Abstract
There are marked differences in the etiology of the major histological types of esophageal cancer (EC)—squamous cell carcinomas (ESCC) and adenocarcinomas (EAC). This study aimed to summarize the current scientific knowledge on modifiable risk factors for EC, by histological type, through a systematic review of meta-analyses referenced in PubMed and ISI Web of Knowledge. We identified 100 meta-analyses on risk factors for ESCC (n = 54), EAC (n = 43), or EC (n = 51). ESCC risk significantly increased with alcohol and maté drinking, smoking, red and processed meat consumption and human papillomavirus infection, while it was negatively associated with body mass index and consumption of fruit, vegetables, white meat, folate, and some carotenoids. Cessation of drinking and smoking significantly reduced ESCC risk. For EAC, an increased risk was reported for smoking, body mass index, and red and processed meat consumption, while risk decreased with Helicobacter pylori infection, low/moderate alcohol drinking, physical activity, and consumption of fruit, vegetables, folate, fiber, beta-carotene, and vitamin C. Differences in results between meta-analyses and mechanisms underlying some of the associations found are discussed. This work reinforces the importance of a separate assessment of EC subtypes to allow for a proper evaluation of incidence trends and planning of prevention/control interventions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There are marked differences between the tumors of the major histological types of esophageal cancer (EC) regarding incidence and mortality trends, which reflect the specificities of each subtype regarding its determinants [1].
Esophageal squamous cell carcinoma (ESCC) comprises most cases of esophageal cancer [2], although its incidence has been steadily decreasing or stabilizing in Western countries [3]. The main risk factors of ESCC occurrence are tobacco smoking and alcohol consumption and many studies have shown both the independent and synergistic effects of these determinants [4].
Esophageal adenocarcinoma (EAC) incidence rates have been steadily increasing in several Western countries [3], although there are differences, either between countries [1] and between regions within the same country [5]. The upward trends are in part due to the increased prevalence of recognized risk factors such as gastroesophageal reflux disease (GERD) and obesity [6], but they may also be explained by variation in other modifiable exposures, such as smoking, diet, and Helicobacter pylori (HP) infection [6,7,8,9].
A large body of research has been devoted to studying the determinants of esophageal cancer, as summarized in several meta-analyses. This study aims to summarize the state of the art on the etiology of EC by systematically reviewing published meta-analyses on the main modifiable factors associated with the occurrence of esophageal cancer by histological type.
Methods
PubMed and ISI Web of Knowledge were searched up to September 2015 to identify published meta-analyses addressing the association between the main modifiable exposures and esophageal cancer. The titles and abstracts of the retrieved articles were read and full texts were obtained for the studies considered potentially relevant. In addition, references cited in the identified articles were manually searched.
Studies were included if a meta-analysis based on published results or an individual participant data meta-analysis was performed to quantify the association between modifiable exposures and EC, ESCC, or EAC. Only full-length papers published in English, Portuguese, Spanish, French, Italian, or Polish were included. Studies focusing on the impact of the cessation of modifiable exposures on the risk of EC, EAC, or ESCC were also kept in our review. Studies were excluded if: (1) EC, ESCC, or EAC was not reported as an outcome of interest; (2) determinants other than alcohol drinking, smoking and smokeless tobacco, HP infection, Human papillomavirus (HPV) infection, obesity/BMI, physical activity or diet were evaluated; (3) no summary estimate was provided in the form of an odds ratio (OR), relative risk (RR), or hazard ratio (HR), along with the correspondent 95% confidence interval (CI); (4) results provided constituted duplicate information from previous studies (i.e., reviews mentioning as summary estimate a result from a meta-analysis already included in our review).
Since EC is a relatively rare and a highly lethal disease, we ignored the distinction between RR, OR, and HR, reporting RR henceforth as the effect estimate. For each study, the following information was extracted: first author’s name, publication year, number of studies included in the meta-analysis and corresponding study design when available, EC histological type evaluated, risk factor assessed, categories of exposure compared, the RR and corresponding 95% CI. Stratified results by sex, study type and geographical area and dose–response RRs were collected, whenever available. If both fixed and random effects estimates were provided, the latter were used as they allow for some heterogeneity between studies.
All studies were assessed independently by two researchers (CC and BP) to determine their eligibility and for data extraction; disagreements were discussed and resolved by consensus or involving a third researcher (NL).
Each meta-analysis obtained from a systematic review was attributed a quality score, ranging from 0 to 11, based on the AMSTAR tool [10]. Results obtained were summarized using a harvest plot, for the most commonly evaluated determinants. Forest plots describing the overall and sex-specific RRs on the main determinants of EAC and ESCC were obtained using Stata Statistical Software, version 11.0 [11].
Results
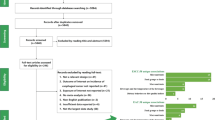
We identified 100 publications reporting results from meta-analyses addressing the association between the aforementioned risk factors and ESCC (n = 54), EAC (n = 43) or EC (n = 51). The systematic review flow-chart is presented as Supplementary Fig. S1. Information extracted for each study is accessible in Supplementary Table S1, and quality assessment is presented in Fig. 1 and Supplementary Table S2. The quality scores ranged between 3 and 10, and 50 meta-analyses had a score of 7 or higher. The main findings are presented below, and a summary of RR for the most commonly described risk factors are presented in Table 1.
Harvest plot of the overall association between the main determinants of esophageal cancer and its occurrence, by subtype, when comparing the highest with the lowest levels of exposure, infected with non-infected patients or dose–response effects. Each bar corresponds to a meta-analysis (based on systematic reviews in black, otherwise in grey) and depicts its quality score; labels correspond to the number of studies included in the estimate provided in each meta-analysis; an horizontal pattern indicates that the estimate was obtained from case–control studies only. Meta-analyses are ordered according to year of publication (x-axis)
Alcohol drinking
Twenty-five studies evaluated the association between alcohol drinking and EC, 11 of which did not include histology-specific RRs [12,13,14,15,16,17,18,19,20,21,22].
The association between ESCC and alcohol consumption was addressed in 11 studies (Fig. 2a) [23,24,25,26,27,28,29,30,31,32,33]. When comparing ever with never drinkers, the RR for ESCC was 3.7 among men and 2.1 among women [25]. Dose–response effects were reported in several meta-analyses [23,24,25, 28,29,30, 32, 33]. No significant differences were found between case–control and cohort studies [23, 24], nor between different geographical areas (Asia/Non-Asia [29], Europe/Asia [31] and Europe/Asia/North America [23, 24]).
Six meta-analyses reported on the relation between alcohol drinking and EAC (Fig. 2b) [27, 28, 30, 33,34,35]. In general, point estimates increased with alcohol consumption, but no significant associations were found in most meta-analyses, even at high levels of consumption [27, 30, 33, 35].
Tobacco smoking
Fourteen studies evaluated the association between tobacco smoking and EC, EAC, or ESCC [17, 25,26,27, 30, 31, 33, 34, 36,37,38,39,40,41]. Using never smokers as the reference category, results obtained from the five studies not providing histology-specific RRs yielded no significant differences between sexes, study designs (case–control and cohort studies), ethnicity (African Americans, Asians, and Caucasians, though Asians had a lower point estimate) and geographical areas [17, 36, 37, 39, 40].
Six meta-analyses focused on tobacco smoking and ESCC (Fig. 3a) [25,26,27, 30, 31, 33]. Current smokers had a significantly higher risk of ESCC than never smokers (RR = 5.1 among men, RR = 3.1 among women) [25] and presented twice the risk of former smokers (RR = 3.13, 95% CI 2.53, 3.86 vs. RR = 1.68, 95% CI 1.44, 1.96) [31]. Dose–response effects were reported with the number of cigarettes smoked per day or per week [25, 33], the number of smoking years [25] and the number of pack-years [27, 30], using non-smokers as reference. Prabhu et al. [31] found a lower ESCC risk in Asia (RR = 2.31, 95% CI 1.78, 2.99) than in Europe (RR = 4.21, 95% CI 3.13, 5.66) when comparing current with never smokers.
Six studies reported on the association between tobacco smoking and EAC (Fig. 3b) [27, 30, 33, 34, 38, 41]. The only meta-analysis providing sex-specific estimates showed a non-significantly higher EAC risk among men (RR = 2.10 for men, RR = 1.74 for women) [38], with the strength of association being much lower than that of ESCC. As for ESCC, there was a dose–response relation [30, 33, 34, 38]. When comparing ever with never smokers, the association between smoking and EAC was found significant in two meta-analyses (RR ≈ 1.9) [38, 41], but not in a third one (RR = 0.96, 95% CI 0.82, 1.12, n = 4) [27].
Alcohol drinking/tobacco smoking cessation
Time since cessation of alcohol drinking (Supplementary Fig. S2A) or tobacco smoking (Supplementary Fig. S2B) was assessed in four meta-analyses [25, 26, 42, 43].
Ten or more years since cessation did not suffice to reduce ESCC risk to the values observed among never drinking men nor among never smoking men; among women, 5 and 10 years since cessation were enough to reach similar values to the ones obtained for never drinkers and never smokers, respectively [25]. The risk of ESCC among men was shown to decrease by 4% per year since cessation of alcohol drinking (RR = 0.96, 95% CI 0.94, 0.98) and by 2% per year since cessation of tobacco smoking (RR = 0.98, 95% CI 0.97, 0.99) [26]. No meta-analyses were found on the association between alcohol drinking cessation or tobacco smoking cessation and EAC.
Smokeless tobacco
Three meta-analyses evaluated the effects of overall smokeless tobacco on EC and yielded a significantly higher risk among ever users, although conflicting results were reported when stratifying analyses by geographical area [44,45,46]. Akl et al. [47] reported a non-significantly higher risk of EC among current waterpipe smokers, in comparison with never smokers (RR = 1.85, 95% CI 0.95, 3.58). Akhtar et al. [48] focused on areca nut (also commonly referred to as betel nut) chewing, and reported an increased risk of ESCC for chewers in comparison with non-chewers (RR = 3.05, 95% CI 2.41, 3.87). Among never tobacco smokers, the use of snus significantly increased the risk of ESCC (RR = 3.5, 95% CI 1.6, 7.6) but not EAC (RR = 0.2, 95% CI 0.0, 1.9) [49].
HP infection
The effect of HP infection was evaluated by five meta-analyses that reported results for both ESCC and EAC (Fig. 4) [50,51,52,53,54]. All showed no association between HP and ESCC, while for EAC a protective effect of HP infection was found (RR ≈ 0.5). In 2013, protective effects of HP infection (RR = 0.66, 95% CI 0.43, 0.89) and infection with CagA-positive strains (RR = 0.77, 95% CI 0.65, 0.92) were reported for ESCC, when analyses were restricted to studies from Iran and China [52].
HPV infection
Seven studies focused on the association between HPV infection and the occurrence of ESCC [55,56,57,58,59,60,61], while one other did not provide histology-specific estimates [62]. A positive association between HPV infection and ESCC was reported in all studies, with overall RRs ranging between 2.69 [55] and 3.32 [56]. However, results varied according to geographical areas (no significant associations found for Europe and America [55, 56, 61], while a significantly increased risk of ESCC was reported in Asia, with RRs of approximately 4 being reported for China [57, 58]) and HPV subtype (HPV-16 was consistently reported as being associated with ESCC, contrarily to HPV-18 and other subtypes [60]).
BMI and central adiposity
Twelve publications assessed the effects of BMI on EC (Supplementary Fig. S3), one of which did not provide histology-specific RRs [63]. ESCC was focused on by four meta-analyses [22, 27, 64, 65]. A study found no significant change in ESCC risk with an increment of 1 kg/m2 [22], while another described a significant reduction with an increment of 5 kg/m2 (RR = 0.71 for men and RR = 0.57 for women) [64]. A meta-analysis published in 2015 found a significantly reduced ESCC risk among individuals with a BMI over 25 (RR = 0.8, 95% CI 0.67, 0.95), but not among obese individuals (RR = 1.05, 95% CI 0.76, 1.46), when compared to those with a normal weight [27].
EAC was focused on by 11 meta-analyses, eight of which reported a significant dose–response effect of BMI [22, 34, 64,65,66,67,68,69]. EAC risk was found increasing by 13% per 5 kg/m2 (RR = 1.13, 95% CI 1.11, 1.16) [69], while non-significant associations were found when comparing obese with normal weight men (RR = 1.23, 95% CI 0.58, 2.60) [70]. When comparing the highest with the lowest categories of central adiposity, an RR of 2.51 (95% CI 1.56, 4.04) was reported [71].
Physical activity
Five meta-analyses focused on the association between physical activity and EC [27, 72,73,74,75], one of which did not provide histology-specific estimates [74]. The remaining four meta-analyses compared the highest with the lowest levels of exercise, finding no significant association with ESCC [27, 72, 73, 75]. Two found a significant protective effect of physical activity on EAC risk (RR = 0.79 [72] and RR = 0.68 [75]), while the other two found no significant association [27, 73].
Further stratified results were only available for EC as a whole. A significantly reduced risk of EC was reported for studies from North America (RR = 0.77, 95% CI 0.64, 0.92), Australia (RR = 0.72, 95% CI 0.57, 0.91), and the Middle East (RR = 0.48, 95% CI 0.29, 0.81) [72], but not from Europe or Asia [72, 73, 75]. A reduced risk of EC was also reported by sex, and in both case–control and cohort studies [72, 73, 75].
Diet
The effects of some dietary aspects were only reported for EC as a whole: while no significant association was described with energy intake [76], barbecued meat [77], eggs [22], milk and dairy products [22], and black tea [78] consumption, protective effects were found regarding citrus fruits [22], raw and non-starchy vegetables [22], lutein and zeaxanthin intake [79]. For the remaining diet-related factors, 12 meta-analyses did not report histology-specific results [17, 22, 76, 80,81,82,83,84,85,86,87,88].
Among meta-analysis addressing histologic type-specific data, ESCC risk was significantly lower among individuals presenting a “healthy dietary pattern” (higher loading of fruits, fresh vegetables, dietary fiber and antioxidants and a lower loading of fat dairy, processed food and meat) (RR = 0.36) and higher for a “drinker/alcohol dietary pattern” (higher loading of wines, beers, and spirits) (RR = 2.34), while it did not significantly change for a “Western dietary pattern” [89]. When comparing the highest with the lowest levels of intake, no significant associations were found for ESCC with dietary glycemic index [90, 91] or consumption of meat (overall [77, 92] and among women [93]), barbecue [93], cereals [93], fat (among women) [93], fiber [95], salt (among women) [93], acrylamide [85, 96], zinc [97], beta-carotene [79], green tea [78], coffee [78, 93, 98], coffee with milk [93], or soft drinks [99]. A significantly increased ESCC risk was found with the consumption of pickled vegetables (RR = 2.08) [100], meat (RR = 1.46 among men) [93], red meat (RRs between 1.55 and 1.86) [77, 92, 101, 102], fat (RR = 1.57 among men) [93], salt (RR = 2.11 among men) [93], maté (RR = 1.34 among men, RR = 2.20 among women) [93, 94] and regarding the temperature at which foods and beverages were consumed (RR = 1.6) [103], while a significantly decreasing ESCC risk was reported for fruit and vegetables (RRs between 0.4 and 0.6, with marked differences between sexes and geographical regions, see Supplementary Fig. S4) [93, 104], white meat (RR = 0.63) [92], folate (RR ≈ 0.65) [105, 106], alpha-carotene (RR = 0.82) [79], beta-cryptoxanthin (RR = 0.83) [79], lycopene (RR = 0.74) [79] and tea (RR = 0.53 among men) [93]. For poultry [77, 92], fish [77, 92, 107], processed meat [77, 92, 101, 102], and glycemic load [91], results were inconsistent between meta-analyses. When evaluating dose–response effects, increments of 100 g/day in consumption were found to decrease ESCC risk by nearly 40 and 60%, for fruit and vegetables, respectively [104], and increasing ESCC risk by 41% for red meat [102].
For EAC, when comparing the highest with the lowest levels of intake, no significant associations were found with the consumption of poultry [77, 92], white meat [92], fish [77, 92, 107], acrylamide [85, 96], zinc [97], vitamin E [108], coffee [78, 98], soft drinks [99] and the temperature at which foods and beverages were consumed [103], while a decreasing risk was found regarding folate (RR ≈ 0.5) [105, 106], fiber (RR = 0.66) [95], beta-carotene (RR = 0.46) [79, 108] and vitamin C intake (RR = 0.49) [108] and an increasing risk was reported with the consumption of total meat (RR = 1.96) [92], red meat (RR between 1.2 and 1.4) [77, 92, 101, 109] and processed meat (RR ≈ 1.4) [77, 92, 101, 109]. Increments of 100 g/day in consumption were found to decrease EAC risk by 13 and 9%, for fruit and vegetables, respectively [110], and to increase EAC risk by 45% for red meat [102].
Interactions between risk factors
Seven studies evaluated the interaction between some of the aforementioned risk factors for EC, EAC, or ESCC [4, 17, 25, 36, 48, 67, 93].
For ESCC, significant interactions were found between areca nut chewing and tobacco smoking [48], tobacco smoking and alcohol drinking [4], and between the consumption of maté at very hot temperatures and drinking more than 1.5 l of maté per day [93].
Ishikawa et al. [17] evaluated the potential effect modifications of smoking (current), alcohol (daily), and green tea (≥3 cups/day) consumption on EC risk, by analyzing combined categories of these variables and using people with none of the exposures as reference. The interactions between smoking and alcohol drinking, smoking and green tea consumption, alcohol and green tea consumption, and all three variables yielded RRs of 9.23 (95% CI 2.10, 40.60), 4.99 (95% CI 1.11, 22.43), 2.97 (95% CI 0.53, 16.58) and 11.10 (95% CI 2.63, 46.51), respectively. Ansary-Moghaddam et al. [36] found a significant interaction between smoking and alcohol for the occurrence of EC.
Discussion
The association between the most well-known risk factors for esophageal cancer and its occurrence have been extensively described in the literature and an increasing number of meta-analyses have been published focusing on those determinants. Although methodological limitations are inherent to the primary studies included in the meta-analyses, this review depicts the state-of-the-art on the modifiable risk factors for EC, showing marked differences between its subtypes regarding the strength of association with each determinant. In most situations, risk estimates did not differ significantly between meta-analyses focusing on the same risk factors, but there were some exceptions that should be discussed.
Most meta-analyses found no significant association between alcohol drinking and EAC, even at high levels of consumption. However, two meta-analyses, originated from pooled analysis of studies included in the International Barrett’s and Esophageal Adenocarcinoma Consortium (BEACON), suggested a protective effect of low/moderate alcohol consumption on EAC risk [28, 34]. The authors argued that these results could depict a true association, as alcohol consumption may have favorable effects on insulin resistance or levels of serum lipids and lipoproteins [111], which may be important for EAC risk. In 2015, two meta-analyses compared ever with never drinkers: while Drahos et al. [34] found a significant protective effect of alcohol consumption on EAC risk, at all ages and in people aged 70 or older; Fahey et al. [27] found no significant association. Although results obtained by Fahey et al. were based on a systematic review, only two case–control studies (from Sweden and the USA) were used to obtain the summary estimates, while Drahos et al. used individual data from eight BEACON case–control studies (from Australia, Ireland, Sweden, and the USA). Therefore, results provided by the latter are more reliable, since a larger number of studies were included in the analysis, and the use of individual data allows for the adjustment of each study’s results to the same variables, ensuring the comparability of results.
Tobacco consumption was found to increase the risk of EAC in all meta-analyses, with the exception of Fahey et al. [27], who reported no significant association. As before, this lack of association is probably due to the smaller number of studies included in the meta-analysis, in comparison with the other studies performing similar evaluations [34, 38, 41].
Our study has shown that a significant reduction in ESCC risk could be obtained from alcohol drinking and tobacco smoking cessation, with RRs reaching similar values to the ones observed in individuals who never drank or smoked, within some years after cessation. This depicts the importance of planning interventions aimed to reduce the consumption of both alcohol and tobacco. Future studies focusing on EAC to provide such estimates would also be useful, especially given the marked increase in EAC incidence trends observed in Western countries in the last decades [1, 112]. Two meta-analyses [72, 75] found an inverse association between physical activity and EAC risk, while two others [27, 73] found no significant association. Those not showing a significant result were the ones including the smaller number of studies in the analyses.
In all meta-analyses identified in our study, HP infection was consistently described as having a protective effect of EAC risk, while no significant association was found with ESCC. The mechanisms underlying the inverse association between HP infection and EAC are not clear, but it has been suggested that hypoacidity in association with gastric atrophy may have a role [113].
Overweight and obesity were consistently reported as risk factors for EAC, but a protective effect of BMI for ESCC was also observed. A possible explanation for this inverse association is a negative confounding of the BMI and cancer association by smoking intensity [114], which has been supported by studies presenting an inverse association between BMI and ESCC risk among smokers, but not among non-smokers [115].
Although our quality assessment has shown that most meta-analyses published are of good quality, the key limitation on the interpretability of our findings is the heterogeneity between (and within each of) the meta-analyses selected for inclusion in our review. Among the 81 studies performing a systematic review of literature, some focused on a specific geographical area (e.g., Japan [20, 40]) or included only studies of a given design (e.g., cohort studies [83, 88]); six of the 12 studies obtained through pooled analyses used data from BEACON, three used data from studies conducted in South America, two in Asia, and one in Italy. Thus, cultural aspects, customs, and lifestyles of each geographical area are likely explanations for the differences found between summary estimates provided for some determinants, namely regarding diet.
Furthermore, moderate-to-high degrees of heterogeneity were observed in several meta-analyses, with many authors mentioning the difficulties in using available data from observational studies, since there is no standardization in data collection and reporting [27, 114].
Our inclusion criteria focused on environmental risk factors, leading to the exclusion of many studies focusing on pharmacological treatments (e.g., nonsteroidal anti-inflammatory drugs) and genetic factors. However, some of the latter are widely recognized as significantly associated with the risk of EC, and therefore their role cannot be disregarded. For example, the biologic effects of alcohol intake on EC depend on the individuals’ genotype. Subjects with the ALDH2 (aldehyde dehydrogenase 2) Lys487 allele have a deficiency of ALDH2, leading to a higher risk of EC than that observed in individuals with no deficiency and consuming the same amount of alcohol [116]. GERD and Barrett’s esophagus are among the most commonly mentioned risk factors for EAC in epidemiological studies, but they were also excluded from the present study, as it is arguable whether they are modifiable factors. Few meta-analyses have focused on these determinants, mainly due to the high heterogeneity found between individual studies included in existing systematic reviews. Nevertheless, the existing meta-analyses reported a gradually increased risk of EAC with the increasing frequency and duration of GERD symptoms [34, 117, 118]. There are some comorbidities that have also been assessed through meta-analyses and may be worth analyzing in future studies. Examples include an increased risk of ESCC found in people with gastric atrophy (RR = 1.94, 95% CI 1.48, 2.55) [119] and an increased risk of EAC in the presence of diabetes mellitus (RR = 2.12, 95% CI 1.01, 4.46) [120].
In conclusion, this comprehensive systematic review summarizes the state-of-the-art on the etiology of EC, showing evident differences between ESCC and EAC regarding some risk factors. This reinforces the importance of a separate assessment of EC subtypes to allow for a proper discussion of incidence trends and a suitable planning of interventions towards the reduction of cancer burden in the population.
References
Castro C, Bosetti C, Malvezzi M, et al. Patterns and trends in esophageal cancer mortality and incidence in Europe (1980–2011) and predictions to 2015. Ann Oncol Off J Eur Soc Med Oncol ESMO. 2014;25(1):283–90 (epub 2013/12/21).
Corley DA, Buffler PA. Oesophageal and gastric cardia adenocarcinomas: analysis of regional variation using the Cancer Incidence in Five Continents database. Int J Epidemiol. 2001;30(6):1415–25 (epub 2002/02/01).
Jemal A, Center MM, DeSantis C, et al. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomark Prev. 2010;19(8):1893–907 (epub 2010/07/22).
Prabhu A, Obi KO, Rubenstein JH. The synergistic effects of alcohol and tobacco consumption on the risk of esophageal squamous cell carcinoma: a meta-analysis. Am J Gastroenterol. 2014;109(6):822–7 (epub 2014/05/03).
Kubo A, Corley DA. Marked regional variation in adenocarcinomas of the esophagus and the gastric cardia in the United States. Cancer. 2002;95(10):2096–102 (epub 2002/11/02).
Mayne ST, Navarro SA. Diet, obesity and reflux in the etiology of adenocarcinomas of the esophagus and gastric cardia in humans. J Nutr. 2002;132(11):3467S–70S.
De Ceglie A, Fisher DA, Filiberti R, et al. Barrett’s esophagus, esophageal and esophagogastric junction adenocarcinomas: the role of diet. Clin Res Hepatol Gastroenterol. 2011;35(1):7–16 (epub 2010/10/26).
Pera M, Manterola C, Vidal O, et al. Epidemiology of esophageal adenocarcinoma. J Surg Oncol. 2005;92(3):151–9 (epub 2005/11/22).
Vaughan TL, Davis S, Kristal A, et al. Obesity, alcohol, and tobacco as risk factors for cancers of the esophagus and gastric cardia: adenocarcinoma versus squamous cell carcinoma. Cancer Epidemiol Biomark Prev. 1995;4(2):85–92 (epub 1995/03/01).
Yuan JM, Knezevich AD, Wang R, et al. Urinary levels of the tobacco-specific carcinogen N′-nitrosonornicotine and its glucuronide are strongly associated with esophageal cancer risk in smokers. Carcinogenesis. 2011;32(9):1366–71 (epub 2011/07/08).
StataCorp. Stata Statistical Software: release 11. College Station, TX: StataCorp LP; 2009.
Bagnardi V, Blangiardo M, La Vecchia C, et al. A meta-analysis of alcohol drinking and cancer risk. Br J Cancer. 2001;85(11):1700–5 (epub 2001/12/18).
Bosetti C, La Vecchia C, Negri E, et al. Wine and other types of alcoholic beverages and the risk of esophageal cancer. Eur J Clin Nutr. 2000;54(12):918–20 (epub 2000/01/11).
Corrao G, Bagnardi V, Zambon A, et al. Exploring the dose-response relationship between alcohol consumption and the risk of several alcohol-related conditions: a meta-analysis. Addiction (Abingdon, England). 1999;94(10):1551–73 (epub 2000/05/03).
Corrao G, Bagnardi V, Zambon A, et al. A meta-analysis of alcohol consumption and the risk of 15 diseases. Prev Med. 2004;38(5):613–9 (epub 2004/04/07).
Holman CD, English DR, Milne E, et al. Meta-analysis of alcohol and all-cause mortality: a validation of NHMRC recommendations. Med J Aust. 1996;164(3):141–5 (epub 1996/02/05).
Ishikawa A, Kuriyama S, Tsubono Y, et al. Smoking, alcohol drinking, green tea consumption and the risk of esophageal cancer in Japanese men. J Epidemiol Jpn Epidemiol Assoc. 2006;16(5):185–92 (epub 2006/09/05).
Li Y, Mao Y, Zhang Y, et al. Alcohol drinking and upper aerodigestive tract cancer mortality: a systematic review and meta-analysis. Oral Oncol. 2014;50(4):269–75.
Li Y, Yang H, Cao J. Association between alcohol consumption and cancers in the Chinese population—a systematic review and meta-analysis. PLoS One. 2011;6(4):e18776 (epub 2011/04/29).
Oze I, Matsuo K, Wakai K, et al. Alcohol drinking and esophageal cancer risk: an evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Jpn J Clin Oncol. 2011;41(5):677–92 (epub 2011/03/25).
Roerecke M, Shield KD, Higuchi S, et al. Estimates of alcohol-related oesophageal cancer burden in Japan: systematic review and meta-analyses. Bull World Health Organ. 2015;93(5):329–38 (epub 2015/08/01).
World Cancer Research Fund/American Institute for Cancer Research. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Washington DC: AICR; 2007.
Bagnardi V, Rota M, Botteri E, et al. Light alcohol drinking and cancer: a meta-analysis. Ann Oncol Off J Eur Soc Med Oncol ESMO. 2013;24(2):301–8 (epub 2012/08/23).
Bagnardi V, Rota M, Botteri E, et al. Alcohol consumption and site-specific cancer risk: a comprehensive dose-response meta-analysis. Br J Cancer. 2015;112(3):580–93 (epub 2014/11/26).
Castellsague X, Munoz N, De Stefani E, et al. Independent and joint effects of tobacco smoking and alcohol drinking on the risk of esophageal cancer in men and women. Int J Cancer J Int du Cancer. 1999;82(5):657–64 (epub 1999/07/27).
Castellsague X, Munoz N, De Stefani E, et al. Smoking and drinking cessation and risk of esophageal cancer (Spain). Cancer Causes Control CCC. 2000;11(9):813–8 (epub 2000/11/15).
Fahey PP, Mallitt KA, Astell-Burt T, et al. Impact of pre-diagnosis behavior on risk of death from esophageal cancer: a systematic review and meta-analysis. Cancer Causes Control: CCC; 2015 (epub 2015/07/27).
Freedman ND, Murray LJ, Kamangar F, et al. Alcohol intake and risk of oesophageal adenocarcinoma: a pooled analysis from the BEACON Consortium. Gut. 2011;60(8):1029–37 (epub 2011/03/17).
Islami F, Fedirko V, Tramacere I, et al. Alcohol drinking and esophageal squamous cell carcinoma with focus on light-drinkers and never-smokers: a systematic review and meta-analysis. Int J Cancer J Int du Cancer. 2011;129(10):2473–84 (epub 2010/12/31).
Lubin JH, Cook MB, Pandeya N, et al. The importance of exposure rate on odds ratios by cigarette smoking and alcohol consumption for esophageal adenocarcinoma and squamous cell carcinoma in the Barrett’s Esophagus and Esophageal Adenocarcinoma Consortium. Cancer Epidemiol. 2012;36(3):306–16 (epub 2012/04/17).
Prabhu A, Obi KO, Rubenstein JH. Systematic review with meta-analysis: race-specific effects of alcohol and tobacco on the risk of oesophageal squamous cell carcinoma. Aliment Pharmacol Ther. 2013;38(10):1145–55 (epub 2013/10/02).
Rota M, Bellocco R, Scotti L, et al. Random-effects meta-regression models for studying nonlinear dose-response relationship, with an application to alcohol and esophageal squamous cell carcinoma. Stat Med. 2010;29(26):2679–87 (epub 2010/09/03).
Zeka A, Gore R, Kriebel D. Effects of alcohol and tobacco on aerodigestive cancer risks: a meta-regression analysis. Cancer Causes Control CCC. 2003;14(9):897–906 (epub 2003/12/20).
Drahos J, Xiao Q, Risch HA, et al. Age-specific risk factor profiles of adenocarcinomas of the esophagus: a pooled analysis from the international BEACON consortium. Int J Cancer J Int du Cancer. 2015. (epub 2015/07/16).
Tramacere I, Pelucchi C, Bagnardi V, et al. A meta-analysis on alcohol drinking and esophageal and gastric cardia adenocarcinoma risk. Ann Oncol Off J Eur Soc Med Oncol ESMO. 2012;23(2):287–97 (epub 2011/05/10).
Ansary-Moghaddam A, Huxley RR, Lam TH, et al. The risk of upper aero digestive tract cancer associated with smoking, with and without concurrent alcohol consumption. Mt Sinai J Med N Y. 2009;76(4):392–403 (epub 2009/07/31).
Ansary-Moghaddam A, Martiniuk A, Lam TH, et al. Smoking and the risk of upper aero digestive tract cancers for men and women in the Asia-Pacific region. Int J Environ Res Publ Health. 2009;6(4):1358–70 (epub 2009/05/15).
Cook MB, Kamangar F, Whiteman DC, et al. Cigarette smoking and adenocarcinomas of the esophagus and esophagogastric junction: a pooled analysis from the international BEACON consortium. J Natl Cancer Inst. 2010;102(17):1344–53 (epub 2010/08/19).
Gandini S, Botteri E, Iodice S, et al. Tobacco smoking and cancer: a meta-analysis. Int J Cancer. 2008;122(1):155–64.
Oze I, Matsuo K, Ito H, et al. Cigarette smoking and esophageal cancer risk: an evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Jpn J Clin Oncol. 2012;42(1):63–73 (epub 2011/12/02).
Tramacere I, La Vecchia C, Negri E. Tobacco smoking and esophageal and gastric cardia adenocarcinoma: a meta-analysis. Epidemiology (Cambridge, Mass). 2011;22(3):344–9 (epub 2011/02/19).
Jarl J, Gerdtham UG. Time pattern of reduction in risk of oesophageal cancer following alcohol cessation–a meta-analysis. Addiction (Abingdon, England). 2012;107(7):1234–43 (epub 2011/12/20).
Rehm J, Patra J, Popova S. Alcohol drinking cessation and its effect on esophageal and head and neck cancers: a pooled analysis. Int J Cancer J Int du Cancer. 2007;121(5):1132–7 (epub 2007/05/10).
Boffetta P, Hecht S, Gray N, et al. Smokeless tobacco and cancer. Lancet Oncol. 2008;9(7):667–75 (epub 2008/07/05).
Lee PN, Hamling J. Systematic review of the relation between smokeless tobacco and cancer in Europe and North America. BMC Med. 2009;7:36 (epub 2009/07/30).
Siddiqi K, Shah S, Abbas SM, et al. Global burden of disease due to smokeless tobacco consumption in adults: analysis of data from 113 countries. BMC Med. 2015;13:194.
Akl EA, Gaddam S, Gunukula SK, et al. The effects of waterpipe tobacco smoking on health outcomes: a systematic review. Int J Epidemiol. 2010;39(3):834–57 (epub 2010/03/09).
Akhtar S. Areca nut chewing and esophageal squamous-cell carcinoma risk in Asians: a meta-analysis of case-control studies. Cancer Causes Control CCC. 2013;24(2):257–65 (epub 2012/12/12).
Lee PN. Summary of the epidemiological evidence relating snus to health. Regul Toxicol Pharmacol RTP. 2011;59(2):197–214 (epub 2010/12/18).
Islami F, Kamangar F. Helicobacter pylori and esophageal cancer risk: a meta-analysis. Cancer Prev Res. 2008;1(5):329–38 (epub 2009/01/14).
Rokkas T, Pistiolas D, Sechopoulos P, et al. Relationship between Helicobacter pylori infection and esophageal neoplasia: a meta-analysis. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2007;5(12):1413–7 (epub 2007/11/13).
Xie FJ, Zhang YP, Zheng QQ, et al. Helicobacter pylori infection and esophageal cancer risk: an updated meta-analysis. World J Gastroenterol WJG. 2013;19(36):6098–107 (epub 2013/10/10).
Zhuo X, Zhang Y, Wang Y, et al. Helicobacter pylori infection and oesophageal cancer risk: association studies via evidence-based meta-analyses. Clin Oncol (R Coll Radiol G B). 2008;20(10):757–62 (epub 2008/09/17).
Nie S, Chen T, Yang X, et al. Association of Helicobacter pylori infection with esophageal adenocarcinoma and squamous cell carcinoma: a meta-analysis. Dis Esophagus Off J Int Soc Dis Esophagus ISDE. 2014;27(7):645–53 (epub 2014/03/19).
Hardefeldt HA, Cox MR, Eslick GD. Association between human papillomavirus (HPV) and oesophageal squamous cell carcinoma: a meta-analysis. Epidemiol Infect. 2014;142(6):1119–37 (epub 2014/04/12).
Li X, Gao C, Yang Y, et al. Systematic review with meta-analysis: the association between human papillomavirus infection and oesophageal cancer. Aliment Pharmacol Ther. 2014;39(3):270–81 (epub 2013/12/07).
Liu H, Li J, Diao M, et al. Statistical analysis of human papillomavirus in a subset of upper aerodigestive tract tumors. J Med Virol. 2013;85(10):1775–85.
Liyanage SS, Rahman B, Gao Z, et al. Evidence for the aetiology of human papillomavirus in oesophageal squamous cell carcinoma in the Chinese population: a meta-analysis. BMJ Open. 2013;3(11):e003604 (epub 2013/11/19).
Liyanage SS, Rahman B, Ridda I, et al. The aetiological role of human papillomavirus in oesophageal squamous cell carcinoma: a meta-analysis. PLoS One. 2013;8(7):e69238.
Sitas F, Egger S, Urban MI, et al. InterSCOPE study: associations between esophageal squamous cell carcinoma and human papillomavirus serological markers. J Natl Cancer Inst. 2012;104(2):147–58 (epub 2012/01/10).
Yong F, Xudong N, Lijie T. Human papillomavirus types 16 and 18 in esophagus squamous cell carcinoma: a meta-analysis. Ann Epidemiol. 2013;23(11):726–34 (epub 2013/08/07).
Zhang SK, Guo LW, Chen Q, et al. The association between human papillomavirus 16 and esophageal cancer in Chinese population: a meta-analysis. BMC Cancer. 2015;15:1096 (epub 2015/03/18).
Guh DP, Zhang W, Bansback N, et al. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Publ Health. 2009;9:88 (epub 2009/03/27).
Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet (London, England). 2008;371(9612):569–78 (epub 2008/02/19).
Smith M, Zhou M, Whitlock G, et al. Esophageal cancer and body mass index: results from a prospective study of 220,000 men in China and a meta-analysis of published studies. Int J Cancer J Int du Cancer. 2008;122(7):1604–10 (epub 2007/12/07).
Hampel H, Abraham NS, El-Serag HB. Meta-analysis: obesity and the risk for gastroesophageal reflux disease and its complications. Ann Intern Med. 2005;143(3):199–211 (epub 2005/08/03).
Hoyo C, Cook MB, Kamangar F, et al. Body mass index in relation to oesophageal and oesophagogastric junction adenocarcinomas: a pooled analysis from the International BEACON Consortium. Int J Epidemiol. 2012;41(6):1706–18 (epub 2012/11/14).
Kubo A, Corley DA. Body mass index and adenocarcinomas of the esophagus or gastric cardia: a systematic review and meta-analysis. Cancer Epidemiol Biomark Prev. 2006;15(5):872–8 (epub 2006/05/17).
Turati F, Tramacere I, La Vecchia C, et al. A meta-analysis of body mass index and esophageal and gastric cardia adenocarcinoma. Ann Oncol Off J Eur Soc Med Oncol ESMO. 2013;24(3):609–17 (epub 2012/08/18).
Dobbins M, Decorby K, Choi BC. The association between obesity and cancer risk: a meta-analysis of observational studies from 1985 to 2011. ISRN Prev Med. 2013;2013:680536 (epub 2013/01/01).
Singh S, Sharma AN, Murad MH, et al. Central adiposity is associated with increased risk of esophageal inflammation, metaplasia, and adenocarcinoma: a systematic review and meta-analysis. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2013;11(11):1399–412.e7 (epub 2013/05/28).
Behrens G, Jochem C, Keimling M, et al. The association between physical activity and gastroesophageal cancer: systematic review and meta-analysis. Eur J Epidemiol. 2014;29(3):151–70 (epub 2014/04/08).
Chen Y, Yu C, Li Y. Physical activity and risks of esophageal and gastric cancers: a meta-analysis. PLoS One. 2014;9(2):e88082 (epub 2014/02/12).
Schmid D, Leitzmann MF. Television viewing and time spent sedentary in relation to cancer risk: a meta-analysis. J Natl Cancer Inst. 2014; 106(7):1–19.
Singh S, Devanna S, Varayil JE, et al. Physical activity is associated with reduced risk of esophageal cancer, particularly esophageal adenocarcinoma: a systematic review and meta-analysis. BMC Gastroenterol. 2014;14:101 (epub 2014/06/03).
Yu XF, Wang YQ, Zou J, et al. A meta-analysis of the effects of energy intake on risk of digestive cancers. World J Gastroenterol WJG. 2012;18(48):7362–70 (epub 2013/01/18).
Salehi M, Moradi-Lakeh M, Salehi MH, et al. Meat, fish, and esophageal cancer risk: a systematic review and dose-response meta-analysis. Nutr Rev. 2013;71(5):257–67 (epub 2013/04/18).
Zheng JS, Yang J, Fu YQ, et al. Effects of green tea, black tea, and coffee consumption on the risk of esophageal cancer: a systematic review and meta-analysis of observational studies. Nutr Cancer. 2013;65(1):1–16 (epub 2013/02/02).
Ge XX, Xing MY, Yu LF, et al. Carotenoid intake and esophageal cancer risk: a meta-analysis. Asian Pac J Cancer Prev APJCP. 2013;14(3):1911–8 (epub 2013/05/18).
Lock K, Pomerleau J, Causer L, et al. The global burden of disease attributable to low consumption of fruit and vegetables: implications for the global strategy on diet. Bull World Health Organ. 2005;83(2):100–8.
Riboli E, Norat T. Epidemiologic evidence of the protective effect of fruit and vegetables on cancer risk. Am J Clin Nutr. 2003;78(3 Suppl):559s–69s (epub 2003/08/26).
Soerjomataram I, Oomen D, Lemmens V, et al. Increased consumption of fruit and vegetables and future cancer incidence in selected European countries. Eur J Cancer (Oxford, England: 1990). 2010;46(14):2563–80 (epub 2010/09/17).
Yu XF, Zou J, Dong J. Fish consumption and risk of gastrointestinal cancers: a meta-analysis of cohort studies. World J Gastroenterol WJG. 2014;20(41):15398–412 (epub 2014/11/12).
Liu YX, Wang B, Wan MH, et al. Meta-analysis of the relationship between the methylenetetrahydrofolate reductase C677T genetic polymorphism, folate intake and esophageal cancer. Asian Pac J Cancer Prev APJCP. 2011;12(1):247–52 (epub 2011/04/27).
Pelucchi C, Bosetti C, Galeone C, et al. Dietary acrylamide and cancer risk: an updated meta-analysis. Int J Cancer J Int du Cancer. 2015;136(12):2912–22 (epub 2014/11/19).
Zheng P, Zheng HM, Deng XM, et al. Green tea consumption and risk of esophageal cancer: a meta-analysis of epidemiologic studies. BMC Gastroenterol. 2012;12:165 (epub 2012/11/23).
Sang LX, Chang B, Li XH, et al. Green tea consumption and risk of esophageal cancer: a meta-analysis of published epidemiological studies. Nutr Cancer. 2013;65(6):802–12 (epub 2013/08/06).
Yu X, Bao Z, Zou J, et al. Coffee consumption and risk of cancers: a meta-analysis of cohort studies. BMC Cancer. 2011;11:96 (epub 2011/03/17).
Liu X, Wang X, Lin S, et al. Dietary patterns and oesophageal squamous cell carcinoma: a systematic review and meta-analysis. Br J Cancer. 2014;110(11):2785–95 (epub 2014/04/10).
Mulholland HG, Murray LJ, Cardwell CR, et al. Glycemic index, glycemic load, and risk of digestive tract neoplasms: a systematic review and meta-analysis. Am J Clin Nutr. 2009;89(2):568–76 (epub 2008/12/18).
Turati F, Galeone C, Gandini S, et al. High glycemic index and glycemic load are associated with moderately increased cancer risk. Mol Nutr Food Res. 2015;59(7):1384–94 (epub 2015/02/20).
Zhu HC, Yang X, Xu LP, et al. Meat consumption is associated with esophageal cancer risk in a meat- and cancer-histological-type dependent manner. Dig Dis Sci. 2014;59(3):664–73 (epub 2014/01/08).
Castellsague X, Munoz N, De Stefani E, et al. Influence of mate drinking, hot beverages and diet on esophageal cancer risk in South America. Int J Cancer J Int du Cancer. 2000;88(4):658–64 (epub 2000/11/04).
Andrici J, Eslick GD. Mate consumption and the risk of esophageal squamous cell carcinoma: a meta-analysis. Dis Esophagus Off J Int Soc Dis Esophagus ISDE. 2013;26(8):807–16 (epub 2012/08/16).
Coleman HG, Murray LJ, Hicks B, et al. Dietary fiber and the risk of precancerous lesions and cancer of the esophagus: a systematic review and meta-analysis. Nutr Rev. 2013;71(7):474–82 (epub 2013/07/03).
Pelucchi C, La Vecchia C, Bosetti C, et al. Exposure to acrylamide and human cancer—a review and meta-analysis of epidemiologic studies. Ann Oncol Off J Eur Soc Med Oncol ESMO. 2011;22(7):1487–99 (epub 2011/01/18).
Li P, Xu J, Shi Y, et al. Association between zinc intake and risk of digestive tract cancers: a systematic review and meta-analysis. Clin Nutr (Edinburgh, Scotland). 2014;33(3):415–20 (epub 2013/10/24).
Turati F, Galeone C, La Vecchia C, et al. Coffee and cancers of the upper digestive and respiratory tracts: meta-analyses of observational studies. Ann Oncol Off J Eur Soc Med Oncol ESMO. 2011;22(3):536–44 (epub 2010/10/15).
Boyle P, Koechlin A, Autier P. Sweetened carbonated beverage consumption and cancer risk: meta-analysis and review. Eur J Cancer Prev Off J Eur Cancer Prev Organ (ECP). 2014;23(5):481–90 (epub 2014/03/15).
Islami F, Ren JS, Taylor PR, et al. Pickled vegetables and the risk of oesophageal cancer: a meta-analysis. Br J Cancer. 2009;101(9):1641–7 (epub 2009/10/29).
Choi Y, Song S, Song Y, et al. Consumption of red and processed meat and esophageal cancer risk: meta-analysis. World J Gastroenterol WJG. 2013;19(7):1020–9 (epub 2013/03/08).
Qu X, Ben Q, Jiang Y. Consumption of red and processed meat and risk for esophageal squamous cell carcinoma based on a meta-analysis. Ann Epidemiol. 2013;23(12):762–770.e1 (epub 2013/11/02).
Chen Y, Tong Y, Yang C, et al. Consumption of hot beverages and foods and the risk of esophageal cancer: a meta-analysis of observational studies. BMC Cancer. 2015;15:449 (epub 2015/06/03).
Liu J, Wang J, Leng Y, et al. Intake of fruit and vegetables and risk of esophageal squamous cell carcinoma: a meta-analysis of observational studies. Int J Cancer J Int du Cancer. 2013;133(2):473–85 (epub 2013/01/16).
Larsson SC, Giovannucci E, Wolk A. Folate intake, MTHFR polymorphisms, and risk of esophageal, gastric, and pancreatic cancer: a meta-analysis. Gastroenterology. 2006;131(4):1271–83 (epub 2006/10/13).
Tio M, Andrici J, Cox MR, et al. Folate intake and the risk of upper gastrointestinal cancers: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2014;29(2):250–8 (epub 2013/11/15).
Han YJ, Li J, Huang W, et al. Fish consumption and risk of esophageal cancer and its subtypes: a systematic review and meta-analysis of observational studies. Eur J Clin Nutr. 2013;67(2):147–54 (epub 2013/01/17).
Kubo A, Corley DA. Meta-analysis of antioxidant intake and the risk of esophageal and gastric cardia adenocarcinoma. Am J Gastroenterol. 2007;102(10):2323–30 (epub 2007/06/22).
Huang W, Han Y, Xu J, et al. Red and processed meat intake and risk of esophageal adenocarcinoma: a meta-analysis of observational studies. Cancer Causes Control CCC. 2013;24(1):193–201 (epub 2012/11/28).
Li B, Jiang G, Zhang G, et al. Intake of vegetables and fruit and risk of esophageal adenocarcinoma: a meta-analysis of observational studies. Eur J Nutr. 2014;53(7):1511–21 (epub 2014/01/23).
Agarwal DP. Cardioprotective effects of light-moderate consumption of alcohol: a review of putative mechanisms. Alcohol Alcohol. 2002;37(5):409–15 (epub 2002/09/10).
Hur C, Miller M, Kong CY, et al. Trends in esophageal adenocarcinoma incidence and mortality. Cancer. 2013;119(6):1149–58 (epub 2013/01/11).
Blaser MJ. Disappearing microbiota: Helicobacter pylori protection against esophageal adenocarcinoma. Cancer Prev Res. 2008;1(5):308–11 (epub 2009/01/14).
Renehan AG, Soerjomataram I, Leitzmann MF. Interpreting the epidemiological evidence linking obesity and cancer: a framework for population-attributable risk estimations in Europe. Eur J Cancer (Oxford, England: 1990). 2010;46(14):2581–92 (epub 2010/09/17).
Steffen A, Schulze MB, Pischon T, et al. Anthropometry and esophageal cancer risk in the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol Biomark Prev. 2009;18(7):2079–89 (epub 2009/07/02).
International Agency for Research on Cancer. World Cancer Report 2014. Lyon: 2014.
Rubenstein JH, Taylor JB. Meta-analysis: the association of oesophageal adenocarcinoma with symptoms of gastro-oesophageal reflux. Aliment Pharmacol Ther. 2010;32(10):1222–7 (epub 2010/10/20).
Cook MB, Corley DA, Murray LJ, et al. Gastroesophageal reflux in relation to adenocarcinomas of the esophagus: a pooled analysis from the Barrett’s and Esophageal Adenocarcinoma Consortium (BEACON). PLoS One. 2014;9(7):e103508 (epub 2014/07/31).
Islami F, Sheikhattari P, Ren JS, et al. Gastric atrophy and risk of oesophageal cancer and gastric cardia adenocarcinoma—a systematic review and meta-analysis. Ann Oncol Off J Eur Soc Med Oncol ESMO. 2011;22(4):754–60 (epub 2010/09/24).
Huang W, Ren H, Ben Q, et al. Risk of esophageal cancer in diabetes mellitus: a meta-analysis of observational studies. Cancer Causes Control CCC. 2012;23(2):263–72 (epub 2011/11/19).
Acknowledgements
This work was supported by FEDER funds through the Operational Competitiveness Programme and by national funding from the Foundation for Science and Technology–FCT (Portuguese Ministry of Science, Technology and Higher Education) (FCOMP-01-0124-FEDER-021181), under the project “Determinants of gastric and esophageal cancers incidence in the north of Portugal” (PTDC/SAU-EPI/122460/2010), and the Unidade de Investigação em Epidemiologia - Instituto de Saúde Pública da Universidade do Porto (EPIUnit) (POCI-01-0145-FEDER-006862; UID/DTP/04750/2013). An individual Postdoc grant attributed to BP (SFRH/BPD/75918/2011) was co-funded by the FCT and the "Programa Operacional Capital Humano" (POCH/FSE).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
535_2017_1375_MOESM2_ESM.eps
Forest plots of overall and sex-specific associations between alcohol drinking cessation (A) and tobacco smoking cessation (B) and the occurrence of esophageal cancer. RR: relative risk, CI: confidence interval, ESCC: esophageal squamous cell carcinoma, EC: esophageal cancer. (EPS 60 kb)
535_2017_1375_MOESM3_ESM.eps
Forest plot of overall and sex-specific associations between BMI and the occurrence of esophageal cancer, by histological subtype. RR: relative risk, CI: confidence interval, ESCC: esophageal squamous cell carcinoma, EAC: esophageal adenocarcinoma. (EPS 53 kb)
535_2017_1375_MOESM4_ESM.eps
Forest plot of overall and sex-specific associations between fruit and vegetable consumption and the occurrence of esophageal cancer, by histological subtype. RR: relative risk, CI: confidence interval, ESCC: esophageal squamous cell carcinoma, EAC: esophageal adenocarcinoma. (EPS 89 kb)
Rights and permissions
About this article
Cite this article
Castro, C., Peleteiro, B. & Lunet, N. Modifiable factors and esophageal cancer: a systematic review of published meta-analyses. J Gastroenterol 53, 37–51 (2018). https://doi.org/10.1007/s00535-017-1375-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-017-1375-5