Abstract
Background
Diverticular disease is a significant burden on healthcare systems that is managed, surgically or medically, mainly as an emergency or acute condition. There are no standardized treatment recommendations for symptomatic uncomplicated disease. We hypothesized that a probiotic would reduce abdominal pain in such patients.
Methods
We conducted a single-center, double-blind, placebo-controlled trial of probiotic treatment (Symprove) in adult patients with moderate-to-severe chronic, non-acute symptomatic diverticular disease. 143 patients were randomized to receive 1 mL/kg/day of probiotic liquid (N = 72) or placebo (N = 71) daily for 3 months. The primary endpoint was abdominal pain severity. Secondary endpoints consisted of the change in the frequency of eight abdominal symptoms and the level of intestinal inflammation (fecal calprotectin).
Results
120 patients completed the trial. Abdominal pain score, the primary end point, decreased in both groups, but no significant difference between the groups was found (P = 0.11). In relation to placebo, the probiotic significantly decreased the frequency of four of the eight secondary endpoints: constipation, diarrhea, mucorrhea, and back pain (P < 0.04). No significant differences were found in frequency of abdominal pain, PR bleeding, dysuria, and bloating.
Conclusions
Multi-strain liquid probiotic did not improve abdominal pain scores significantly, but significantly improved the frequency of four other symptoms associated with chronic, non-acute symptomatic diverticular disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diverticular disease accounts for 2.6 million visits to outpatient clinics per year in the United States (Peery et al. 2012). About 60% of people over 60 have colonic diverticula, and some 25% of these will develop abdominal symptoms (Hobson and Roberts 2004; Wolff and Boostrom 2012). The development of diverticula is traditionally linked to a low-fiber diet, with acute diverticulitis, with peri-colonic inflammation, caused by poorly understood sequence of events (Painter and Burkitt 1971). Acute diverticulitis is divided into complicated and uncomplicated. Complicated diverticulitis is often treated with surgery or drainage of abscesses, while uncomplicated diverticulitis is managed empirically with antibiotics, intravenous fluids, and a low residue diet. There is little guidance on whether patients with diverticulitis require follow-up or other treatment and the current focus of interest is on whether there is a risk of recurrent attack of diverticulitis (estimated between 15 and 35% over 5 years). The risk of developing complications during repeat attacks and requiring surgery is much lower (about 5%). Accordingly, the Association of Coloproctology of Great Britain and Ireland, the American Society of Colon and Rectal Surgeons, and other professional bodies have removed earlier guidance on elective surgery after two attacks and recommend individualized management of patients.
However, there is far greater number of patients that have chronic symptoms associated with diverticulosis, so-called symptomatic uncomplicated diverticular disease (SUDD). Such elderly patients have previously been considered in the context of irritable bowel syndrome (IBS) (Spiller 2012). However, this is somewhat problematic as the colon is morphologically and functionally abnormal in diverticular disease and the symptoms may follow an episode of well-documented diverticulitis. Indeed, it is suggested that the symptoms of SUDD following an episode of diverticulitis represent a distinct nosologic entity termed post-diverticulitis IBS (Cohen et al. 2013; Yamada et al. 2014; Spiller 2014), which has similarities with post-infectious IBS (Dai and Jiang 2012; Schwille-Kiuntke et al. 2011; Marshall et al. 2007; Marshall et al. 2010), although the conditions stand apart. The similarities of symptoms in IBS, post-infectious IBS, and SUDD suggest the potential of common therapeutic manipulations either by medication or by probiotics (Wolff and Boostrom 2012; Daniels et al. 2014; Hall 2014). There is accumulating evidence to implicate low-grade inflammation and alterations of the gut microbiome in the pathogenesis of the IBS like symptoms of SUDD (Daniels et al. 2014; Hall 2014). Probiotics are, therefore, emerging as a potential treatment strategy for SUDD, as they may alter the gut microbiome. Previous studies have focused on the role of probiotics (when given with mesalazine) in preventing a repeat attack of diverticulitis rather than symptomatic treatment (Tursi et al. 2013; Stollman et al. 2013).
Symprove is a liquid multi-strain probiotic that has been beneficial in reducing symptom scores in patients with classic IBS (Sisson et al. 2014). We performed a randomized, double-blind, placebo-controlled trial to assess the ability of Symprove to reduce abdominal pain and other symptoms in elderly patients with SUDD. The null hypothesis tested throughout is that of no difference between the groups receiving Symprove or placebo over 3 months, in terms of the average abdominal pain scores, level of inflammatory markers, and other related symptoms as well as diverticulitis-episode-free time.
Methods
Study design
This was a single-center, double-blind, placebo-controlled trial of a 3-month probiotic treatment (Symprove) in adult patients with moderate-to-severe chronic, non-acute symptomatic diverticular disease. Patients attending a dedicated Diverticular Disease Clinic at King’s College Hospital [a tertiary referral hospital in south London (Tibble et al. 2001) that specializes in the management of moderately-to-severe diverticular disease], from April 2013 to October 2014, were approached to participate.
We enrolled consecutive patients presenting with persistent abdominal symptoms (of at least 3-month duration) with an established diagnosis of uncomplicated diverticulosis (diagnosis of diverticulosis established by colonoscopy and/or CT scan, with or without raised inflammatory markers, and without a past diagnosis of IBS). Patients who had surgery for diverticulitis or its attendant complications, patients with right sided diverticulitis, and those with predominant bleeding symptoms were excluded. Similarly excluded were patients with complicated diverticulitis (phlegmon, perforation, abscesses, fistula, bleeding, etc.), those with co-existing inflammatory bowel or other chronic gastrointestinal disease, as well as patients with severe neurologic, psychiatric, musculoskeletal, respiratory, renal, cardiac, and neurologic diseases. Patients with substance misuse were also excluded as were women at risk of pregnancy.
Demographic and clinical data were recorded for each patient. Following an informed written consent to participate in the study and randomization (performed with a computerized protocol provided by the Department of Pharmacy, King’s College Hospital), patients were asked to attend the clinic every 4 weeks for 4 months, to return their symptom-based questionnaires as well as to receive supplies of their study medication. All data were collected and double entered into a customized database created for the study (EpiInfo, version 3.3.2). Duplicate databases were compared electronically and discrepancies resolved from the primary source.
Study medication and administration
Symprove (Symprove Ltd, Farnham, Surrey, UK) contains four strains of bacteria with a total of 109 colony forming units: (Lactobacillus rhamnosus NCIMB 30174, Lactobacillus plantarum NCIMB 30173, Lactobacillus acidophilus NCIMB 30175, and Enterococcus faecium NCIMB 30176) in a water-based suspension of barley extract. The placebo was an identical liquid in appearance and taste, containing distilled water (99.22%), mango and passion fruit natural flavour (0.50%), ascorbic acid (0.26%), and beta-carotene (0.02%) (Sisson et al. 2014). The placebo and probiotic were packaged in identical sealed boxes, identified by a trial batch/code number only. Validation of strains was performed by King’s College London (Ken Bruce, personal communication). Patients were instructed to keep study medication refrigerated (between 2 and 7 °C) throughout the study and to self-administer 1 mL/kg each morning on an empty stomach, with breakfast, if desired, no less than 10 min later.
Laboratory testing
Patients submitted a stool sample prior to initiating treatment and again at treatment completion, allowing for quantification of intestinal inflammation with fecal calprotectin. Fecal calprotectin testing was performed as per previous standardized description (Tibble et al. 2001) using an enzyme-linked immunosorbent assay (ELISA) from Bühlmann (EK-CAL, Bühlmann AG, Switzerland). Calprotectin results are expressed as ug/g feces. Intra-assay precision in the range 52.5–1246 ug/g has a coefficient of variation of 2.7–8.1%, while inter-assay precision is 6.6–14.5% in the range 18.1–1764 ug/g. The limit of detection of the calprotectin method was 10 µg/g. As these values were not normally distributed (Shapiro–Wilk test P < 0.0001), patient results were log normalized for analysis.
Patients also underwent baseline hematology and chemistry profiling, including estimated glomerular filtration rate (eGFR) using ADVIA analysers from Siemens Diagnostics (2120 for haematology and 2400 for chemistry, Siemens Diagnostics, Frimley, Surrey, UK). As eGFR values were skewed, values trichotomized into normal (at least 85 mL/min), mild impairment (70–84 mL/min), and moderate impairment (less than 70 mL/min) (Lab tests online 2015).
In this paper, we present only the complete case analysis, which is appropriate given that the “missingness” of outcome at time 3 was shown to be independent from any covariates and measures of the primary outcome at the previous times.
Primary endpoint
The primary endpoint was the severity of the abdominal pain experienced and it was measured longitudinally through the study. Patients scored this outcome four times during the trial: prior to treatment initiation, and 1, 2, and 3 months thereafter. Each time, seven consecutive days were assessed on a five-point scale (0—no pain, 1—mild discomfort, 2—mild pain, 3—moderate pain, and 4—severe pain) yielding a continuous score ranging from 0 to 28. A logarithmic transformation or categorization of this outcome was envisaged in case of a highly skewed distribution.
Secondary endpoints
Secondary endpoints were eight symptoms, common in patients with diverticular disease: abdominal pain, constipation, diarrhea, PR bleeding, mucorrhea, dysuria, back pain, and bloating. These symptoms were recorded as an ordinal score indicating the frequency of the symptom, from never to constantly (0—never; 1—once per month; 2—a few times per month; 3—a few times per week; 4—a few times per day; 5—a few times per hour; and 6—constantly). A dichotomization of these outcomes into “high (daily) frequency” (a few times per day, per hour, or constant symptoms) and “sporadic frequency” (never, once, a few times per month, or a few times per week) was envisaged in the case of highly skewed distributions.
The changes in fecal calprotectin and occurrence of episodes of diverticulitis were also assessed during the trial. The diagnosis of acute diverticulitis was made by clinicians independent of the study team (during an urgent attendance to their general physician, accident or emergency departments, or admission to hospital) and made clinically based on symptoms and examination and elevated inflammatory markers (ESR and/or CRP).
Statistical analysis plan
Balance of groups in all baseline patient characteristics were examined using univariate tests: two-sample t- or Mann–Whitney test for the continuous outcomes, Pearson Chi square for categorical outcomes, and Chi square for linear trends for ordered categorical outcomes. For the main analysis, mixed effect repeated measures (multilevel) modelling was used for the primary and secondary outcomes, to account for the four repeated measures (0, 1, 2, and 3 months) performed in these longitudinal study.
The two treatment groups were further compared in terms of the baseline-to-month 3 changes in the continuous outcomes, using analysis of covariance, to adjust for baseline level, with multivariate models (linear, ordinal, or logistic regressions as appropriate) used to adjust for the effects of potential confounders. Kaplan–Meier analyses were used to compare occurrence of acute diverticulitis between groups during the trial. Analysis was performed on an intention-to-treat basis. The complete case analysis is followed by an assessment of the impact of any missing data.
The sample size calculation was based on preliminary measurements: the baseline mean abdominal pain severity (primary endpoint) was estimated as 9, with a standard deviation of 3.5. It was predicted that, after 3 months, a mean decrease would be observed in the probiotic group, with a means of 2 and 0 in the probiotic and placebo groups, respectively, and a standard deviation of 3.5. Assuming an allocation ratio of 1:1, to guarantee 80% power to detect an effect size of 0.57 (mean difference of 2 with standard deviation of 3.5) for the abdominal pain severity between probiotic and placebo, a total effective sample size of 100 was required. Anticipating a drop-out rate of 20%, we needed to enroll 125 patients. Randomization was performed with a two-staged computerized protocol provided by the Department of Pharmacy, King’s College Hospital.
Approval
The study was funded by King’s College Hospital NHS Trust, London, in part via an unrestricted research grant from Symprove Ltd. This trial is registered with Clinicaltrials.gov, number NCT02115867 and with the King’s College Hospital Research Ethics Committee for London-Riverside, reference 12/LO/1695.
Results
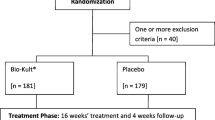
193 patients met the eligibility for the clinical trial, with 143 patients ultimately consenting and undergoing randomization (Fig. 1) (Moher and Schulz 2001). Of the 143 patients randomized, 120 patients completed the trial. Of the 23 patients who did not complete the trial, four were lost to follow-up, ten experienced an adverse event, eight patients were unable to complete the trial due to life circumstances not related to the trial, and one patient was unblinded following hospitalization with acute diverticulitis. Fifteen patients on probiotic and eight patients on placebo (P = 0.10) did not complete the trial.
Table 1 shows that a total of 25 patients experienced 28 adverse events, 15 were on the probiotic, and 13 on placebo (Chi-square test, P = 0.64), 10 of which resulted in withdrawal of the trial: 8 were on the probiotic and 2 on placebo (Fisher’s exact test, P = 0.27). Patients completing the trial had nearly uniformly high treatment compliance, with 89% of patients taking at least 95% of recommended doses, while patients who discontinued therapy missed a median of 65% of doses (IQR 54–70).
Baseline characteristics
Table 2 shows the demographic details of the patients. The vast majority of patients 87% had abdominal pain and only 2% reported normal bowel habits. The majority of patients (73.4%) had never been exposed to probiotics or probiotic yogurts prior to enrolling in the trial.
91 patients (64%) had a prior episode of acute diverticulitis, while 52 patients (36%) had de novo symptomatic diverticular disease without a documented acute episode of diverticulitis, with no difference between groups (P > 0.30). With the exception of eGFR, the two groups were well balanced for all demographic and clinical characteristics. Due to the eGFR imbalance, the effect of this variable was explored for all the models and, when necessary, adjusted for.
Primary outcome: abdominal pain severity
For the primary outcome, the baseline total pain score ranged from 0 to 22 and had a mean of 9.5 (SD = 7.7) in the probiotic group; it ranged from 0 to 28 with a mean of 7.5 (SD = 7.0) in the placebo group. Although the mean in the probiotic group was slightly higher, no significant difference in baseline abdominal pain severity between the two arms of the trial was found (mean difference = 2; 95% CI −0.62 to 4.6; P = 0.13).
Abdominal pain severity was first analyzed in terms of the sum of the seven daily scores, yielding a continuous outcome ranging from 0 to 28, with larger values indicating worse abdominal pain. Using a multilevel linear regression on this outcome, controlling for eGFR, a significant decrease across time was found (P < 0.001). This reduction appeared gradually, seen only at the second and third months; no significant reduction was found between the first and second months (P = 0.72). The reduction in mean pain severity from baseline was 1.6 (Coef = −1.63; 95% CI −2.6 to −0.62; P < 0.002) at month 2 and 2.7 (Coef = −2.7; 95% CI −3.7 to −1.6; P < 0.001) at month 3. The time-by-treatment interaction was found to be non-significant (P = 0.18) indicating that the reduction over time was similar in the probiotic and placebo groups.
The mean change of total abdominal pain in the probiotic group from baseline 9.5 (SD = 7.7; range 0–28; N = 62) to 3 months to 5.9 (SD = 6.8; range 0–24; N = 49), based on the corresponding paired data, was 3.2 (SD = 5.8; range −14 to 20; N = 47). The mean total abdominal pain for the placebo group was 7.5 (SD = 7.0; range 0–22; N = 62) at baseline and 6.2 (SD = 6.5; range 0–23; N = 55) at 3 months. The mean reduction after 3 months, based on the corresponding paired data for the placebo group, was 2.2 (SD = 4.7; range −8 to 14; N = 53). On a multivariate linear regression that controlled for the baseline value and eGFR category, the mean difference between probiotic and placebo in terms of the reduction at 3 months was found to be −0.54 (95% CI −2.4 to 1.3; P = 0.56). This difference was found to be non-significant. It is important to note that the primary outcome abdominal pain severity was somewhat skewed, and for this reason, the model was also fit to the logarithmic transformation of the main outcome; the results were consistent.
Maximum abdominal pain during the weeks of assessment
Abdominal pain severity was summarized in terms of the maximum pain reported over the daily scores of the corresponding week, yielding an ordinal outcome with categories 0 (best) to 4 (worst). Summarizing the outcome with the maximum of 7 days offers a natural way to avoid imputing missing data for which one has to make assumptions that are often unjustified. In addition, it avoids the distributional problems that are encountered with the continuous form of the outcome. We analyzed this ordinal outcome in two ways: the first one, using an ordinal logistic regression for the outcome with the five levels (0– 4), as defined and the second one, using a binary logistic regression for the dichotomization 0–2 vs 3–4.
Using an ordinal logistic regression on the outcome with five levels (0–4), we found that the odds of a worse level for the maximum pain reported over the week decreased in 50% between baseline and month 3. This reduction over time was found to be statistically significant (OR = 0.50; 95% CI 0.31–0.81; P = 0.004), although a non-significant time-by-treatment interaction indicated that this time effect was similar for both treatment groups (OR = 1.3; 95% 0.82–2.1; P = 0.25). Using a logistic regression that modelled the likelihood of a high level for the maximum pain reported (e.g., maximum = 3 or 4), the odds of high level were about half for the probiotic group in relation to placebo, but this difference did not reach statistical significance (OR = 0.49; 95% CI 0.15–1.6; P = 0.22).
The missing data observed in the principal outcome in our study followed a monotonic pattern, in the sense that were due to patients withdrawing from the study at some point. The exception was only for three patients: one with missing values for 3 days in month 1, another with missing values for the last 4 days in month 2 and one with a missing day in month 3. Using a logistic regression model, the “missingness” of outcome at time 3 was shown to be independent from any covariates and measures of the primary outcome at the previous times. Supplementary analysis of the main outcome inputting missing data was consistent with the complete case analysis. To assess the effect of missing data, a sensitivity analysis was conducted in which the study sample was augmented to have complete data at month 3 for a total of 111 patients per group. An effective sample size of 111 would have been required if a standard deviation of 5.3 (our pooled standard deviation for the baseline to month 3 changes) had been assumed. The additional data were simulated under the hypothesis of no treatment difference for the placebo group and a mean reduction of 2 for the probiotic group (e.g., giving an advantage to the probiotic), and nevertheless, the result was consistent with that obtained in the actual study. Even giving such an advantage to the probiotic, the additional patients did not reverse the finding of no significant treatment difference (Coeff = −0.30; 95% CI −2.0 to 1.4; P = 0.73) in the augmented sample. In addition, it should be observed that the maximum abdominal pain, which provided the added advantage of utilizing weekly data in the presence of missing days, did not reach statistical significance for treatment difference, consistent with the analysis of the total abdominal pain.
Secondary outcomes
Symptom scores
The complete case analysis of the eight abdominal symptoms is presented in Table 3 (for the ordinal scores) and Table 4 (for the dichotomization indicating whether the abdominal symptom presented with a high (at least daily) or a sporadic frequency). The baseline summaries, overall and per treatment group, for the corresponding outcome are presented on the left portion of each table.
The linear regressions modeling the (baseline to month 3) reduction in the ordinal scores for the eight abdominal symptoms, adjusting for the corresponding symptom score, and the eGFR at baseline (Table 3) showed a significant advantage for the probiotic in terms of back pain (coeff = 0.71; 95% CI 0.001–1.38; P = 0.04). The mixed logistic regressions modelling the likelihood of experiencing a high (at least daily) frequency in the abdominal symptoms, adjusting for the time effect and the baseline eGFR (Table 4), also showed a significant advantage for the probiotic in terms of back pain (OR = 0.33; 95% CI 0.11–0.99; P = 0.047). A significant time-by-treatment interaction was detected (P = 0.07), and on further inspection, a significant increase in high (at least daily) frequency of back pain over time was found in the placebo group (P = 0.003), not in the probiotic group (P = 0.46). The advantage of Symprove over placebo is not significant at month 1 (P = 0.14); it emerges at month 2 (OR = 0.31; 95% CI 0.10–0.99; P = 0.045) and is maintained to the end of the study period.
Although the mean reductions in constipation and mucorrohea were not statistically significant (Table 3), taking into account that the study was not powered for the secondary outcomes, an advantage for Symprove may be suggested in terms of these two abdominal symptoms given that, on the one hand, the odds of high (at least daily) frequency in constipation are significantly less for the probiotic (Table 4). The odds ratio is 0.36 (95% CI 0.13–1.02; P = 0.05). This significant advantage for Symprove emerges at month 1 (P = 0.01), is seen at month 2 (P = 0.046), and maintained to the end of the study period. On the other hand, in terms of mucorrohea, the mean score reduction is larger for Symprove and its confidence interval lies mostly on the positive axis (coeff = 0.46; 95% CI −0.11 to 1.04; P = 0.11). Likewise, the odds of at least daily frequency in the mucorrohea symptom are less for the probiotic and its 95% confidence interval lies mostly to the left of 1 (OR = 0.39; 95% 0.14–1.07; P = 0.07) suggesting a borderline significance. This borderline advantage for Symprove is seen at month 1 (P = 0.6), and maintained to the end of the study period.
No significant effect of Symprove was found in terms of abdominal pain (P = 0.28), diarrhea (P = 0.09), bloating (P = 0.33), rectal bleeding (P = 0.09), and dysuria (P = 0.10). Considering that the study was not powered for the secondary outcomes and given that the point estimates of the odds ratios are all below 1, the results suggest that the high frequency of these symptoms may have been slightly moderated by the probiotic.
Fecal calprotectin changes
Baseline fecal calprotectin ranged from <10 to 1032 µg/g, with a mean of 92 µg/g and a median of 42 µg/g and there were no significant differences between the two groups at the start of the study.
Month 3 values of fecal calprotectin ranged from <10 to 715 µg/g, with a mean of 89 µg/g and a median of 39 µg/g, which did not differ significantly from baseline. The mean change in fecal calprotectin was an increase of 3 µg/g with the probiotic and a decrease of 3.2 µg/g with placebo. Multiple linear regression was performed to assess predictor variables for the log of high final fecal calprotectin. In both a full model and all reduced models tested, the best predictor of the final fecal calprotectin amongst all patients was baseline fecal calprotectin (P < 0.0001). Male patients had a 0.45 log higher fecal calprotectin than did females, after controlling for other variables.
Given the initial imbalance in fecal calprotectin across genders, the logistic regression was performed on male patients only. In male patients, the 3-month trial of probiotic reduced the log of baseline fecal calprotectin by 75%. In non-adjusted terms, male patients on probiotic had a fecal calprotectin that went from 36 µg/g (IQR 14–103) to 42 µg/g (IQR 16–118), while patients on placebo went from 66 µg/g (IQR 34–135) to 106 µg/g (IQR 52–162). Thus, in male patients, the probiotic prevented a rise in fecal calprotectin during the 3-month trial (P = 0.05).
Acute diverticulitis episodes
Only one patient was hospitalized with diverticulitis during the study period, at which time the patient was withdrawn from the trial (the patient was on placebo). A total of 11 patients (7.7%) developed acute diverticulitis during the trial: 4.5% (N = 3) on the probiotic and 12% (N = 8) on placebo. An exact logistic regression indicated that this difference was not statistically significant (OR = 0.34; 95% CI 0.36–1.5; P = 0.19). Since intervals between episodes of acute diverticulitis are often longer than the study period (90 days), the median survival time cannot be estimated from the data. Instead, we estimated the proportion of patients that were episode-free at the end of the study: 88% (95% CI 77–94%) for the placebo group and 96% (95% CI 87–99%) for the Symprove group. The hazard ratio was 0.35 (95% CI 0.09–1.34), suggesting a tendency to longer episode-free intervals in Symprove.
Furthermore, on further inspection, we found indication of a possible interaction effect between treatment and number of prior episodes in those patients that had 3 or more prior episodes; the proportion episode-free by the end of our study period was 96% (95% CI 73–99%) in the Symprove group and 68% (95% CI 43–84%) for placebo; and the hazard ratio for this group of patients was 0.12 (95% 0.01–0.97).
Discussion
In this randomized clinical trial, no statistically significant benefit of the probiotic Symprove over placebo was detected in terms of the primary outcome, namely, reduction in abdominal pain severity in patients with symptomatic diverticular disease. In terms of secondary outcomes, however, the probiotic was found to significantly decrease the frequency of constipation and back pain. The probiotic also appeared to prevent exacerbation of intestinal inflammation in male patients and was associated with fewer episodes of diverticulitis during the trial than placebo in patient with frequent episodes of diverticulitis.
Diverticular disease has a wide spectrum of varying clinical and pathological manifestations, which often creates confusion to terminology. Treatment has historically been either surgical or with medication and probiotics attempting to prevent relapse of acute attacks. SUDD is viewed as an entity in its own right (Kvasnovsky and Papagrigoriadis 2015). However, many researchers have highlighted similarities with the symptoms of IBS, and in those with a documented episode of diverticulitis followed by IBS like symptoms, the sequence appears similar to that seen in patients with post-infection IBS like symptoms. The patients (64%) in this study belonged to SUDD following a documented attack of diverticulitis and the rest had SUDD without a documented diverticulitis episode, but rather, the diverticular disease was discovered because of de novo abdominal symptoms.
The primary endpoint for the present study was an assessment of abdominal pain severity, as this was one of the main beneficial effect of the probiotic Symprove in patients with classic IBS (Sisson et al. 2014). Symptoms of pain in SUDD are likely to be multifactorial, and perhaps, a combination of functional and mechanical abnormality as well as due to inflammatory induced alteration of visceral nerve endings, so-called visceral hypersensitivity, similar to that of IBS (Humes et al. 2012). The symptoms of somatization such as back pain may also be created with similar mechanism. The probiotic did not improve pain severity significantly, which contrasts with its effect in patients with classic IBS (Sisson et al. 2014), which suggests that the pathogenesis of pain differs in the two conditions (Spiller 2012; Cuomo et al. 2013). It is possible that some of the abdominal pain in SUDD represents the so-called “post-diverticulitis-IBS” which seems to have some common features to post-infectious IBS, which is a recognized complication of severe food infections. Post-infectious IBS appears to have a distinctively different natural history to classic IBS and may not respond to the conventional IBS treatments (Schwille-Kiuntke et al. 2011; Marshall et al. 2007; Marshall et al. 2010; Neal et al. 1997; Morken et al. 2009), despite that it falls within the IBS terminology. Nevertheless, the probiotic Symprove had a beneficial action on some secondary outcome measures, such as bowel habits, which is important as there are so few proven treatments for these patients.
Probiotics are likely to be able to modulate the immune system (as so-called “immunobiotics”); however, to date, we have little data as to which strains, in what proportions, and for what disease processes will have the best effect. Even within the genus Lactobacillus, different strains appear to stimulate immunity in different sites of the human body(Marranzino et al. 2012).
There are some limitations and specific strengths to this single-center research. The recruitment from a specialized diverticular disease clinic may have targeted a patient population that has more severe disease than the average ‘diverticular’ patient. Our study population, although not uniform in line with most study populations with diverticular disease, excluded patients with a firm previous IBS diagnosis (Cohen et al. 2013; Boostrom et al. 2012) and hence may be more representative of patients who are genuinely affected by SUDD (Kvasnovsky et al. 2015).
The study followed patients for 3 months only and most of these patients had ongoing long-term symptoms. There was a variability of previous diverticulitis episodes and the fibrosis caused by repeat inflammatory episodes may have influenced the effect of the probiotic on pain alteration, something that the power of the present of the study has not been able to bring out. It is possible that patients with a more acute presentation would have been more likely to benefit from probiotic intervention. Other randomized controlled trials of probiotics have included a treatment range of 4–52 weeks (Kvasnovsky et al. 2015), but have addressed different aspects of the disease, mainly possible prevention of recurrence of diverticulitis and not the management of symptoms. However, Lahner and colleagues found improvement in abdominal pain between 3 and 6 months of treatment with Lactobacillus paracasei and a high fiber diet in patients with symptomatic non-inflamed diverticulosis that may have a comparable study population to ours (Lahner et al. 2012).
A strength of this research is that it addresses a well-defined group of symptomatic patients without pre-existing IBS, where alternative treatment options are by and large empiric and unproven. We believe that our clinic population may represent a representative cross section of a broad group of patients with unmet therapeutic needs, and suspect that this pragmatic study design will be useful to clinicians encountering patients with symptomatic diverticular disease in any context (Roland and Torgerson 1998). Patients completing the trial had nearly uniformly high treatment compliance, with 89% of patients taking at least 95% of recommended doses. Meanwhile, patients who discontinued therapy missed a median of 65% of doses (IQR 54–70).
The probiotic Symprove prevented an increase in fecal calprotectin in men. Men may have a stronger inflammatory component in diverticular disease than women (Kvasnovsky et al. 2015), although there was no significant change in white blood cell count or CRP in patients of either gender in the clinical trial. In previous research, the probiotic appeared to decrease the excretion of calprotectin somewhat in patients with IBS and elevated fecal calprotectin (Sisson et al. 2014) and it decreased fecal calprotectin significantly in patients with quiescent ulcerative colitis (Sisson et al. 2015). The probiotic may have a local anti-inflammatory effect within the colon which decreases fecal calprotectin. The importance of this kind of inflammation in diverticular disease was addressed by Tursi and colleagues who assessed 54 patients recovering from acute uncomplicated diverticulitis over nearly 2 years (Tursi et al. 2014). Patients with a recurrence of diverticulitis over that period were more likely to have an elevated fecal calprotectin 2 weeks after their index episode. Thus, preventing an elevation in fecal calprotectin, as in the patients in this study, could hypothetically decrease their risk of future episodes. Although this area clearly requires further targeted study, alterations of microbiota certainly correlate with intestinal mucosal inflammation (Angriman et al. 2014; Hold et al. 2014).
While there was no difference in time to acute diverticulitis episode between the two groups, there was a strong indication that the probiotic prevented diverticulitis episodes in patients with three or more diverticulitis episodes. To date, no medication has been shown to consistently reduce the risk of recurrent diverticulitis, although there are data to suggest that mesalamine may do so (Stollman et al. 2013). The current study was not powered to assess the effectiveness of the probiotic at preventing episodes of acute diverticulitis—but nevertheless, it is an undeniably important outcome experienced by 11 patients (7.7%); 3 and 8 on probiotic and placebo, respectively. Likewise, in terms of the binary outcome ‘maximum pain’ reported high (Wolff and Boostrom 2012; Painter and Burkitt 1971) or low (0–2), we observed that from baseline to month 3, the level of pain worsened in 5 out of 53 patients on placebo (9.4%), and in 1 out of 47 (2%) patients on the probiotic. Collectively, these may be further indications of a potential benefit of the probiotic Symprove in relation to placebo.
In conclusion, probiotics have a sound theoretical underpinning as a treatment modality for SUDD. In this study, the probiotic Symprove improved certain symptoms associated with SUDD, not least bowel habits that are often a major issue for these patients. Symprove and treatment with L. paracasei and a high fiber diet appear to be the only currently available proven means of treatment of abnormal bowel symptoms in SUDD. If confirmed with further studies, these probiotics may be a major benefit and a useful adjunct for these patients.
References
Angriman I, Scarpa M, Castagliuolo I (2014) Relationship between pouch microbiota and pouchitis following restorative proctocolectomy for ulcerative colitis. World J Gastroenterol 20:9665–9674
Boostrom SY, Wolff BG, Cima RR, Merchea A, Dozois EJ, Larson DW (2012) Uncomplicated diverticulitis, more complicated than we thought. J Gastrointest Surg 16:1744–1749
Cohen E, Fuller G, Bolus R, Modi R, Vu M, Shahedi K et al (2013) Increased risk for irritable bowel syndrome after acute diverticulitis. Clin Gastroenterol Hepatol 11:1614–1619
Cuomo R, Barbara G, Andreozzi P, Bassotti GC, Asetti T, Grassini M et al (2013) Symptom patterns can distinguish diverticular disease from irritable bowel syndrome. Eur J Clin Invest 43:1147–1155
Dai C, Jiang M (2012) The incidence and risk factors of post-infectious irritable bowel syndrome: a meta-analysis. Hepatogastroenterology 59:67–72
Daniels L, Philipszoon LE, Boermeester MA (2014) A hypothesis: important role for gut microbiota in the etiopathogenesis of diverticular disease. Dis Colon Rectum 57:539–543
Hall JF (2014) The microbiome and diverticulitis: a new target for medical therapy? Dis Colon Rectum 57:544–545
Hobson KG, Roberts PL (2004) Etiology and pathophysiology of diverticular disease. Clin Colon Rectal Surg 17:147–153
Hold GL, Smith M, Grange C, Watt ER, El-Omar EM, Mukhopadhya I (2014) Role of the gut microbiota in inflammatory bowel disease pathogenesis: what have we learnt in the past 10 years? World J Gastroenterol 20:1192–1210
Humes DJ, Simpson J, Smith J, Sutton P, Zaitoun A, Bush D et al (2012) Visceral hypersensitivity in symptomatic diverticular disease and the role of neuropeptides and low grade inflammation. Neurogastroenterol Motil 24:318-e163
Kvasnovsky CL, Papagrigoriadis S (2015) Symptoms in patients with diverticular disease should not be labelled as IBS. Int J Colorectal Dis 30:995
Kvasnovsky CL, Bjarnason I, Papagrigoriadis S (2015) What colorectal surgeons should know about probiotics: a review. Colorectal Dis 17:840–848
Lab tests online (2015) https://labtestsonline.org/understanding/analytes/gfr/tab/test/. 30 July 2015
Lahner E, Esposito G, Zullo A, Hassan C, Cannaviello C, Paolo MC et al (2012) High-fibre diet and Lactobacillus paracasei B21060 in symptomatic uncomplicated diverticular disease. World J Gastroenterol 18:5918–5924
Marranzino G, Villena J, Salva S, Alvarez S (2012) Stimulation of macrophages by immunobiotic Lactobacillus strains: influence beyond the intestinal tract. Microbiol Immunol 56(11):771–781
Marshall JK, Thabane M, Borgaonkar MR, James C (2007) Postinfectious irritable bowel syndrome after a food-borne outbreak of acute gastroenteritis attributed to a viral pathogen. Clin Gastroenterol Hepatol 5:457–460
Marshall JK, Thabane M, Garg AX, Clark WF, Moayyedi P, Collins SM, Walkerton Health Study Investigators (2010) Eight year prognosis of postinfectious irritable bowel syndrome following waterborne bacterial dysentery. Gut 59:605–611
Moher D, Schulz KF, Altman DG, CONSORT group (Consolidated Standards of Reporting Trials) (2001) The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. Ann Intern Med 134:657–662
Morken MH, Lind RA, Valeur J, Wilhelmsen I, Berstad A (2009) Subjective health complaints and quality of life in patients with irritable bowel syndrome following Giardia lamblia infection: a case control study. Scand J Gastroenterol 44:308–313
Neal KR, Hebden J, Spiller R (1997) Prevalence of gastrointestinal symptoms six months after bacterial gastroenteritis and risk factors for development of the irritable bowel syndrome: postal survey of patients. BMJ 314:779–782
Painter NS, Burkitt DP (1971) Diverticular disease of the colon: a deficiency disease of Western civilization. Br Med J 2:450–454
Peery AF, Dellon ES, Lund J, Crockett SD, McGowan CE, Bulsiewicz WJ et al (2012) Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology 143:1179–1187
Roland M, Torgerson DJ (1998) Understanding controlled trials: what are pragmatic trials? BMJ 316(7127):285 (Clinical research ed)
Schwille-Kiuntke J, Enck P, Zendler C, Krieg M, Polster AV, Klosterhalfen S et al (2011) Postinfectious irritable bowel syndrome: follow-up of a patient cohort of confirmed cases of bacterial infection with Salmonella or Campylobacter. Neurogastroenterol Motil 23:479–488
Sisson G, Ayis S, Sherwood RA, Bjarnason I (2014) Randomised clinical trial: a liquid multi-strain probiotic vs. placebo in the irritable bowel syndrome–a 12 week double-blind study. Aliment Pharmacol Ther 40:51–60
Sisson G, Hayee B, Bjarnason I (2015) Assessment of a multi strain probiotic (Symprove) in IBD. Gastroenterology 148:S-531
Spiller R (2012) Is it diverticular disease or is it irritable bowel syndrome? Dig Dis 30:64–69
Spiller R (2014) New thoughts on the association between diverticulosis and irritable bowel syndrome. Am J Gastroenterol 109:1906–1908
Stollman N, Magowan S, Shanahan F, Quigley EM, DIVA Investigator Group (2013) A randomized controlled study of mesalamine after acute diverticulitis: results of the DIVA trial. J Clin Gastroenterol 47(7):621–629. doi:10.1097/MCG0b013e31828003f6
Tibble JSG, Foster R, Sherwood R, Fagerhol M, Bjarnason I (2001) Faecal calprotectin and faecal occult blood tests in the diagnosis of colorectal carcinoma and adenoma. Gut 49:402–408
Tursi A, Brandimarte G, Elisei W, Picchio M, Forti G, Pianese G et al (2013) Randomised clinical trial: mesalazine and/or probiotics in maintaining remission of symptomatic uncomplicated diverticular disease–a double-blind, randomised, placebo-controlled study. Aliment Pharmacol Ther 38:741–751
Tursi A, Elisei W, Picchio M, Brandimarte G (2014) Increased faecal calprotectin predicts recurrence of colonic diverticulitis. Int J Colorectal Dis 29:931–935
Wolff BG, Boostrom SY (2012) Prophylactic resection, uncomplicated diverticulitis, and recurrent diverticulitis. Dig Dis 30:108–113
Yamada EI, Namori M, Uchida E, Tanida E, Izumi M, Takeshita K et al (2014) Association between the location of diverticular disease and the irritable bowel syndrome: a multicenter study in Japan. Am J Gastroenterol 109:1900–1905
Acknowledgements
We thank the patients and staff at the Diverticular Disease clinic at King’s College Hospital, especially Ingvar Bjarnason for his assistance.
Author information
Authors and Affiliations
Contributions
Guarantor of the article: Mr. Savvas Papagrigoriadis. Specific author contributions: Study conception and design: CK, IB, and SP. Acquisition of data: CK, IB, RS, and SP. Analysis and interpretation of data: CK, IB, AND, RS, and SP. Drafting of article: CK, IB, and SP. Critical revision for important intellectual content: CK, IB, AND, RS, and SP. Final approval of version to be published: CK, IB, AND, RS, and SP. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest and source of funding
Ingvar Bjarnason has served as a speaker and advisor board member for Symprove Ltd. Authors CLK, SP, and IB worked on this clinical trial of a probiotic, Symprove, funded by King’s College Hospital, London, via an unrestricted research grant from Symprove Ltd. No funding or other support was obtained for the writing of this manuscript.
Rights and permissions
About this article
Cite this article
Kvasnovsky, C.L., Bjarnason, I., Donaldson, A.N. et al. A randomized double-blind placebo-controlled trial of a multi-strain probiotic in treatment of symptomatic uncomplicated diverticular disease. Inflammopharmacol 25, 499–509 (2017). https://doi.org/10.1007/s10787-017-0363-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-017-0363-y