index was quantified by counting at least 500 cells in “hot spots”.
Laboratory tests were performed in all patients at least every 3–6 months. Computed tomography or magnetic resonance imaging with a bolus injection of contrast medium was also performed at the same intervals and at least two radiologists diagnosed the progression and relapse. The progression-free survival (PFS) rate was calculated from the day of surgical operation to the first date of recurrence, or the day of the initial non-surgical treatments to the first day when progressive disease defined by response evaluation criteria in solid tumor (RECIST) was observed. We conducted a prognostic survey of all patients in October 2018. Information on outcomes after treatment after more than 5 years was collected by personal interview for patients observed in other hospitals. Written informed consent was obtained from each subject and all study procedures were approved by an institutional review board (Human Research Ethics Committee, Tokyo Medical and Dental University ID: 1080).
Statistical analyses were performed using IBM SPSS Statistics for Windows, version 23.0 (IBM Corp., Armonk, NY). Data were presented as the median (range). P < 0.05 was considered statistically significant. χ2 or Fisher’s exact tests were used to analyze categorical variables. Mann–Whitney tests were used to analyze differences between continuous values of two independent groups. Survival curves were constructed using the Kaplan–Meier methods and compared using log-rank test results. After univariate analysis, the significant variables were subjected to multivariate analysis using a Cox proportional hazards model.
Results
Baseline characteristics
This study included 217 Pan-NET patients who received treatment at a single high-volume center in Japan. The 152 patients received R0 resection. The median observation period was 1415 days. The baseline characteristics are listed in Table 1. The median age was 55 years. Eighteen patients had genetic diseases such as multiple endocrine neoplasms type 1 and von Hippel–Lindau disease. Nonfunctional tumors, insulinoma, gastrinoma, glucagonoma, and VIPoma were observed in 173, 23, 14, 4, and 3 patients, respectively; 82 and 125 tumors occurred in the pancreatic head and body/tail, and 10 occurred diffusely. Appropriate operative procedures were selected for R0 resection. The median primary tumor size was 20 mm. The 26 patients had multiple tumors in the pancreas; 79 and 60 patients had synchronous lymph node and liver metastases, respectively. The median Ki-67 and mitotic indices were 2.7% and 1/10 HPF, respectively. Positive staining for chromogranin A, synaptophysin, and NCAM was observed in 192 (88.5%), 207 (95.4%), and 190 (87.6%) patients, respectively. Triple-positive staining of the three NE markers was identified in 172 patients. Vascular invasion and macroscopic local invasion were observed in 13 and 24 patients, respectively. Following the 2017 WHO classification, 100, 92, and 25 patients had NET-G1, NET-G2, and NET-G3, respectively.
Risk factors predicting the prognoses of patients
Table 2 shows which risk factors determined the PFS after treatment. In univariate analysis, tumor size (P < 0.0001), macroscopic local invasion (P = 0.005), vascular invasion (P = 0.048), lymph node metastasis (P < 0.0001), liver metastasis (P < 0.0001), Ki-67 index (P < 0.0001), mitotic rate (P < 0.0001), R0 resection (P < 0.0001), and triple-positive NE marker staining (P < 0.0001) were identified as risk factors. In multivariate analysis, triple-positive staining (hazard ratio [HR] 0.4, P < 0.0001), simultaneous liver metastasis (HR 2.3, P = 0.03), and Ki-67 index (HR 4.8, P < 0.0001) were identified as risk factors for PFS. R0 resection was not the risk factor for PFS (P = 0.4), because 65 out of 217 patients involved the patients with chemotherapy only. Their tumor progression is not same as that after R0 resection.
Table 3 shows the risk factors associated with OS. The univariate analysis identified tumor size (P = 0.002), lymph node metastasis (P < 0.0001), liver metastasis (P < 0.0001), Ki-67 index (P < 0.0001), mitotic rate (P < 0.0001), R0 resection (P < 0.0001), and triple-positive NE marker staining (P = 0.02) as significant risk factors. In multivariate analysis, R0 resection (P = 0.049), Ki-67 index (P = 0.01), and triple-positive NE marker staining (P = 0.02) decided OS. These results suggest that triple-positive NE marker staining was one of the most important factors for both PFS and OS.
Figure 3 shows whether triple-positive NE marker staining decides both PFS and OS. As shown in Fig. 3a, the 5-year PFS of patients with triple-positive staining and non-triple-positive staining was 56.3% and 23.8%, respectively (P < 0.0001). Moreover, as shown in Fig. 3b, the 5-year OS rates of patients with triple-positive staining and non-triple-positive staining were 88.8%, and 66.4%, respectively (P = 0.014).
Characteristics of patients with triple-positive NE marker staining
These results led us to determine the characteristics of patients with or without triple-positive NE marker staining (Table 4). Non-triple-positive staining was associated with large tumors (P = 0.04), high Ki-67 index (P < 0.0001) and high mitotic rate (P = 0.003). Age, gender, hereditary disease, multiple tumors, lymph node metastasis, liver metastasis, macroscopic invasion, and vascular invasion were not related to the number of NE markers with positive staining.
Discussion
The three key factors in WHO classifications, such as Ki-67 index, mitotic rate, and tumor differentiation, have played pivotal roles in estimating malignant potential. The present study, excluding poor-differentiated tumors, validated the importance of the Ki-67 index for predicting the PFS and OS in Pan-NET patients, though mitosis could not predict the prognoses. However, this study identified that positive staining for three NE markers was the most important factor to predict both PFS and OS. This triple-positive staining more accurately predicted the OS than mitoses, a key factor in the 2017 WHO classification. Moreover, large tumor size, and high mitotic rate and high Ki-67 index were associated in patients with non-triple-positive marker staining. To our knowledge, this is the first report to elucidate the link between prognosis and positivity for these all three NE markers.
R0 resection turned out to be an important risk factor for OS (Table 3). We divided 217 patients with or without R0 resection into two groups according to triple-positive staining status, because the number of patients with R0 resection was limited. Debulking or palliative operations were more often selected rather than curative resections in patients diagnosed with synchronous distant metastasis or large tumors involving major arteries. As shown in Table 1, the median age, gender, and rate of nonfunctioning tumors were consistent with those in a previous report of a large study of Pan-NENs after resection [23]. Sixty percent of tumors were detected in the body or tail of the pancreas and the location and the selection of surgical procedure were similar to those of a previous study of the AJCC and ENETS-staging classifications [15]. The 5-year DFS and OS in patients with R0 resection were 71.2% and 96.7%, respectively (data not shown). These survival rates were consistent with those of a previous study [24].
In multivariate analysis of PFS, simultaneous liver metastasis, Ki-67 index, and triple-positive NE marker staining were identified as risk factors for disease progression (Table 2). In multivariate analysis of OS, Ki-67 index, R0 resection and triple-positive staining was identified as risk factors (Table 3). Some key factors of the WHO classifications, ENETS staging, and AJCC staging, such as mitosis, tumor size, lymph node metastases, macroscopic invasion, and liver metastases, did not predict the OS. In the previous study, Ki-67 index has been reported as an important determinant of prognoses. Tumors > 4 cm and Ki-67 index > 9% were identified as the prognostic risks for DFS and OS in univariate analysis and only tumor size was an independent risk factor for OS in multivariate analysis [25]. Ki-67 index, larger tumor size, and positive lymph nodes were risk factors for recurrence in patients with well-differentiated tumors after R0/R1 resection [26]. Higher tumor grade, lymph node metastasis, and perineural invasion were identified in patients with G1 and G2 nonfunctioning Pan-NETs without distant metastases [27].
The present study demonstrated the prognostic value of triple-positive staining for better PFS and OS. It was consistent with the previous studies. The prognostic value of the three NE markers was reported in lung large cell NEC [28]. The expression of single markers did not predict survival, while positive staining for both chromogranin A and NCAM or one of each showed worse recurrence-free survival. The results of the other studies without multivariate analysis remain controversial.
Table 4 shows that tumors in the non-triple-positive group had more aggressive behavior such as large tumor size, high mitotic index, and high Ki67 index. However, they were not associated with lymph node metastases, liver metastases, macroscopic invasion, and vascular invasion, the key parameter of the WHO 2004 classification and TNM classification such as AJCC and ENETS. In the future, evaluation of individual hormones may reveal the relevance of NE marker expression and hormone production. The number of hormone production tended to reduce the risk of metachronous liver metastasis in univariate analysis in our previous study [29]. Due to the varieties and heterogeneity of hormone production, a much larger study is required for a precise analysis.
Several biomarkers have been proposed to predict the malignant potential after primary tumor resection. In our previous study, downregulated pancreatic beta cell genes involving PAX6 in primary tumors predicted metachronous liver metastasis and poor OS in Pan-NEN patients. Patients with low PAX6 expression had a higher Ki-67 index and mitotic rate [29]. B cell lymphoma 2 (BCL2) has been evaluated as a biomarker in Pan-NENs. The overexpression was correlated with a higher mitotic rate and Ki-67 index and was suggested to distinguish Pan-NECs from Pan-NETs [30].
The present study has several limitations because it is an observational retrospective study. During the long period of enrollment from 2002 to 2018, diagnostic imaging technique, such as MRI, multi-detector CT (MDCT), and octreoscan with MDCT, evolved significantly in the last 16 years. All the treatments were performed to predominately Asian patients. However, the large number of patients of this cohort may help to minimize selection bias.
In conclusion, the present study identified that triple-positive staining for the NE markers predicts the prognosis of patients with well-differentiated Pan-NETs. Since these three NE markers have been widely used in routine pathological diagnosis, we hope that this practical and simple prognostic predictor could be extensively applied in all Pan-NEN patients with or without surgical resection.
Abbreviations
- CI:
-
Confidence interval
- HPF:
-
High power field
- HR:
-
Hazard ratio
- MEN:
-
Multiple endocrine neoplasia
- NCAM:
-
Neural cell adhesion molecule
- NE:
-
Neuroendocrine
- NET:
-
Neuroendocrine tumor
- NEC:
-
Neuroendocrine carcinoma
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- VHL:
-
von Hippel–Lindau
- WHO:
-
World Health Organization
References
Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–72.
Ito T, Igarashi H, Nakamura K, et al. Epidemiological trends of pancreatic and gastrointestinal neuroendocrine tumors in Japan: a nationwide survey analysis. J Gastroenterol. 2015;50:58–64.
Kimura W, Kuroda A, Morioka Y. Clinical pathology of endocrine tumors of the pancreas. Analysis of autopsy cases. Dig Dis Sci. 1991;36:933–42.
Hallet J, Law CH, Cukier M, et al. Exploring the rising incidence of neuroendocrine tumors: a population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer. 2015;121:589–97.
Fesinmeyer MD, Austin MA, Li CI, et al. Differences in survival by histologic type of pancreatic cancer. Cancer Epidemiol Biomark Prev. 2005;14:1766–73.
Frilling A, Modlin IM, Kidd M, et al. Recommendations for management of patients with neuroendocrine liver metastases. Lancet Oncol. 2014;15:e8–21.
Gratian L, Pura J, Dinan M, et al. Impact of extent of surgery on survival in patients with small nonfunctional pancreatic neuroendocrine tumors in the United States. Ann Surg Oncol. 2014;21:3515–21.
Falconi M, Eriksson B, Kaltsas G, et al. ENETS consensus guidelines update for the management of patients with functional pancreatic neuroendocrine tumors and non-functional pancreatic neuroendocrine tumors. Neuroendocrinology. 2016;103:153–71.
National Comprehensive Cancer Network. NCCN guidelines: neuroendocrine and adrenal tumors. Version 3.2018;2018.
Kunz PL, Reidy-Lagunes D, Anthony LB, et al. Consensus guidelines for the management and treatment of neuroendocrine tumors. Pancreas. 2013;42:557–77.
Schurr PG, Strate T, Rese K, et al. Aggressive surgery improves long-term survival in neuroendocrine pancreatic tumors: an institutional experience. Ann Surg. 2007;245:273–81.
Ferrone CR, Tang LH, Tomlinson J, et al. Determining prognosis in patients with pancreatic endocrine neoplasms: can the WHO classification system be simplified? J Clin Oncol. 2007;25:5609–15.
Rindi G, Falconi M, Klersy C, et al. TNM staging of neoplasms of the endocrine pancreas: results from a large international cohort study. J Natl Cancer Inst. 2012;104:764–77.
Hochwald SN, Zee S, Conlon KC, et al. Prognostic factors in pancreatic endocrine neoplasms: an analysis of 136 cases with a proposal for low-grade and intermediate-grade groups. J Clin Oncol. 2002;20:2633–42.
Strosberg JR, Cheema A, Weber JM, et al. Relapse-free survival in patients with nonmetastatic, surgically resected pancreatic neuroendocrine tumors: an analysis of the AJCC and ENETS staging classifications. Ann Surg. 2012;256:321–5.
Lloyd RV, Osamura RY, Klöppel G, et al. WHO classification of tumours of endocrine organs. Lyon Cedex: International Agency for Research on Cancer (IARC); 2017. p. 209–40.
Basturk O, Yang Z, Tang LH, et al. The high-grade (WHO G3) pancreatic neuroendocrine tumor category is morphologically and biologically heterogenous and includes both well differentiated and poorly differentiated neoplasms. Am J Surg Pathol. 2015;39:683–90.
Lowe K, Khithani A, Liu E, et al. Ki-67 labeling: a more sensitive indicator of malignant phenotype than mitotic count or tumor size? J Surg Oncol. 2012;106:724–7.
Hijioka S, Hosoda W, Matsuo K, et al. Rb loss and KRAS mutation are predictors of the response to platinum-based chemotherapy in pancreatic neuroendocrine neoplasm with grade 3: a Japanese multicenter pancreatic NEN-G3 study. Clin Cancer Res. 2017;23:4625–32.
Nasir A, Coppola D. Neuroendocrine tumors: review of pathology, molecular and therapeutic advances. 1st ed. New York: Springer; 2016.
Garin-Chesa P, Fellinger EJ, Huvos AG, et al. Immunohistochemical analysis of neural cell adhesion molecules. Differential expression in small round cell tumors of childhood and adolescence. Am J Pathol. 1991;139:275–86.
Farinola MA, Weir EG, Ali SZ. CD56 expression of neuroendocrine neoplasms on immunophenotyping by flow cytometry: a novel diagnostic approach to fine-needle aspiration biopsy. Cancer. 2003;99:240–6.
Bilimoria KY, Talamonti MS, Tomlinson JS, et al. Prognostic score predicting survival after resection of pancreatic neuroendocrine tumors: analysis of 3851 patients. Ann Surg. 2008;247:490–500.
Teo R, Goh BKP, Tai DWM, et al. Validation and comparison between current prognostication systems for pancreatic neuroendocrine neoplasms: a single-institution experience with 176 patients. Surgery. 2017;161:1235–45.
Genc CG, Falconi M, Partelli S, et al. Recurrence of pancreatic neuroendocrine tumors and survival predicted by Ki67. Ann Surg Oncol. 2018;25:2467–74.
Hamilton NA, Liu TC, Cavatiao A, et al. Ki-67 predicts disease recurrence and poor prognosis in pancreatic neuroendocrine neoplasms. Surgery. 2012;152:107–13.
Genc CG, Jilesen AP, Partelli S, et al. A new scoring system to predict recurrent disease in grade 1 and 2 nonfunctional pancreatic neuroendocrine tumors. Ann Surg. 2018;267:1148–54.
Eichhorn F, Dienemann H, Muley T, et al. Predictors of survival after operation among patients with large cell neuroendocrine carcinoma of the lung. Ann Thorac Surg. 2015;99:983–9.
Kudo A, Akahoshi K, Ito S, et al. Downregulated pancreatic beta cell genes indicate poor prognosis in patients with pancreatic neuroendocrine neoplasms. Ann Surg. 2018. https://doi.org/10.1097/SLA.0000000000002911.
Yachida S, Vakiani E, White CM, et al. Small cell and large cell neuroendocrine carcinomas of the pancreas are genetically similar and distinct from well-differentiated pancreatic neuroendocrine tumors. Am J Surg Pathol. 2012;36:173–84.
Funding
This work was supported by Grant-in-Aid for Scientific Research (C) Grant Number 15K10046.
Author information
Authors and Affiliations
Contributions
BL contributed to the study design, clinical and pathological data acquisition, data analysis, and manuscript drafting. AK contributed to the study conception and design, data interpretation, critical article revision, and provided final approval of the version to be published. YK contributed to the acquisition of pathological data. TO contributed to the acquisition of clinical data. KO contributed to the acquisition of clinical data. HO contributed to the acquisition of clinical data. YM contributed to the acquisition of clinical data. DB contributed to the acquisition of clinical data. ST revised the article critically for intellectual content. TA contributed to the acquisition of pathological data. MT contributed to clinical data acquisition and critical article revision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, B., Kudo, A., Kinowaki, Y. et al. A simple and practical index predicting the prognoses of the patients with well-differentiated pancreatic neuroendocrine neoplasms. J Gastroenterol 54, 819–828 (2019). https://doi.org/10.1007/s00535-019-01570-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-019-01570-0