Abstract
Purpose
To evaluate the effectiveness of antioxidants in the prevention and management of oral mucositis in adults undergoing radiotherapy and/or chemotherapy with diagnosed head and neck cancer (HNC) compared to placebo intervention.
Methods
Cochrane, EMBASE, PubMed, and Web of Science databases were used to search for randomized controlled trials (RCTs) comparing oral or topical antioxidants with placebo in clinically diagnosed HNC adult patients receiving radiotherapy with/without chemotherapy. The primary outcome was to assess the efficacy of the antioxidant to prevent and decrease the incidence/prevalence and severity of oral/oropharyngeal mucositis. The risk of bias was assessed following Cochrane’s guidelines.
Results
The database search resulted in 203 records up to February 19, 2021. Thirteen RCTs were included with 650 HNC-diagnosed patients. Included studies showed a statistically significant improvement in mucositis severity score for all antioxidants except melatonin. However, further studies are needed as only one study reported outcomes for zinc, propolis, curcumin, and silymarin. Patients receiving vitamin E were 60% less likely to develop severe mucositis grade 2 or higher than those receiving placebo in one study (P = 0.040). Patients receiving zinc were 95% less likely to develop severe mucositis (grades 3–4) in one study compared to placebo (P = 0.031). One meta-analysis showed no statistical difference in the risk of having severe mucositis (grades 3–4) with 199 patients compared to placebo for honey (n = 2 studies, P = 0.403). Meta-analyses could not be conducted for zinc, propolis, curcumin, melatonin, silymarin, and selenium due to the lack of studies reporting similar outcomes for the same intervention.
Conclusion
Though oral and topical antioxidants significantly improved mucositis severity scores in HNC patients receiving radiotherapy with/without chemotherapy in individual studies, the quality of the evidence was low due to the small number of studies and unclear/high-risk bias. Additionally, large RCTs are needed to confirm these results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oral mucositis (OM) is defined as inflammation of the oral mucosa, which can range from erythema to ulceration [1]. Head and neck cancer (HNC) patients undergoing radiotherapy (RT) or chemoradiotherapy (CRT) can experience symptoms of OM [2]. For patients receiving a high dose of RT or CRT for HNC, OM can be a dose-limiting complication, resulting in severe pain, difficulty in eating, poor quality of life, and weight loss. Bacteremia and sepsis in immunocompromised states may result in longer hospital admissions, increased number of inpatient visits, and greater economic burden for patients and health systems [3]. Underreporting of oral sequelae and the use of multiple grading systems make the prevalence of OM during HNC treatment difficult to generalize [4]. The severity and incidence of OM are dependent on the type, dose, and duration of therapy, as well as complex biological and genetic level factors. Higher incidence is reported in patients receiving RT and CRT versus chemotherapy (CT) alone [5].
The development of OM in patients undergoing RT starts at the epithelial surface due to the generation of reactive oxygen species (ROS) which results in DNA damage [6]. OM resulting from CT begins in the basal layer of the epithelium, when the anti-cancer agent penetrates from submucosal vasculature. Chemotherapy-induced OM can be aggravated due to immunosuppression [6]. Many theories include microbial flora in the etiology and propagation of OM; however, conclusive evidence is lacking [7].
The World Health Organization (WHO), the Radiation Therapy Oncology Group (RTOG), the National Cancer Institute (NCI), and the Mucositis Study Group (1999) [8] have developed OM grading scales that are widely utilized in clinical and research settings.
Antioxidants are substances that may prevent oxidative damage to cells and act as a scavenging system against free radicals [9]. Free radicals (or ROS) may be generated through exposure to environmental factors such as foods, chemicals, industrial byproducts, and trauma and may play an important part in the pathogenesis of cancer [1, 10]. ROS are mediators of cell death, yet most cancer therapies depend on ROS production for efficient tumor eradication [11]. Antioxidants could be endogenous or exogenous, enzymatic or non-enzymatic, and belong to various categories (Online Resource Table 1). Antioxidants can act on cell membranes, mitochondria, and cytosol to exert anti-inflammatory and anti-apoptotic effects. Examples of antioxidants include vitamins, flavonoids, and minerals [12].
Systematic reviews focusing on randomized controlled trials (RCTs) have reported the efficacy of antioxidants on the management of OM [13, 14]. In the 2020 edition of MASCC/ISOO Clinical Practice Recommendations [15], previous recommendations supporting zinc as a preventive measure in the 2014 edition [3] were reversed to no guidelines possible due to lack of level I and II evidence. Glutamine oral tablets in 30–60-mg doses were added as a suggestion but are not recommended in par-enteral form due to severe adverse effects. Honey was added as a suggestion in 2020, in both topical and oral forms, for the prevention of OM. Additionally, concentrated calcium phosphate mouthwash was added, but no guidelines could be recommended due to conflicting results among populations [15].
Objectives
The goal of this systematic review with meta-analyses was to assess the effects of oral and topical antioxidants in the prevention and/or management of oral and oropharyngeal mucositis in HNC-diagnosed patients treated with RT or CRT.
Methods
Research question
RCTs included studies investigating the efficacy of oral and topical antioxidants for the management or prevention of oral and oropharyngeal mucositis in HNC patients undergoing RT or CRT. This systematic review with meta-analyses used PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement as a guideline and was registered with PROSPERO #CRD42021231153. The PICOS question under study was:
-
Population: HNC patients undergoing radiotherapy with or without chemotherapy.
-
Intervention: Oral or topical antioxidants.
-
Comparison: Placebo (color and taste matched tablets).
-
Outcomes: The primary outcomes were the incidence/prevalence of oral/oropharyngeal mucositis and the severity of mucositis. The secondary outcomes included prevalence and severity of xerostomia and erythema; wound formation; normalcy of eating and drinking; quality of life; pain intensity; the need for other medication; duration of mucositis (time); adverse events.
-
Settings: oncology departments of universities and hospitals.
Inclusion/exclusion criteria
Studies included were English language publications of RCTs on the efficacy of antioxidants to treat and prevent OM in HNC patients receiving RT with/without CT. Reviews, systematic reviews, animal studies, pilot studies, editorials, abstracts, meta-analyses, and clinical guidelines were excluded, as well as studies comparing to interventions different than a placebo.
Database search, collection, extraction, and management
Four databases were searched (EMBASE, Cochrane, Medline, and Web of Science), and the search strategy is described in Online Resource Table 2.
Two authors (N.K. and A.R.) assessed half of the references each after the removal of duplicates, and their inclusion/exclusion results were reviewed by a third author (C.E.). If a disagreement among the reviewers existed, a fourth author (R.E.) helped make the final decision. The reference sections of all included studies, systematic reviews, and clinical guidelines were reviewed by three authors (N.K., A.R., and C.E.). New studies were submitted to inclusion/exclusion criteria again by the same three authors (N.K., A.R., and C.E.). The full article was reviewed, with a fourth author (R.E.) making the final inclusion/exclusion decision in agreement with the three authors. Three reviewers obtained data regarding the studies independently (AR, NK, CE), including study design, funding, number of centers, recruitment period, inclusions/exclusions criteria, and control and intervention groups' demographics, along with intervention methods and sample size and the outcomes of the results. After discussion, the authors agreed on the final tables.
Risk of bias assessment
The risk of bias was evaluated by three reviewers separately (A.R., N.K., C.E.) and the corresponding author (R.E), following the protocol in the Cochrane handbook [16].
Statistical analysis and evidence quality
Trials reporting OM in patients with HNC undergoing RT, with/without CT, receiving oral or topical antioxidants compared to placebo were included in the meta-analyses. Review authors calculated weighted means and standard deviations based on tabular frequency data reported in two studies [17, 18] using standard formulas. When original studies reported means and 95% confidence intervals (CIs), we calculated the standard error of the mean (SEM) as (95%_UPPER – 95%_LOWER)/3.92 based on Cochrane’s manual. When authors reported interquartile range (IQR) = (Q25, Q75) and medians (m), we calculated mean = (Q25 + m + Q75)/3 and the standard deviation (SD) as (Q75 – Q25)/1.35. When SEM was reported, we calculated SD as SEM × sqrt(N), with N = sample size.
For mucositis scores (0–4 WHO OMAS or 0–4 NCI-CTC v2 or 0–4 RTOG OM), treatment effects were analyzed as the difference in means (DM) of post-treatment mucositis scores with 95% CI. For the incidence of severe mucositis (subjects with severe mucositis grades 2–3 in OMAS scale or mucositis grade 2 or higher in RTOG scale) and the number of people requiring analgesics, treatment effects were analyzed as risk ratios (RRs) with 95% CI.
We calculated Cochran’s Q test [19] and I2 statistic [20] to assess heterogeneity and used random-effects model if Q-test P < 0.10 and fixed-effect model if P ≥ 0.10. Comprehensive Meta-Analysis software (version 3) was used (Biostat, Englewood, NJ, USA).
Subgroup analyses were conducted for each oral/topical antioxidant. Unfortunately, sensitivity analyses for low versus unclear/high risk though planned could not be conducted due to the small number of studies per intervention.
The quality of evidence was reviewed, and the results were summarized with the help of GRADE profiler (GRADEpro) software [21].
Results
Summary of search results
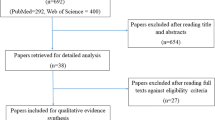
The primary database searching provided 353 references plus 18 additional records from other sources. The duplicates were removed; after that, 202 references were scanned by three review authors (N.K., A.R., C.E.), which were reduced to 39 records based on the titles and abstracts. The 39 manuscripts were fully analyzed for inclusion based on the full text. Thirteen manuscripts were included. The reasons for exclusion were no HNC patients (n = 2), no placebo group (n = 6), proceedings abstract (n = 3), no antioxidant (n = 9), more than one antioxidant (n = 1), not an RCT (n = 1), protocol (n = 1), different outcomes (n = 1), and different condition (n = 3). The same search (Online Resource Table 2) was conducted again on February 12, 2021, to include any new records, and one relevant study was found [22]. The PRISMA diagram in Fig. 1 presents the search results.
Included studies
A total of thirteen double-blinded RCTs were included in the qualitative analysis [17, 18, 22,23,24,25,26,27,28,29,30,31,32] with details presented in Tables 1 and 2.
Population
The age of the research subjects included in the systematic review ranged from 15 to 87 years old [22, 32] (Table 1). The RCTs were conducted in Iran [17, 22, 23, 26, 29, 30, 32], the USA [25], England [27], Cyprus [28], Turkey [18], Brazil [31], and Thailand [24] (Table 1). Centers providing the intervention varied from radiotherapy/oncology departments [18, 25, 28,29,30], RT centers [23, 26], and oncology centers [22, 24, 31]. Additionally, not all patients received the same treatment: some patients received only RT [26, 28, 31, 32], some received CRT [17, 18, 22,23,24,25, 27, 29, 30]. Patients had a diagnosis of squamous cell carcinoma (SCC) of the head and neck [23, 26, 28], oral cancer [18, 22, 24, 29, 30], or pharyngeal cancer [25, 27, 31] (Table 2).
Interventions
Interventions included oral/topical antioxidants (Table 1), such as:
TOPICAL AND SYSTEMIC
-
Manuka honey: Patients were advised to rinse with manuka honey (20 mL), and to swallow it slowly, 4 times per day for 4 weeks during RT, and for 2 weeks after treatment [27].
-
Melatonin: In one study [24], both topical 10-mL melatonin oral gargle (local) 15 min before CT and 20 mg oral melatonin capsules once daily for 7 weeks (systemic).
-
One 400 mg vitamin E oil capsule was dissolved in the saliva, then patient will rinse the oral cavity for 5 min and swallow it 5 min before RT and after 8–12 h at home [31].
-
Aloe vera: 20 mL swish and swallow 4 times per day, beginning on the first day of RT until the completion of the RT course [25].
-
Propolis 3% 15 mL solution, instructed to rinse and swallow, 3 times per day for 5 weeks [32].
TOPICAL
-
Thyme honey mouthrinse: Subjects were instructed to dilute 20 mL of thyme honey in purified water and gargle 3 times per day for 7 weeks [28].
-
Calendula: Patients undergoing RT or CRT were advised to use oral mouthwash calendula 5 mL 2 times a day [26].
-
Propolis 3% mouthrinse (30% extract), 5 mL of solution swish and rinse, 3 times per day for 7 days [17].
SYSTEMIC
-
Silymarin: Treatment group received 140 mg silymarin tablets 3 times per day for 6 weeks [30].
-
Curcumin: 80 mg (1 capsule) once daily throughout the RT period [29].
-
Zinc sulfate capsules: 50 mg thrice daily, starting day 1 of RT until 6 weeks after treatment [18] or 30 mg capsule 3 times per day, starting 10 days before RT until 8 weeks after end of RT [23].
-
Selenium 200 mcg 2 times a day throughout the RT period [22].
Co-interventions
Seven studies had co-interventions, such as local anesthetic solutions and analgesic agents [18]; mouthrinses in any combination of baking soda, Benadryl, nystatin, viscous lidocaine, and antibiotics as well as systemic antifungal agents for active infection and dietary supplements as needed [25], topical niosome suspension [24], saline rinses [22, 27, 32], paracetamol/codeine, or dipyrone analgesics as needed were used [31].
Outcomes
Primary outcomes included OM severity and incidence reported using WHO mucositis score (0–4 scale) [33]; the National Cancer Institute Common Terminology Criteria (NCI-CTC v2 and v3) grading system (0–4 scale); Radiation Therapy Oncology Group grading system (RTOG) on a 0–4 scale [34, 35]; and Oral Mucositis Assessment Score (OMAS) on a 0–3 scale [36].
Risk of bias
RCTs were assessed for risk of bias (Online Resource Tables 3 and 4; Fig. 2). In summary, nine studies were unclear [17, 22,23,24, 27, 29,30,31,32], and four were at high risk of bias [18, 25, 26, 28].
Adverse events
Online Resource Table 5 presents the adverse events. Eight of the studies did not report any side effects/adverse events in the intervention group as well as in the placebo group [17, 23, 24, 26, 28,29,30, 32]. Two studies [27, 31] presented mild adverse events, such as taste and consistency intolerability of placebo gel, mild nausea, vomiting, and fever. One study [18] presented moderate grade 3 vomiting in the intervention group, and another trial presented patients with renal failure [22].
Results of meta-analysis
Incidence of severe mucositis
One meta-analysis showed no statistical difference in the risk of having severe mucositis (grades 3–4) with 199 patients compared to placebo for honey (n = 2 studies, P = 0.403), Fig. 3.
Results of included studies
Meta-analyses could not be conducted for most of the outcomes as only one study was found for some of the interventions; however, we present in this review the Forest plots for visualization purposes (Fig. 4).
Results of Forest plots comparing antioxidants to placebo intervention for H&N cancer patients in original studies: a difference in post-treatment mucositis severity (scale 0–4); b incidence of severe mucositis (grade 3 or 4); c incidence of severe mucositis (RTOG score 2 or higher); d patients who required analgesics
Post-treatment severity of oral mucositis
Forest plots for curcumin, honey, melatonin, propolis, silymarin, and zinc compared to placebo are shown in Fig. 4a for the differences in average post-treatment mucositis severity (0–4 scale). Studies reported OM severity using the WHO mucositis score [33], RTOG grading system [34, 35], and NCI-CTC v2-v3 criteria. All antioxidants except melatonin showed a statistically significantly lower post-treatment mucositis score than placebo interventions (P ≤ 0.001); however, more studies are needed as only one study reported outcomes for zinc, curcumin, honey, melatonin, propolis, and silymarin (Fig. 4a).
Incidence of severe mucositis
Forest plots for incidence of severe mucositis (grades 3 or 4 with WHO OM score or RTOG) for melatonin, propolis, selenium, vitamin E, and zinc compared to placebo are shown in Fig. 4b. Patients receiving zinc were 95.2% less likely to develop severe mucositis (RR = 0.048; 95% CI = 0.003 to 0.753; P = 0.031; Fig. 4b) in one study [18]. Forest plots showed no statistical difference in the risk of having severe mucositis (grades 3–4) compared to placebo for selenium (n = 1 study, P = 0.449), melatonin (n = 1 study, P = 0.427), and propolis (n = 1 study, P = 0.237), Fig. 4b.
Patients receiving vitamin E were 60% less likely to develop severe mucositis RTOG grade 2 or higher in one study [25] than those receiving placebo (RR = 0.400; 95% CI = 0.167 to 0.958; P = 0.040; Fig. 4c). Forest plots showed no statistical difference in the risk of having severe mucositis (RTOG grade 2 or higher) for aloe vera compared to placebo (n = 1 study, P = 0.205; Fig. 4c).
Number of patients requiring analgesics
Forest plots showed no statistically significant difference in the risk of requiring analgesics for aloe vera (n = 1 study, P = 0.573), honey (n = 1 study, P = 0.850), or melatonin (n = 1, P = 0.276) compared to the placebo group, Fig. 4d.
Summary of evidence quality
The quality of the evidence was low for incidence of severe mucositis with honey (Table 3) due to the small sample size, the small number of studies, and the unclear/high risk of bias. The quality of the evidence was very low for all other outcomes and interventions as only one study was available for each intervention.
Discussion
Main findings
Patients with HNC are managed with one or more treatment modalities, including surgical resection, radiotherapy, and systemic therapy. The goal of therapy may be curative or palliative, with the efficacy of the cancer outcome balanced against morbidity and quality of life impacts of the disease and its treatment. In severe cases, OM may lead to difficulty eating, dysphagia, severe pain, and dryness of mouth, resulting in increased hospital visits, cessation of treatment, and further worsening of quality of life [37]. In our systematic review, we only included RCTs comparing oral/topical antioxidants with placebo. The risk of bias for all studies was unclear/high (Fig. 2).
All antioxidants except melatonin and selenium showed statistically significantly lower mucositis severity scores than placebo interventions in the original studies. Meta-analyses could not be conducted for most of the outcomes as only one study was found for zinc, curcumin, propolis, silymarin, selenium, and vitamin E. However, we are showing the Forest plots for visualization purposes. In summary, the quality of the evidence was low for incidence of severe mucositis with honey (Table 3) and very low for all other outcomes.
Agreements/disagreements with other studies and reviews
Honey
Khanal et al. [38] compared topical honey with lidocaine in patients undergoing RT and showed that honey could limit the severity of mucositis. In another study, manuka honey did not improve OM, but both manuka honey and syrup were associated with a decrease in bacterial infection [27]. Pure natural honey (prophylactic) was effective in reducing OM resulting from RT with/without CT in one study [39]. Despite its limitations, one study [28] showed thyme honey’s favorable effects on the management of RT-induced OM. It also showed that honey was effective in improving swallowing, dysphagia, and oral and throat pain, and decreased HNC patients’ weight loss[28]. Manuka and kanuka essential oils (mouthwash) showed a delayed onset of RT-induced mucositis and reduced pain and oral symptoms [40]. Due to the small sample size, additional investigations are warranted [39]. In one trial [41], patients in Malaysia with HNC, who were undergoing RT, swished and swallowed honey, both prior to and after RT. Honey was effective in improving and preventing OM compared to no intervention. According to one review [42], manuka honey did not show positive results.
Propolis
One pilot study showed extract of propolis prevented and healed RT-induced mucositis [32], while an additional double-blinded RCT [17] concluded propolis mouthwash used during CT decreased the severity of OM, wound and erythema scores compared to placebo mouthrinse [17, 32].
Vitamin E
Ferreira et al. [31] concluded HNC patients undergoing RT experienced reduced symptoms and incidence of OM while undergoing RT, when receiving a vitamin E–containing mouthrinse. One trial [43] reported a favorable effect of vitamin E in CT-induced mucositis, while another investigation [44] concluded that vitamin E did not improve OM. Confirmatory studies with a larger number of patients need to be undertaken to verify these results [45].
Calendula
Two studies included in our systematic review showed that calendula officinal is an effective treatment for decreasing OM severity in patients treated with CT [26] and RT [23].
Aloe vera
Severe mucositis incidence was significantly lower in the aloe vera group versus placebo in patients treated with RT [46]. However, our subgroup analysis showed no difference in the incidence of severe mucositis (RTOG 2 or higher) nor a decreased need for analgesics [25].
Zinc
Forest plots showed that zinc significantly improved the severity of OM and agree with the conclusions by De Freitas Cuba et al. [47], that oral zinc can be considered beneficial in preventing OM during RT ± CT. Two studies [18, 48] found no differences between zinc supplementation versus placebo for the prevention of any severity of mucositis in patients with HNC receiving RT.
Silymarin
According to one trial [30], oral formulation of 420 mg silymarin administered in 3 doses may reduce the severity and delay the onset of RT-induced OM.
Curcumin
One RCT [29] demonstrated a favorable effect of nanomicelle curcumin in the prevention and reduction of the severity of RT-induced OM.
Selenium
One trial [49] failed to demonstrate selenium supplements reduced side effects of CT and RT, or the effects of surgery. Further investigation agreed that selenium supplementation during radiation had no effect on the severity/incidence of OM [22].
Melatonin
According to one study [24], adjuvant melatonin delayed the onset of OM and reduced the need for opioid pain management in patients receiving CRT. However, melatonin did not significantly improve post-treatment mucositis severity (0–4 grade; Fig. 4a), nor the incidence of severe mucositis (grade 3–4; Fig. 4b), nor the need for analgesics (Fig. 4c) in this study [24] according to the included studies.
Applicability of evidence
Our search was conducted in four databases up to 02/19/2021 and limited to English published articles. The results of this systematic review are applicable to 15–87-year-old patients and a treatment duration of 1 to 8 weeks (short term). These results are applicable to males and females and apply to patients mostly from Iran, though there were also patients included from USA, Europe, England, Brazil, and Thailand.
Heterogeneity of the review
Heterogeneity found in this systematic review was due to the diversity of oral/topical interventions used with varying routes of administration (oral tablets, mouthrinses) as described in Table 1. Duration of the intervention/control measures in the included RCTs ranged widely from 1 to 8 weeks. Seven studies had differing co-interventions including local anesthetic solutions and analgesic agents [18], mouthrinses [25], topical niosome suspension [24], saline rinses [22, 27, 32], and paracetamol/codeine [31].
Another factor that induced heterogeneity was the different grading criteria used to assess OM. Due to the heterogeneity in the outcome measures described above and the different interventions, these resulted in a small number of studies pooled for each subgroup analysis with a final small sample size resulting in low quality of the evidence. Additionally, not all patients had the same type of cancer or received the same treatment, some patients received only RT [26, 28, 31, 32], some received CRT [17, 18, 22,23,24,25, 27, 29, 30].
Implications for research
Our findings coincide with previous research results that oral and topical antioxidants can have a beneficial effect on reducing the severity of OM, but high-quality randomized placebo-controlled trials are needed to make recommendations or guidelines for using natural products. Future RCTs should minimize biases, have no co-interventions, be double-blinded, not be funded by the manufacturer of the products under study, have long-term follow-up, and study different dosages and large sample size. This systematic review can be used as a guideline for further studies. In particular, larger high-quality studies are needed for zinc, propolis silymarin, and vitamin E topical oil as they show promising results in reducing the severity of OM.
There is controversy about antioxidants interfering with radiotherapy in cancer patients among oncologists. However, Moss (2007) [50] concluded that “dietary antioxidants do not conflict with the use of radiotherapy in the treatment of a wide variety of cancers and may significantly mitigate the adverse effects of that treatment.“ In a recent review, Salehi et al. [51] concluded that antioxidants available as supplements have “at best little value in preventing chronic disease course,” or could even be harmful in cancer patients. An understanding of “smart antioxidants” is needed that could cause a redox imbalance in tumor cells but not in normal tissues [51].
Implications for clinical practice
HNC patients are treated with a variety of regimens that may include surgery, radiotherapy, and chemotherapy. OM can cause severe pain with difficulties in chewing, speech, and mastication. Pharmacological treatments such as anesthetic gels or rinses have been used for mucositis, but they may come with side effects and merely palliate discomfort. Oral or topical antioxidants may act as supplements to combat mucositis. Due to a small number of studies and sample size, further research is needed to assess the efficacy of antioxidants in clinical practice.
Conclusions
Though oral and topical antioxidants provided a significant improvement in mucositis severity scores in individual studies, only one study was found for each intervention, and the quality of the evidence was low due to the small number of studies and unclear or high-risk bias. Additional studies are needed to confirm these results.
Availability of data and material
Results of the search and the meta-analyses are presented in tables and figures. Any intermediate data is available by email to renciso@usc.edu.
Code availability
Not applicable.
References
Lalla RV, Sonis S, Peterson D (2008) Management of oral mucositis in patients who have cancer. Dent Clin North Am 52:61–77. https://doi.org/10.1016/j.cden.2007.10.002
Epstein JB, Saunders DP (2015) Managing oral mucositis cancer therapy. Oral Health Group. Available at: https://www.oralhealthgroup.com/features/managing-oral-mucositis-cancer-therapy/
Lalla RV, Bowen J, Barasch A et al (2014) MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 120:1453–1461. https://doi.org/10.1002/cncr.28592
Villa A, Sonis ST (2016) Pharmacotherapy for the management of cancer regimen-related oral mucositis. Expert Opin Pharmacother 17:1801–1807. https://doi.org/10.1080/14656566.2016.1217993
Raber-Durlacher JE, Elad S, Barasch A (2010) Oral mucositis. Oral Oncol 46:452–456. https://doi.org/10.1016/j.oraloncology.2010.03.012
Sonis ST (2004) The pathobiology of mucositis. Nat Rev Cancer 4:277–284. https://doi.org/10.1038/nrc1318
Basile D, Di Nardo P, Corvaja C et al (2019) Mucosal injury during anti-cancer treatment: from pathobiology to bedside. Cancers (Basel) 11:1–22. https://doi.org/10.3390/cancers11060857
Trotti A, Byhardt R, Stetz J et al (2000) Common toxicity criteria: version 2.0. An improved reference for grading the acute effects of cancer treatment: impact on radiotherapy. Int J Radiat Oncol Biol Phys 47:13–47. https://doi.org/10.1016/S0360-3016(99)00559-3
Halliwell B (1991) Reactive oxygen species in living systems: source, biochemistry, and role in human disease. Am J Med 91:14S-22S. https://doi.org/10.1016/0002-9343(91)90279-7
Schreiber RD, Old LJ, Smyth MJ (2011) Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 331(6024):1565–1570. https://doi.org/10.1126/science.1203486
Panieri E, Gogvadze V, Norberg E et al (2013) Reactive oxygen species generated in different compartments induce cell death, survival, or senescence. Free Radic Biol Med 57:176–187. https://doi.org/10.1016/j.freeradbiomed.2012.12.024
Sahu PK, Sahu PK, Sahu PL, Agarwal DD (2016) Structure activity relationship, cytotoxicity and evaluation of antioxidant activity of curcumin derivatives. Bioorganic Med Chem Lett 26:1342–1347. https://doi.org/10.1016/j.bmcl.2015.12.013
Block KI, Koch AC, Mead MN et al (2007) Impact of antioxidant supplementation on chemotherapeutic efficacy: a systematic review of the evidence from randomized controlled trials. Cancer Treat Rev 33:407–418. https://doi.org/10.1016/j.ctrv.2007.01.005
Worthington HV, Clarkson JE, Bryan G et al (2011) Interventions for preventing oral mucositis for patients with cancer receiving treatment. Cochrane Database Syst Rev (4):CD000978. https://doi.org/10.1002/14651858.CD000978.pub5
Elad S, Cheng KKF, Lalla RV et al (2020) MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 126:4423–4431. https://doi.org/10.1002/cncr.33100
Higgins J, Green S (editors) (2011) Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. Available at: https://handbook-5-1.cochrane.org/
Akhavan-Karbassi MH, Yazdi MF, Ahadian H, SadrAbad MJ (2016) Randomized double-blind placebo-controlled trial of propolis for oral mucositis in patients receiving chemotherapy for head and neck cancer. Asian Pac J Cancer Prev 17:3611–3614. https://doi.org/10.14456/apjcp.2016.142
Ertekin MV, Koç M, Karslioǧlu I, Sezen O (2004) Zinc sulfate in the prevention of radiation-induced oropharyngeal mucositis: a prospective, placebo-controlled, randomized study. Int J Radiat Oncol Biol Phys 58:167–174. https://doi.org/10.1016/S0360-3016(03)01562-1
Cochran W (1954) The combination of estimates from different experiments. Biometrics 10:101–129
Higgins J, Thompson S (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558
Guyatt G, Oxman AD, Akl EA et al (2011) GRADE guidelines: 1. Introduction - GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 64:383–394. https://doi.org/10.1016/j.jclinepi.2010.04.026
Laali E, Manifar S, Kazemian A et al (2020) Effect of selenium on incidence and severity of mucositis during radiotherapy in patients with head and neck cancer. Oral Health Prev Dent 18:765–772. https://doi.org/10.3290/j.ohpd.a45080
Moslemi D, Babaee N, Damavandi M et al (2014) Oral zinc sulphate and prevention of radiation-induced oropharyngealmucositis in patients with head and neck cancers: a double blind, randomized controlled clinical trial. Int J Radiat Res 12(3):235–241. Available at: http://ijrr.com/article-1-1281-en.html
Onseng K, Johns NP, Khuayjarernpanishk T et al (2017) Beneficial effects of adjuvant melatonin in minimizing oral mucositis complications in head and neck cancer patients receiving concurrent chemoradiation. J Altern Complement Med 23:957–963. https://doi.org/10.1089/acm.2017.0081
Su CK, Mehta V, Ravikumar L et al (2004) Phase II double-blind randomized study comparing oral aloe vera versus placebo to prevent radiation-related mucositis in patients with head-and-neck neoplasms. Int J Radiat Oncol Biol Phys 60:171–177. https://doi.org/10.1016/j.ijrobp.2004.02.012
Babaee N, Moslemi D, Khalilpour M et al (2013) Antioxidant capacity of calendula officinalis flowers extract and prevention of radiation induced oropharyngeal mucositis in patients with head and neck cancers: a randomized controlled clinical study. Daru 21(1):18. https://doi.org/10.1186/2008-2231-21-18
Bardy J, Molassiotis A, Ryder WD et al (2012) A double-blind, placebo-controlled, randomised trial of active manuka honey and standard oral care for radiation-induced oral mucositis. Br J Oral Maxillofac Surg 50:221–226. https://doi.org/10.1016/j.bjoms.2011.03.005
Charalambous M, Raftopoulos V, Paikousis L et al (2018) The effect of the use of thyme honey in minimizing radiation - induced oral mucositis in head and neck cancer patients: A randomized controlled trial. Eur J Oncol Nurs 34:89–97. https://doi.org/10.1016/j.ejon.2018.04.003
Delavarian Z, Pakfetrat A, Ghazi A et al (2019) Oral administration of nanomicelle curcumin in the prevention of radiotherapy-induced mucositis in head and neck cancers. Spec Care Dentist 39:166–172. https://doi.org/10.1111/scd.12358
Elyasi S, Hosseini S, Niazi Moghadam MR et al (2016) Effect of oral silymarin administration on prevention of radiotherapy induced mucositis: a randomized, double-blinded, placebo-controlled clinical trial. Phyther Res 30:1879–1885. https://doi.org/10.1002/ptr.5704
Ferreira PR, Fleck JF, Diehl A et al (2004) Protective effect of alpha-tocopherol in head and neck cancer radiation-induced mucositis: a double-blind randomized trial. Head Neck 26:313–321. https://doi.org/10.1002/hed.10382
Javadzadeh Bolouri A, Pakfetrat A, Tonkaboni A et al (2015) Preventing and therapeutic effect of propolis in radiotherapy induced mucositis of head and neck cancers: a triple-blind, randomized, placebo-controlled trial. Iran J cancer Prev 8:e4019. https://doi.org/10.17795/ijcp-4019
World Health Organization (1979) WHO Handbook for Reporting Results of Cancer Treatment. In: WHO Offset publication no. 48. Geneva, Switzerland, World Health Organization. pp 15–27. Available at: https://apps.who.int/iris/bitstream/handle/10665/37200/WHO_OFFSET_48.pdf
Ferretti GA, Raybould TP, Brown AT et al (1990) Chlorhexidine prophylaxis for chemotherapy- and radiotherapy-induced stomatitis: a randomized double-blind trial. Oral Surg Oral Med Oral Pathol 69:331–338. https://doi.org/10.1016/0030-4220(90)90295-4
Epstein JB, Stevenson-Moore P, Jackson S et al (1989) Prevention of oral mucositis in radiation therapy: a controlled study with benzydamine hydrochloride rinse. Int J Radiat Oncol Biol Phys 16:1571–1575. https://doi.org/10.1016/0030-4220(90)90295-4
Ferretti GA, Ash RC, Brown AT et al (1988) Control of oral mucositis and candidiasis in marrow transplantation: a prospective, double-blind trial of chlorhexidine digluconate oral rinse. Bone Marrow Transplant 3:483–493
Peterman A, Cella D, Glandon G et al (2001) (2001) Mucositis in head and neck cancer: economic and quality-of-life outcomes. J Natl Cancer Inst Monogr 29:45–51. https://doi.org/10.1093/oxfordjournals.jncimonographs.a003440
Khanal B, Baliga M, Uppal N (2010) Effect of topical honey on limitation of radiation-induced oral mucositis: an intervention study. Int J Oral Maxillofac Surg 39:1181–1185. https://doi.org/10.1016/j.ijom.2010.05.014
Rodríguez-Caballero A, Torres-Lagares D, Robles-García M et al (2012) Cancer treatment-induced oral mucositis: a critical review. Int J Oral Maxillofac Surg 41:225–238. https://doi.org/10.1016/j.ijom.2011.10.011
Maddocks-Jennings W, Wilkinson JM, Cavanagh HM, Shillington D (2009) Evaluating the effects of the essential oils Leptospermum scoparium (manuka) and Kunzea ericoides (kanuka) on radiotherapy induced mucositis: a randomized, placebo controlled feasibility study. Eur J Oncol Nurs 13:87–93. https://doi.org/10.1016/j.ejon.2009.01.002
Regupriya M (2020) Effect of topical application of honey on oral mucosa among patients with head and neck cancer undergoing Radiation therapy at Sri Ramakrishna Hospital, Coimbatore. Asian J Nurs Educ Res 10(2):127–129. https://doi.org/10.5958/2349-2996.2020.00028.2 Available at: https://ajner.com/AbstractView.aspx?PID=2020-10-2-3
Münstedt K, Männle H (2020) What is wrong with the meta-analyses on honey and oral mucositis due to cancer therapies? Complement Ther Med 49:102286. https://doi.org/10.1016/j.ctim.2019.102286
El-Housseiny AA, Saleh SM, El-Masry AA, Allam AA (2007) The effectiveness of vitamin “E” in the treatment of oral mucositis in children receiving chemotherapy. J Clin Pediatr Dent 31:167–170. https://doi.org/10.17796/jcpd.31.3.r8371x45m42l10j7
Sung L, Tomlinson GA, Greenberg ML et al (2007) Serial controlled N-of-1 trials of topical vitamin E as prophylaxis for chemotherapy-induced oral mucositis in paediatric patients. Eur J Cancer 43:1269–1275. https://doi.org/10.1016/j.ejca.2007.02.001
Wadleigh RG, Redman RS, Graham ML et al (1992) Vitamin E in the treatment of chemotherapy-induced mucositis. Am J Med 92:481–484. https://doi.org/10.1016/0002-9343(92)90744-v
Puataweepong P, Dhanachai M, Dangprasert S, Sithatani C, Sawangsilp T et al (2009) The efficacy of oral Aloe vera juice for radiation induced mucositis in head and neck cancer patients: a double-blind placebo-controlled study. Asian Biomed 3:375–382. https://doi.org/10.5372/ABM.V3I4.233
de Freitas CL, Salum F, Cherubini K, Zancanaro de Figueiredo M (2015) Antioxidant agents: a future alternative approach in the prevention and treatment of radiation-induced oral mucositis? Altern Ther Health Med 21:36–41
Lin LC, Que J, Lin LK, Lin FC (2006) Zinc supplementation to improve mucositis and dermatitis in patients after radiotherapy for head-and-neck cancers: a double-blind, randomized study. Int J Radiat Oncol Biol Phys 65:745–750. https://doi.org/10.1016/j.ijrobp.2006.01.015
Dennert G, Horneber M (2006) Selenium for alleviating the side effects of chemotherapy, radiotherapy and surgery in cancer patients. Cochrane Database Syst Rev 2006(3):CD005037. https://doi.org/10.1002/14651858.CD005037.pub2
Moss RW (2007) Do antioxidants interfere with radiation therapy for cancer ? Integr Cancer Therapies 6:281–292. https://doi.org/10.1177/1534735407305655
Salehi B, Martorell M, Arbiser JL et al (2018) Antioxidants : positive or negative actors ? Biomolecules 8:1–11. https://doi.org/10.3390/biom8040124
Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP (1982) Toxicology and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5:649–655
Pandey KB, Rizvi SI (2010) Markers of oxidative stress in erythrocytes. Oxid Med Cell Longev 3:2–12. https://doi.org/10.4161/oxim.3.1.10476
Kellogg EW, Fridovich I (1977) Liposome oxidation and erythrocyte lysis by enzymically generated superoxide and hydrogen peroxide. J Biol Chem 252:6721–6728. https://doi.org/10.1016/s0021-9258(17)39909-x
He H, Oo TL, Huang W et al (2019) Nitric oxide acts as an antioxidant and inhibits programmed cell death induced by aluminum in the root tips of peanut (Arachis hypogaea L.). Sci Rep 9:1–12. https://doi.org/10.1038/s41598-019-46036-8
Mellors A, Tappel AL (1966) The inhibition of mitochondrial peroxidation by ubiquinone and ubiquinol. J Biol Chem 241:4353–4356. https://doi.org/10.1016/s0021-9258(18)99728-0
Ziberna L, Martelanc M, Franko M, Passamonti S (2016) Bilirubin is an endogenous antioxidant in human vascular endothelial cells. Sci Rep 6:1–6. https://doi.org/10.1038/srep29240
Parrow NL, Fleming RE, Minnick MF (2013) Sequestration and scavenging of iron in infection. Infect Immun 81:3503–3514. https://doi.org/10.1128/IAI.00602-13
Hall ED (1991) Inhibition of lipid peroxidation in CNS trauma. J Neurotrauma 8:S31-40
Tsuchiya M, Scita G, Freisleben H et al (1992) Antioxidant radical-scavenging activity of carotenoids and retinoids compared to alpha-tocopherol. Methods Enzym 213:460–472. https://doi.org/10.1016/0076-6879(92)13148-q
Gibbs PNB, Gore MG, Jordan PM (1985) Investigation of the effect of metal ions on the reactivity of thiol groups in human 5-aminolaevulinate dehydratase. Biochem J 225:573–580. https://doi.org/10.1042/bj2250573
Searle AJF, Tomasi A (1982) Hydroxyl free radical production in iron-cysteine solutions and protection by zinc. J Inorg Biochem 17:161–166. https://doi.org/10.1016/S0162-0134(00)80085-9
Abidi TF, Laskin JD, Conney AH (1991) Inhibitory effects of curcumin on in vitro lipoxygenase and cyclooxygenase activities in mouse epidermis. Cancer Res 51:813–819
Heijnen CGM, Haenen GRMM, Van Acker FAA et al (2001) Flavonoids as peroxynitrite scavengers: the role of the hydroxyl groups. Toxicol Vitr 15:3–6. https://doi.org/10.1016/S0887-2333(00)00053-9
Sakao K, Fujii M, Hou DX (2009) Clarification of the role of quercetin hydroxyl groups in superoxide generation and cell apoptosis by chemical modification. Biosci Biotechnol Biochem 73:2048–2053. https://doi.org/10.1271/bbb.90253
Singh RP, Dhanalakshmi S, Rao AR (2000) Chemomodulatory action of aloe vera on the profiles of enzymes associated with carcinogen metabolism and antioxidant status regulation in mice. Phytomedicine 7:209–219. https://doi.org/10.1016/S0944-7113(00)80006-9
Alencar SM, Oldoni TLC, Castro ML et al (2007) Chemical composition and biological activity of a new type of Brazilian propolis: red propolis. J Ethnopharmacol 113:278–283. https://doi.org/10.1016/j.jep.2007.06.005
Ahmed S, Othman NH (2013) Honey as a potential natural anticancer agent: a review of its mechanisms. Evid Based Complement Alternat Med 2013:829070. https://doi.org/10.1155/2013/829070
Pandi-Perumal SR, Trakht I, Srinivasan V et al (2008) Physiological effects of melatonin: role of melatonin receptors and signal transduction pathways. Prog Neurobiol 85:335–353. https://doi.org/10.1016/j.pneurobio.2008.04.001
Author information
Authors and Affiliations
Contributions
Drs. Raza, Karimyan, Emperumal, Al-Eryani, and Enciso contributed to the study conception and design. Material preparation and data collection were performed by Drs. Raza, Karimyan, and Emperumal under the supervision of Drs. Enciso and Al-Eryani. Dr. Enciso performed all data analyses. Drs. Raza, Karimyan, Emperumal, Al-Eryani, Waters, and Enciso contributed to the first draft of the manuscript and multiple revisions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This systematic review did not require any approval as it did not include any human or animal subjects.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Raza, A., Karimyan, N., Watters, A. et al. Efficacy of oral and topical antioxidants in the prevention and management of oral mucositis in head and neck cancer patients: a systematic review and meta-analyses. Support Care Cancer 30, 8689–8703 (2022). https://doi.org/10.1007/s00520-022-07190-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-022-07190-4