Abstract
A 38-year-old woman presented in early pregnancy with anemia due to an ulcerated gastric tumor which had the typical clinical presentation and endoscopic appearance of a gastric leiomyoma or gastrointestinal stromal tumor. At surgery this was subsequently found to be a mucinous cystic tumor of pancreas. Review of the literature shows that both gastrointestinal hemorrhage and infiltration of stomach are infrequent complications of this tumor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Case report
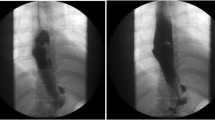
A 38-year-old woman, in the eigth week of pregnancy, presented with a 6-month history of lassitude, dyspnea, and palpitations and a hemoglobin of 6.8 g/dl. Upper gastrointestinal endoscopy revealed an ulcerated lesion, high on the greater curve of the stomach, with the endoscopic appearance of an ulcerated and bleeding leiomyoma or gastrointestinal stromal tumor (GIST; Fig. 1). Following transfusion, her hemoglobin fell overnight from 11 to 9.6 g/dl and a laparotomy with anterior gastrotomy was performed.
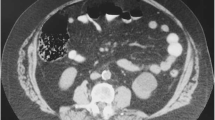
The gastric mass was found to be part of a much larger lesion arising from the pancreas. An intraoperative frozen section revealed a mucinous tumor with a relatively bland cytological appearance (Fig. 2). The tumor was excised as completely as possible, together with the spleen, body and tail of the pancreas, and a wedge of surrounding stomach. A Roux loop of jejunum was anastomosed to the neck of the pancreas. The patient made an uneventful recovery and was discharged on the ninth postoperative day. She continued to full term, delivering a healthy baby. Further pancreatic resection was performed 13 months after the original surgery due to positive resection margins in the first specimen.
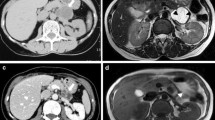
The resected material consisted of the spleen and a tissue mass 10 × 8 × 6 cm containing multiple small cysts. Histological examination showed a mucinous tumor with complex folded glandular structures lined by columnar mucinous cells, goblet cells, and sparse chromogranin-positive endocrine cells, with a surrounding ovarian-type stroma of fibrous connective tissue. Some areas showed a single layer of bland, uniform, small, basally located nuclei, while other areas showed borderline malignant features of multilayered nuclei with rounder, more pleomorphic nuclei, prominent nucleoli, and mitotic figures amounting to moderate and focally severe dysplasia (Fig. 3). Stromal invasion was not identified. The tumor appeared to involve the pancreatic resection margin.
Discussion
Mucinous cystic neoplasms of the pancreas are rare, accounting for only 2–5% of primary exocrine pancreatic tumors. These are subdivided into mucinous cystadenoma, borderline mucinous cystic neoplasm with moderate dysplasia, and cystadenocarcinoma, noninvasive or invasive.
When diagnosed using strict criteria (ovarian-type fibrous stroma and no communication with pancreatic ductal system), these tumors occur predominantly in women, usually in their forties and fifties. Invasive lesions present about 10 years later than cystadenoma and borderline tumors, suggesting an adenoma–carcinoma progression. Men present at an older age. These tumors are usually well circumscribed and arise in the body and tail of the pancreas.
Preoperative assessment typically consists of transabdominal and multislice computed tomography (CT) scanning. Endoscopic ultrasound and fine needle aspiration, if available, enables cytological sampling and assessment of tumor markers such as carcinoembryonic antigen (CEA) in cyst fluid.
Preoperative grading of mucinous tumors in biopsies is problematic because, in common with ovarian mucinous tumors, the histological grade may vary widely from place to place within an individual tumor, and examination of one section per 1 cm of maximum diameter of the resected tumor is recommended. Although, in all the histological sections examined, the present case appeared to be a borderline tumor, the poor circumscription of the tumor and invasion of the stomach wall implied biologically malignant behavior.
In their study of 130 cases, all in women, Thompson et al. [1] highlight the difficulty of reliably distinguishing between borderline and malignant tumors, and include both in a category of mucinous cystic neoplasms with atypia (in this series 60 without and 70 with cytological atypia). When they compared mucinous cystic neoplasms with and without atypia, there was little difference in survival rates. Abdominal pain was the commonest symptom at presentation, affecting 94 out of the 130 patients. Bleeding or anemia was seen in only 7 patients, but the mechanism was not discussed.
Zamboni et al. [2] reported on 56 mucinous cystic neoplasms (22 adenomas, 12 borderline, and 22 adenocarcinomas), all in women. Hemorrhage was not reported as a symptom and gastric invasion was not recorded.
In the Wilentz et al. series [3] of 61 mucinous tumors (27 adenomas, 5 borderline and 29 adenocarcinomas), symptoms at presentation were not discussed. Only cases undergoing complete resection were selected and gastric involvement was not documented in any of these. In this study there were many male patients, with only 78% of cystadenomas, 80% of borderline tumors, and 63% of adenocarcinomas in women.
The French cooperative study of Le Borgne et al. [4] included 398 cystadenomas and cystadenocarcinomas, of which 150 were mucinous cystadenomas (87% women) and 78 were mucinous cystadenocarcinomas (61% women). Hemorrhage was not recorded as a symptom, but evidence of portal hypertension was found at laparotomy in 10% of cases. They comment that mucinous cystadenocarcinomas usually have pushing margins, but that in 26% of their cases surgical dissection was extended into surrounding viscera or the mesenterico-portal vein. Gastrectomy was performed in 4 of the 58 mucinous cystadenocarcinomas undergoing resection. Communication with the pancreatic duct was documented in 6 of the 150 cystadenomas and 10 of the 78 carcinomas, which raises the possibility that at least some of these cases were intraductal papillary mucinous tumors.
Grieshop et al. [5] described 21 cystic neoplasms, including 6 mucinous cystadenomas and 6 mucinous cystadenocarcinomas. One of the latter presented as gastrointestinal hemorrhage from a malignant-looking gastric ulcer 5 years after a gastrotomy for a cystic tumor misdiagnosed as an inflammatory pseudocyst.
Rupture of an intratumoral hematoma through the gastric wall in one patient, and bleeding from hyperemic gastric mucosa caused by tumoral compression of the vasa brevia draining the stomach in another, were described by Campbell and Cruikshank [6] in a series of 14 cases, all in women. Six were serous tumors and the histology of the two cases which bled is not specified. One necessitated partial gastrectomy.
In the Hodgkinson et al. series [7] of 21 patients with mucinous cystadenocarcinoma, 3 patients presented with hematemesis; one had a profuse collateral blood supply around the cyst and one had an occluded splenic vein. In this series 10 patients were men.
Sheers [8] reported a 28-year-old pregnant woman with anemia and bleeding varices due to splenic vein compression by a tumor described simply as a pancreatic cystadenoma, and this author refers to two earlier reports of splenic vein thrombosis due to compression by a papillary cystadenoma and a unilocular cystadenoma, both in middle-aged women, and one case of venous infiltration by mucinous cystadenocarcinoma in a 36-year-old woman.
So far as we are aware, this is the first report of a mucinous cystic neoplasm of the pancreas mimicking a bleeding GIST. Management of this patient presented special difficulties, the first trimester pregnancy precluding definitive radiological investigations, although ultrasound would have been helpful. The risk to the pregnancy had to be weighed against the risk of continuing hemorrhage. Biopsy of tissue from within the ulcer crater would probably have given the correct diagnosis, but at the risk of provoking further hemorrhage, and with uncertainty of the overall grade of tumor due to limited sampling.
Prognosis is uncertain. These are generally slow-growing lesions and even frankly invasive carcinomas may be cured by complete excision. Age over 50, incomplete surgical excision, evidence of capsular invasion, and nuclear immunohistochemical positivity for p53 are adverse features (p53 was negative in our patient). Regular CT surveillance of our patient has shown no evidence of recurrent tumor after 5 years.
References
Thompson LDR, Becker RC, Przygodzki RM, Adair CF, Heffess CS. Mucinous cystic neoplasm (mucinous cystadenocarcinoma of low-grade malignant potential) of the pancreas. Am J Surg Pathol. 1999;23:1–16.
Zamboni G, Scarpa A, Bogina G, Iacono C, Bassi C, Talamini G, et al. Mucinous cystic tumours of the pancreas. Am J Surg Pathol. 1999;23:410–22.
Wilentz RE, Albores-Saavedra J, Zahurak M, Talamini MA, Yeo CJ, Cameron JL, et al. Pathologic examination accurately predicts prognosis in mucinous cystic neoplasms of the pancreas. Am J Surg Pathol. 1999;23:1320–7.
Le Borgne J, de Calan L, Partensky C. Cystadenomas and cystadenocarcinomas of the pancreas. Ann Surg. 1999;230:152–61.
Grieshop NA, Wiebke EA, Kratzer SS, Madura JA. Cystic neoplasms of the pancreas. Am Surg. 1994;60:509–15.
Campbell JA, Cruikshank AH. Cystadenoma and cystadenocarcinoma of the pancreas. J Clin Pathol. 1962;15:432–7.
Hodgkinson DJ, ReMine WH, Weiland LH. A clinicopathological study of 21 cases of pancreatic cystadenocarcinoma. Ann Surg. 1978;188:679–84.
Sheers RA. Pancreatic cystadenoma complicated by varices: case report. Br J Surg. 1980;67:144–5.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Brown, T.H., Menon, V.S., Richards, D.G. et al. Gastrointestinal bleeding in a pregnant woman: mucinous cystic neoplasm of pancreas mimicking gastrointestinal stromal tumor of stomach. J Hepatobiliary Pancreat Surg 16, 681–683 (2009). https://doi.org/10.1007/s00534-009-0159-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00534-009-0159-6