Abstract
Background
We evaluated the clinicopathological factors associated with lymph node metastasis in patients with non-functioning pancreatic neuroendocrine neoplasms (PanNENs), focusing on the risk factors and range of lymph node metastasis for tumors ≤ 2 cm in diameter.
Methods
The subjects of this study were patients with PanNENs consecutively diagnosed at our hospital between January, 2000 and June, 2018. We analyzed 69 patients who underwent R0 resection of a non-functioning sporadic PanNEN with no distant metastasis, as well as 43 patients with tumors ≤ 20 mm in radiological diameter.
Results
Nineteen patients (27.5%), including 7 (16.3%) with a small PanNEN, had lymph node metastasis. A large radiological diameter, a high Ki67 index, and cyst formation correlated significantly with positive lymph node metastasis. In patients with tumors ≤ 20 mm in diameter, a high Ki67 index correlated significantly with lymph node metastasis. When we set the cut-off Ki67 index as 3.3%, 2 of 43 patients had lymph node metastasis. Tumors in the uncinate process readily metastasized to the region around the superior mesenteric artery.
Conclusions
These findings suggest that a high Ki67 index indicates a risk of lymph node metastasis for tumors ≤ 20 mm in diameter and that lymphadenectomy should be performed in the region spatially adjacent to the primary tumor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidence of small pancreatic neuroendocrine neoplasms (PanNENs) is increasing in line with advances in screening technology. However, the optimal strategy for managing small NENs has not been established. For PanNENs ≤ 2 cm, the National Comprehensive Cancer Network (NCCN) guideline proposes resection, ranging from enucleation to standard pancreatectomy, as well as observation in selected cases [1]. The European Neuroendocrine Tumor Society (ENETS) guideline proposes non-operative management for asymptomatic sporadic non-functioning PanNEN ≤ 2 cm, especially when major pancreatic resection is required [2]. In contrast, for patients with PanNEN > 2 cm, both guidelines recommend standard surgical resection. Those guidelines also recommend lymphadeneoctomy, or at least sampling, of the lymph nodes to prevent lymph node metastasis. Several risk factors for lymph node metastasis of PanNEN have been reported, including tumor size, histological grade, location of the pancreas, and lymphovascular invasion [3,4,5,6]. However, no specific indications have defined the risk factors for lymph node metastasis or the range of lymphadenectomy for such small tumors.
Small PanNENs are not rare. According to the Surveillance Epidemiology and End Results database, a large proportion of PanNENs are tumors ≤ 2 cm [7, 8]. In Japan, about 50% of PanNEN patients have tumors ≤ 2 cm in diameter at diagnosis [9]. This reinforces the necessity of identifying the risk factors as well as the ranges of lymph node metastasis associated with such small tumors.
We analyzed the clinicopathological factors, especially for data potentially estimable before surgery, for lymph node metastasis, in patients who underwent resection of PanNENs without distant metastasis, focusing on risk factors, especially for tumors ≤ 2 cm in diameter. We also identified a cut-off for the Ki67 index of 3.3% as being indicative of lymph node metastasis.
Materials and methods
Patients
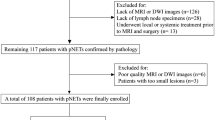
Between January, 2000 and June, 2018 a total of 125 patients underwent surgery for resection of a PanNEN at Kyoto University Hospital. Study protocols were approved by the institutional review committee at Kyoto University (R0455) and met the guidelines of the responsible government agency. Eight PanNEN patients with multiple endocrine neoplasia 1 (MEN1) were excluded. We reviewed 69 patients with non-functioning tumors without metastasis at resection, including 43 with tumors ≤ 20 mm. Tumors were classified as NET grade 1 (n = 39), NET grade 2 (n = 27), and NEC (n = 3) according to the 2017 WHO classification guidelines (Table 1). Tumors were classified as non-functional if they were not associated with distinct clinical manifestations or hormone alterations [10].
Surgical procedures
The operative methods for all non-functioning PanNENs were selected according to the size and appearance of lymph nodes on computed tomography (CT). The standard surgical procedure was either pancreaticoduodenectomy or distal pancreatectomy with regional lymph node dissection. For small tumors (< 10 mm), parenchyma-preserving procedures, including enucleation and central pancreatectomy with lymph node dissection, were performed. Thus, 23 patients underwent pancreaticoduodenectomy, 32 underwent distal pancreatectomy, 3 underwent total pancreatectomy, and 11 underwent parenchyma-preserving tumor resection. The primary tumor and the status of lymph node metastasis were diagnosed pathologically by two pathologists at the time of initial surgical resection. The Ki67 index was measured by immunohistochemistry of the MIB-1 antibody by counting the number of positively labeled cells per 1000 tumor cells to identify the region of the nucleus with the highest positive value. The lymph node status was diagnosed as clinical N0 stage when no swollen lymph node was seen on either CT scans or in the pathological results.
Review of CT and pathological diagnosis
All patients were followed-up at an outpatient clinic, by ultrasonography and CT, every 3–6 months, and by somatostatin receptor scintigraphy (SRS) every year after resection. Lymph node metastasis was diagnosed when a swollen lymph node > 10 mm was seen on CT and confirmed with SRS uptake. For liver metastasis, if a space-occupying lesion (SOL) was detected in the liver on CT, liver metastasis was confirmed by ethoxibenzyl-magnetic resonance imaging (EOB-MRI). Recurrence was diagnosed based on either radiological or biopsy-proven evidence. Disease-free survival (DFS) was calculated as the interval between curative resection and confirmation of recurrence by imaging studies.
Statistical analysis
DFS curves were prepared using the Kaplan–Meier method and differences in survival were examined using the log-rank test. The chi-squared test or Fisher’s exact test was used to compare categorical variables. A value of p < 0.05 was considered significant. Statistical analyses were performed using JMP version 14.0 (SAS Institute, Cary, NC, USA).
Results
Clinicopathological characteristics of PanNEN with and without lymph node metastasis
Eighteen of the 67 patients with non-functioning PanNEN without distant metastasis had lymph node metastasis at resection (Table 1). Thirty-six patients had NETG1 tumors and 27 patients had NETG2 tumors. There was a significant difference in lymph node metastatic rates according to the NET grading score. Both the radiological diameter and the percentage of Ki67 differed significantly between the lymph node-positive and -negative groups. Cyst formation correlated significantly with lymph node metastasis.
There were 42 patients (62.7%) with PanNENs ≤ 20 mm in radiological diameter and 7 of these patients (16.7%) had lymph node metastasis (Table 2). Lymph node metastasis was associated with a significant difference in grading, but not in tumor site. In tumors ≤ 20 mm in diameter, the average radiological diameter was 12.9 mm in the lymph node-negative group and 17.6 mm in the lymph node-positive group. The average Ki67 in the lymph node-negative group was 1.71% and that in the LN-positive group was 9.58%. In the tumors ≤ 20 mm in diameter, only the Ki67 percentage and tumor diameter correlated significantly with lymph node metastasis, whereas early enhancement and cyst formation did not. The Ki67 percentage was also correlated with lymph node metastasis from tumors harvested by endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA).
Recurrence developed after curative resection in four of the patients from the non-functioning small panNEN group. One without lymph node metastasis at resection had recurrence in the liver, while others with lymph node metastasis had liver and lymph node metastasis. In the latter patients, two had recurrences in the liver and one had recurrence in a para-aortic lymph node. All these patients had undergone standard surgery: as pancreaticoduodenectomy in two and as distal pancreatectomy in two.
Uni- and multivariate analysis of factors predictive of lymph node metastasis at resection (Table 3)
We analyzed factors associated with lymph node metastasis, presumed to be estimable before the operation. Univariate analysis revealed that the radiological findings of tumor diameter and Ki67 index were correlated significantly with lymph node metastasis (p = 0.036, p = 0.012, respectively). A multivariate logistic regression test revealed that the Ki67 index (p = 0.043) was correlated independently with lymph node metastasis in tumors ≤ 20 mm. When we evaluated the cut-off value of the Ki67 index by the Receiver Operating Characteristic curve (ROC; Fig. 1a), Ki67 = 3.3% was the most predictive value for lymph node metastasis. Figure 1b shows the ratio of positive lymph node metastasis according to the Ki 67 index > 3.3% in tumors ≤ 20 mm. Less than 5% of tumors with Ki67 < 3.3% were associated with positive lymph node metastasis.
a Receiver operating characteristic (ROC) curve for Ki67 to identify positive lymph node metastasis associated with tumors ≤ 20 mm in diameter. The area under the curve (AUC) equaled 0.808 for a Ki67 cut-off of 3.3. b ROC curve for Ki67 based on endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) to identify positive lymph node metastasis associated with tumors ≤ 20 mm in diameter. The AUC equaled 0.696 for a Ki67 cut-off of 3.0. c Lymph node metastasis of pancreatic neuroendocrine neoplasms (PanNENs) ≤ 20 mm in diameter. Two of the 42 patients had lymph node metastasis for tumors with a Ki67 index < 3.3%. LN lymph node
Location of lymph node metastasis of non-functioning PanNENs (Table 4)
The distribution of metastatic lymph node sites was analyzed with all 18 tumors that had lymph node metastasis. The Union for International Cancer Control (UICC) defined regional lymph nodes for tumors in the head and neck of the pancreas as lymph nodes along the common bile duct, common hepatic artery, portal vein, suprapyloric, subpyloric, proximal mesenteric, celiac, posterior and anterior pancreaticoduodenal vessels, and along the superior mesenteric vein and right lateral wall of the superior mesenteric artery. Regional lymph nodes for tumors in the body and tail were defined as the lymph nodes along the common hepatic artery, celiac axis, splenic artery, and splenic hilum as well as retroperitoneal nodes and lateral aortic nodes. When we spatially classified tumors into four tumor locations, all tumors in the head and uncinate process showed lymph node metastasis to the paraduodenal region (posterior and anterior pancreaticoduodenal vessels); all tumors in the uncinate process showed lymph node metastasis to regions around the SMA; and those in the head of the pancreas showed no metastases to those regions. One of six metastases to regions around the SMA were from tumors in the body of the pancreas, while tumors in the tail of the pancreas showed no metastases around the SMA.
Association of survival with lymph node status in tumors ≤ 20 mm in diameter
The association between lymph node metastasis and DFS was analyzed using the Kaplan–Meier model. Lymph node metastasis was found to be significantly associated with a decrease in DFS (Fig. 2a). The 5- and 10-year DFS rates were 95.7% and 89.7% for patients with N0 disease vs. 71.4% and 53.2%, respectively, for patients with N1 disease (log-rank p = 0.006) Overall survival (OS) rates tended to be shorter for patients with nodal metastasis (N1), but not significantly (log-rank, p = 0.061). The 5- and 10-year OS rates for patients with N1 disease were 85.7% and 67.1%, respectively, vs. 98% for patients with N0 disease (Fig. 2b).
Discussion
This study identified the risk factors for regional lymph node metastasis, which could be evaluated before resection of non-functioning PanNENs ≤ 20 mm in diameter. The Ki67 index and diameter of these tumors were independent risk factors for regional lymph node metastasis. When we defined 3.3% as the cut-off for the Ki67 index, a tumor with a Ki67 index < 3.3% was identified in only 2 of 42 patients, suggesting that we could predict a lower risk of lymph node metastasis for tumors ≤ 20 mm in diameter if the tumor shows a low Ki67 index < 3.3% (WHO Grade 1). Moreover, no tumors with a cystic component showed lymph node metastases in these tumor categories. The current study implied that regional lymph node metastasis is likely to occur adjacent to the primary tumor site. All lymph node-positive tumors in the uncinate process showed lymphatic metastasis to around the SMA in addition to the paraduodenal region, whereas those in the head of the pancreas did not. Tumors in the body of the pancreas showed a small but significant possibility of metastasis around the SMA. These tendencies for lymph node metastasis are informative when we dissect tumors, especially using parenchyma-preserving methods. The disease-free survival rates showed the impact of lymph node metastasis associated with tumors ≤ 20 mm in diameter.
Distinguishing lymph node metastasis preoperatively is often difficult, with tumor size being the only guide to preoperative diagnosis. Tsutsumi et al. set the cut-off line as 15 mm to predict lymph node metastasis and others have also reported a value of 15 mm [3]. However, as the findings of others have shown, tumors < 15 mm, or even < 10 mm may be positive for lymph node metastasis, so size is not a sufficient measure for prediction [4, 5, 11]. Gratian et al. examined 1854 patients with PanNENs and found that 29% presented with regional lymph node metastases [11]. Watzka et al. and Tsutsumi found lymph node metastasis in 12–15% [12] and 9.1% [3], respectively, consistent with our finding of 16.3% with lymph node metastasis, but the rate of lymph nodes ≤ 20 mm is variable in the literature because of the different background tumors and lymphatic sampling rates. One of the major differences was that a large cohort, such as that described by Gratian et al., analyzed patients from the NCDB database, which only collects data on tumors with ICD (International Statistical Classification of Diseases and Related Health Problems) codes 0 to 3, corresponding to “malignant” disease. Their data may thus overestimate the malignant potential of these tumors. Moreover, ethnic background may impact on these differences. Indeed, our ratio of positive lymph nodes was between that described by Tsutsumi et al. and that in the report [11] from the United States.
Our results suggest that tumors with a Ki67 index < 3.3% which is almost WHO Grade1, are unlikely to have lymph node metastases, potentially offering a low risk classification of lymph node metastasis. In other words, tumors ≤ 20 mm in diameter with a low Ki67 index might not need wide-range lymph node dissection, and sampling may suffice. Several arguments have been ongoing regarding whether enucleation is sufficient for preventing recurrence [13,14,15,16] and dissecting regional lymph nodes fully with enucleation is hard. When we applied 3.3% as a cut-off for Ki67, we could estimate the possibility of lymph node metastasis and patients with values < 3.3% and/or WHO G1 could be candidates for enucleation. Our results also showed favorable survival for patients with small tumors without lymph node metastasis. Recent NCCN and ENETS guidelines offer a surveillance strategy for small PanNENs in selected patients. Because 3 to 10% of the autopsy cases had pancreatic neuroendocrine tumors which did not cause symptoms [17], there is a category of non-functioning neuroendocrine tumors that might not need resection if they have no lymph node metastasis. Tumors that are both small and associated with a low Ki67 index could be candidates.
Recently, EUS-FNA has been broadly applied for precise diagnosis and the Ki67 from EUS-FNA specimens, found to be comparable to that from resected specimens. A recent report by Hasegawa of 58 patients with a PanNEN demonstrated a 90% concordance rate between EUS-FNA-identified NETS tumor grade and surgical histopathology when greater > 2000 cells were obtained [18]. Weynaund et al. further attested to the high reproducibility and inter-observer agreement of EUS-FNA for the determination of Ki67 index in PanNENs [19]. Our data showed similar tendencies and a high Ki67 index derived from EUS-FNA was related to positive lymph node metastasis (Table 2), although the number of our EUS-FNA specimens was small. As tumor heterogeneity may be comparatively less in smaller tumors, it is possible that the Ki67 value of a PanNEN can be predicted preoperatively with EUS-FNA, especially for small tumors.
The range of lymph node dissection is also important for operational planning. To the best of our knowledge, this is the first report to identify the range of lymph node metastasis in small PanNENs. The UICC defined “regional lymph node” in a TNM classification for pancreatic neuroendocrine tumors. As no range of dissection for PanNEN has been recommended as yet, all regional lymph nodes must be dissected, even for small tumors. Our results indicate that PanNENs are likely to have lymph node metastases nearby, suggesting the necessity for lymph node dissection near tumors. Conversely, we do not have to dissect lymph nodes around the common bile duct for lesions such as PanNEN in the tail of the pancreas.
Finally, several reports document the important association between lymph node metastasis and poor prognosis [20, 21]. In a meta-analysis, Gao et al. found that lymph node metastasis as well as positive surgical margins, advanced grade, TNM stage, organ metastasis, vascular invasion, and necrosis of the specimen were significantly associated with OS [22]. Our previous study also found that the significant prognostic factors for disease-free survival were lymph node metastasis and vascular invasion in PanNEN [23]. Our current study revealed that positive lymph node metastasis correlates significantly with poor prognosis even for tumors ≤ 20 mm in diameter. These findings are consistent with others showing the impact of lymph node metastasis on the survival of patients with T1 and T2 tumors that are < 40 mm [24].
This study has several limitations. Because it is a retrospective analysis from a single institution, our study was subject to selection and referral biases and the number of patients studied was comparatively small. On the other hand, as a single institutional analysis, we were able to characterize lymph node sampling rates and predictors of lymph node metastasis, which has not been possible using large databases that fail to capture sufficiently granular data. As such, detailed follow-up data are available for many patients. For example, most patients who underwent surgery at our institution have routine CT or MRI examinations every 6 months. The median sampling number from lymph node dissection was two, including sampling as well as dissection. Even so, we could detect a significant correlation with disease-free survival in patients with tumors ≤ 20 mm in diameter.
Conclusions
Our results suggest that a high Ki67 index indicates a risk of lymph node metastasis for tumors ≤ 20 mm in diameter and lymphadenectomy could be applied to the region spatially adjacent to the primary tumor. Furthermore, lymph node status is critically important to estimate the risk of recurrence and thus stratify patients for future trials investigating adjuvant treatment.
References
Shah MH, Goldner WS, Halfdanarson TR, Bergsland E, Berlin JD, Halperin D, et al. NCCN guidelines insights: neuroendocrine and adrenal tumors, version 2.2018. JNCCN. 2018;16(6):693–702. https://doi.org/10.6004/jnccn.2018.0056.
Partelli S, Bartsch DK, Capdevila J, Chen J, Knigge U, Niederle B, et al. ENETS consensus guidelines for standard of care in neuroendocrine tumours: surgery for small intestinal and pancreatic neuroendocrine tumours. Neuroendocrinology. 2017;105(3):255–65. https://doi.org/10.1159/000464292.
Tsutsumi K, Ohtsuka T, Mori Y, Fujino M, Yasui T, Aishima S, et al. Analysis of lymph node metastasis in pancreatic neuroendocrine tumors (PNETs) based on the tumor size and hormonal production. J Gastroenterol. 2012;47(6):678–85. https://doi.org/10.1007/s00535-012-0540-0.
Parekh JR, Wang SC, Bergsland EK, Venook AP, Warren RS, Kim GE, et al. Lymph node sampling rates and predictors of nodal metastasis in pancreatic neuroendocrine tumor resections: the UCSF experience with 149 patients. Pancreas. 2012;41(6):840–4. https://doi.org/10.1097/MPA.0b013e31823cdaa0.
Hashim YM, Trinkaus KM, Linehan DC, Strasberg SS, Fields RC, Cao D, et al. Regional lymphadenectomy is indicated in the surgical treatment of pancreatic neuroendocrine tumors (PNETs). Ann Surg. 2014;259(2):197–203. https://doi.org/10.1097/SLA.0000000000000348.
Wong J, Fulp WJ, Strosberg JR, Kvols LK, Centeno BA, Hodul PJ. Predictors of lymph node metastases and impact on survival in resected pancreatic neuroendocrine tumors: a single-center experience. Am J Surg. 2014;208(5):775–80. https://doi.org/10.1016/j.amjsurg.2014.04.003.
Lawrence B, Gustafsson BI, Chan A, Svejda B, Kidd M, Modlin IM. The epidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin N Am. 2011;40(1):1–18. https://doi.org/10.1016/j.ecl.2010.12.005.
Kuo EJ, Salem RR. Population-level analysis of pancreatic neuroendocrine tumors 2 cm or less in size. Ann Surg Oncol. 2013;20(9):2815–21. https://doi.org/10.1245/s10434-013-3005-7.
Ito T, Sasano H, Tanaka M, Osamura RY, Sasaki I, Kimura W, et al. Epidemiological study of gastroenteropancreatic neuroendocrine tumors in Japan. J Gastroenterol. 2010;45(2):234–43. https://doi.org/10.1007/s00535-009-0194-8.
Han X, Xu X, Jin D, Wang D, Ji Y, Lou W. Clinicopathological characteristics and prognosis-related factors of resectable pancreatic neuroendocrine tumors: a retrospective study of 104 cases in a single Chinese center. Pancreas. 2014;43(4):526–31. https://doi.org/10.1097/MPA.0000000000000065.
Gratian L, Pura J, Dinan M, Roman S, Reed S, Sosa JA. Impact of extent of surgery on survival in patients with small nonfunctional pancreatic neuroendocrine tumors in the United States. Ann Surg Oncol. 2014;21(11):3515–21. https://doi.org/10.1245/s10434-014-3769-4.
Watzka FM, Laumen C, Fottner C, Weber MM, Schad A, Lang H, et al. Resection strategies for neuroendocrine pancreatic neoplasms. Langenbeck’s Arch Surg. 2013;398(3):431–40. https://doi.org/10.1007/s00423-012-1024-7.
Yeo CJ, Wang BH, Anthone GJ, Cameron JL. Surgical experience with pancreatic islet-cell tumors. Arch Surg. 1993;128(10):1143–8.
Fernandez-Cruz L, Molina V, Vallejos R, Jimenez Chavarria E, Lopez-Boado MA, Ferrer J. Outcome after laparoscopic enucleation for non-functional neuroendocrine pancreatic tumours. HPB. 2012;14(3):171–6. https://doi.org/10.1111/j.1477-2574.2011.00422.x.
Sarmiento JM, Farnell MB, Que FG, Nagorney DM. Pancreaticoduodenectomy for islet cell tumors of the head of the pancreas: long-term survival analysis. World J Surg. 2002;26(10):1267–71. https://doi.org/10.1007/s00268-002-6714-9.
Bettini R, Partelli S, Boninsegna L, Capelli P, Crippa S, Pederzoli P, et al. Tumor size correlates with malignancy in nonfunctioning pancreatic endocrine tumor. Surgery. 2011;150(1):75–82. https://doi.org/10.1016/j.surg.2011.0.
Kimura W, Kuroda A, Morioka Y. Clinical pathology of endocrine tumors of the pancreas. Analysis of autopsy cases. Digest Dis Sci. 1991;36(7):933–42.
Hasegawa T, Yamao K, Hijioka S, Bhatia V, Mizuno N, Hara K, et al. Evaluation of Ki-67 index in EUS-FNA specimens for the assessment of malignancy risk in pancreatic neuroendocrine tumors. Endoscopy. 2014;46(1):32–8. https://doi.org/10.1055/s-0033-1344958.
Weynand B, Borbath I, Bernard V, Sempoux C, Gigot JF, Hubert C, et al. Pancreatic neuroendocrine tumour grading on endoscopic ultrasound-guided fine needle aspiration: high reproducibility and inter-observer agreement of the Ki-67 labelling index. Cytopathology. 2014;25(6):389–95. https://doi.org/10.1111/cyt.12111.
Lee LC, Grant CS, Salomao DR, Fletcher JG, Takahashi N, Fidler JL, et al. Small, nonfunctioning, asymptomatic pancreatic neuroendocrine tumors (PNETs): role for nonoperative management. Surgery. 2012;152(6):965–74. https://doi.org/10.1016/j.surg.2012.08.038.
Sharpe SM, In H, Winchester DJ, Talamonti MS, Baker MS. Surgical resection provides an overall survival benefit for patients with small pancreatic neuroendocrine tumors. J Gastrointest Surg. 2015;19(1):117–23 (discussion 23). https://doi.org/10.1007/s11605-014-2615-0.
Gao Y, Gao H, Wang G, Yin L, Xu W, Peng Y, et al. A meta-analysis of prognostic factor of pancreatic neuroendocrine neoplasms. Sci Rep. 2018;8(1):7271. https://doi.org/10.1038/s41598-018-24072-0.
Masui T, Sato A, Nakano K, Uchida Y, Yogo A, Anazawa T, et al. Comparison of recurrence between pancreatic and duodenal neuroendocrine neoplasms after curative resection: a single-institution analysis. Ann Surg Oncol. 2018;25(2):528–34. https://doi.org/10.1245/s10434-017-6260-1.
Conrad C, Kutlu OC, Dasari A, Chan JA, Vauthey JN, Adams DB, et al. Prognostic value of lymph node status and extent of lymphadenectomy in pancreatic neuroendocrine tumors confined to and extending beyond the pancreas. J Gastrointest Surg. 2016;20(12):1966–74. https://doi.org/10.1007/s11605-016-3243-7.
Funding
This work was supported by JSPS KAKENHI Grant number JP18K08677.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We have no conflicts of interest to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Masui, T., Sato, A., Nakano, K. et al. Predictive value of the Ki67 index for lymph node metastasis of small non-functioning pancreatic neuroendocrine neoplasms. Surg Today 49, 593–600 (2019). https://doi.org/10.1007/s00595-019-01779-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-019-01779-9