Abstract
Background
It is unknown how many distressed patients receive the additional supportive care recommended by Australian evidence-based distress management guidelines. The study identifies the (1) distress screening practices of Australian cancer services; (2) barriers to improving practices; and (3) implementation strategies which are acceptable to service representatives interested in improving screening practices.
Method
Clinic leads from 220 cancer services were asked to nominate an individual involved in daily patient care to complete a cross-sectional survey on behalf of the service. Questions related to service characteristics; screening and management processes; and implementation barriers. Respondents indicated which implementation strategies were suitable for their health service.
Results
A total of 122 representatives participated from 83 services (51%). The majority of respondents were specialist nurses or unit managers (60%). Approximately 38% of representatives’ services never or rarely screen; 52% who screen do so for all patients; 55% use clinical interviewing only; and 34% follow referral protocols. The most common perceived barriers were resources to action screening results (74%); lack of time (67%); and lack of staff training (66%). Approximately 65% of representatives were interested in improving practices. Of the 8 implementation strategies, workshops (85%) and educational materials (69%) were commonly selected. Over half (59%) indicated a multicomponent implementation program was preferable.
Conclusions
Although critical gaps across all guideline components were reported, there is a broad support for screening and willingness to improve. Potential improvements include additional services to manage problems identified by screening, more staff time for screening, additional staff training, and use of patient-report measures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Distress in cancer patients and survivors
Cancer-related distress is defined as a multifactorial unpleasant emotional experience of a psychological, social, or spiritual nature and interferes with the ability to cope effectively with the disease, its symptoms, and treatment [1]. A systematic review of symptom prevalence suggests approximately 40% of patients undergoing treatment will experience clinically significant distress [2]. Untreated distress is associated with poor outcomes such as decreased social functioning, more intense physical symptoms, cognitive impairment, poor adherence to treatment, and reduced length of life [3,4,5,6].
Identifying distress in cancer patients
Due to the complex and evolving nature of distress, health professionals often struggle to detect distress in time- or resource-poor clinical settings [7,8,9,10]. For example, distress was detected with only a sensitivity of 64% and a specificity of 65% by oncologists within a sample of 201 advanced cancer patients [11]. The distress levels of cancer survivors may also be poorly identified by health professionals; for example, a large study (n = 2642) of cancer survivors suggested as few as 1 in 10 survivors with distress symptoms were correctly identified by primary care physicians [12]. As health professionals can be inaccurate in gauging the presence and severity of distress, guidelines suggest all patients should be directly asked about emotional wellbeing and offered beneficial supportive care interventions as required [10].
Distress screening is the standardised assessment of patients with the intent to inform referral decisions, specifically if more extensive assessment and psychosocial support services are warranted [13]. Randomised controlled trials have established the importance of screening in reducing emotional distress and improving quality of life, physical symptoms such as pain and breathlessness, patient satisfaction, and patient-professional communication [14, 15]. Evidence from clinical settings suggests timely identification of distress is only effective in improving medical management and patient wellbeing when paired with structured supportive care referrals [10, 16,17,18,19].
Distress screening guidelines and evidence of guideline use
Internationally, there are numerous distress screening and management guidelines providing evidence-based models for application within cancer services. Australian examples include Cancer Australia’s Clinical Guidance for Responding To Suffering In Adults with Cancer [20] and the Clinical Pathway for the screening, assessment, and management of anxiety and depression in adult cancer patients [21]. International examples are outlined in the National Collaborative Cancer Network (NCCN) Guidelines [22], Pan-Canadian Practice Guidelines [23], and by the American Society of Clinical Oncology [24]. While there are some differences, there are four sequential components consistent across guidelines. Box 1 provides a simplified description of each step.
Box 1 Essential components of evidence-based distress screening and management
Screening: Document use of a validated brief screening tool wit clinically meaningful cut points at pivotal moments on cancer trajectories (i.e. diagnosis, treatment start, recurrence), with screening completed once for all patients independent of type/stage ideally within one month of diagnosis. Example of screening tools include the distress thermometer, and Edmonton Symptom Assessment Scale | |
Assessment: Patients who screen positively for distress complete a valid assessment to identify source, contributing factors, and severity of distress. Patients are assessed for depressive/anxiety symptomology. Example include clinical interview, Patient Health Questionnaire(PHQ-90),and General Anxiety Disorder scale(GAD-7) | |
Treatment: Based on assessment, patients are referred to low-, moderate-, or high-intensity psychosocial services. An established referral protocol is used, with stepped-care principles applied. Referral actions are documented. | |
Follow-up/re-assessment: Referral uptake is confirmed. After service referral/uptake, patients are reassessed. If distress is unremitting, additional are offered. Reassessment/referral is documented. |
Although forms of these guidelines have been available for more than a decade, there is evidence emerging that many cancer patients still do not receive evidence-based distress screening and management. Cross-sectional surveys conducted among US NCCN Institutions, which developed distress screening guidelines in 1999, reported only 50% routinely screened all outpatients in 2012 and 30% indicated that routine screening was not occurring in any form at their institution [25].
Although previous research has found that very brief screening takes less than two minutes in an oncology service, there are a number of barriers to implementation especially when considering the broader components of screening, assessment, treatment, and reassessment. For example, participants of a two-year training program in distress management reported experiencing over 65 barriers; the most common barriers included limited staff, staff turn-over, and competing demands [26]. Other well-documented implementation barriers include the lack of support services to action distress screening results [27].
Given these data, it is possible to hypothesise that distress screening rates are similarly low in Australia and there are several large barriers preventing implementation or quality improvement. Surveys of Australian patients receiving care within a small number of metropolitan cancer services found that just over half (57%) were asked about their emotional wellbeing and subsequently offered assistance [28]. Beyond this evidence from only a few sites, it is unclear if and how screening practices may differ for patients across Australian cancer services.
Improving integration of distress screening in all Australian services is an opportunity to improve patient outcomes and experiences and was identified as a top priority by psycho-oncology researchers and clinicians [29]. We conducted this national cross-sectional survey to provide information about the extent to which distress screening is currently integrated into Australian cancer services. Furthermore, this study explored the perceived acceptability of suggested methods for improvement across the four essential components of distress screening and management. Specifically, this study aimed to identify:
- 1.
The distress screening practices of Australian cancer services;
- 2.
The barriers to improving or implementing distress screening; and
- 3.
Whether quality improvement techniques, such as mentoring and bench-marking, are acceptable to service representatives who wish to improve screening rates.
Methods
Study design
This national cross-sectional survey of Australian cancer services’ application of distress screening and management guidelines followed the Strengthening The Reporting of Observational Studies in Epidemiology (STROBE) guidelines [30]. The Hunter New England Human Research Ethics Committee provided approval. Data collection was conducted from January 2017 to August 2017.
Cancer service identification
A list of Australian cancer services was compiled using publicly available information such hospital webpages and CanRefer, a state-specific directory of cancer services (New South Wales and Australian Capital Territory only). A total of 220 health services that provided some form of cancer care were identified. In order to canvas the wide array of services in which patients may receive care, no exclusion criteria related to facility size, staffing, location, or funding structure (i.e. private or public funded) were applied.
Participant recruitment and eligibility
Service managerial or clinical leads were sent a personalised email with a brief study introduction and asked to nominate a team member who could provide detailed information on daily screening practices. If the lead was also the best contact to provide this information, a copy of the survey was subsequently provided. Service leads who did not respond to the initial email received email and telephone reminders.
Nominated individuals were sent a personalised email informing them that they have been identified as a potential respondent on behalf of the health service. The email contained an embedded survey link. Individuals who did not respond to the initial email received email and telephone reminders. This two-staged recruitment approach was followed in order to ensure the most appropriate representative was approached with endorsement from clinical directors.
Study measure
The study-specific survey included three sections:
- 1.
Service characteristics. This included location; availability of mental health services; if public- and/or private-funded; and types of oncology departments if multiple (i.e. radiation, medical, surgical, or other). Respondents specified their role within the service. In the case respondents worked across multiple departments, respondents were asked if screening practices were similar across the departments and asked to complete the survey on behalf the department they were most familiar with.
- 2.
Screening practices. Items exploring screening practices were adapted from a previous US audit with permission [7]. Respondents were asked if they were aware of guidelines and if any patients are screened for distress. Depending on responses, individuals received additional items:
- a.
If distress screening was not conducted in any form, individuals selected from the following list of potential reason(s): not considered to be necessary or worthwhile; would like to but do not have the resources to complete screening; screening completed in another department; would like to but do not have the resources needed to treat the patients identified as distressed; would like to but unsure of how to integrate distress screening into routine care; or other.
- b.
If distress screening was conducted in any form, individuals reported if specific patient groups were screened; when screening was implemented and if practices had been evaluated; measure use; timing of screening; which, if any, health professionals are responsible for screening; rescreening intervals; and how the services used screening results to inform patient care.
- a.
- 3.
Preferred strategies to implement or improve screening practices. Respondents were asked to identify the barriers to implementing screening in their service from their personal perspective. To understand which techniques may be acceptable to service representatives wishing to improve or establish practices, respondents were asked which of eight specific implementation strategies they preferred. The strategies were identified from a systematic review of interventions to improve screening rates [13].
- 4.
This survey was pilot-tested with 10 health professionals.
Statistical analysis
Descriptive statistics were used to report survey data.
Results
Respondent and service characteristics
A total of 122 individuals participated from 83 services (51% response); 39 respondents provided details on a clinic in a multi-service site (i.e. medical oncology, radiation oncology). A total of 21 individuals did not identify their service within the survey.
Table 1 summarises the characteristics of survey respondents and their services. Briefly, all Australian states and territories were represented with the majority of services located in urban areas (63%). Just over half (53%) of the representatives’ services were publicly funded, and approximately a third (29%) were privately funded. While most (75%) representatives’ services had access to a mental health team, a relatively smaller percentage (34%) reported a dedicated psycho-oncology service. The most common service represented by participants was medical oncology (34%), followed by outpatient chemotherapy (19%). The most common type of respondent was oncology or specialist nurses (33%), nurse unit managers (30%), and care coordinators (17%).
Of the 122 representatives, 102 (84%) indicated they were directly involved in identifying or managing emotional distress as part of daily patient care. A total of 54 (44%) reported reading a distress screening guideline prior to survey participation, of which 13 (24%) reported these guidelines influenced their personal practice to a large degree and 26 (48%) to a small degree.
Distress screening practices
Although 22 (19%) representatives indicated their service never screened and 22 (19%) rarely screened cancer outpatients, the majority indicated their service usually or always screened (39% and 23%, respectively). Of the services that never screened, the most common reasons provided by representatives were uncertainty on how to integrate screening into routine workflows (37%), lack of time or resources to screen (27%), and lack of time or resources to action screening results (14%). Two service representatives indicated that the screening was done in a different department only, but neither had access to the screening results. None of the 22 service representatives indicated a lack of evidence of the benefits of screening as a reason for non-screening.
Of the 89 service representatives who reported some form of screening, the majority of representatives reported that the processes were not implemented as part of research studies (80%) but rather as a result of a service directive. According to most service representatives, screening practices had been implemented more than 12 months prior to survey completion (78%). Approximately 23% of representatives indicated that the processes had been evaluated since implementation. A complete description of screening processes reported by the 89 service representatives is included in Table 2 with a brief summary provided below:
Screening and assessment: Approximately 50% of representatives indicated their service screen all patients regardless of clinic or demographic characteristics, with 26% indicating only those patients who seem to be distressed are screened. A small proportion of representatives (13%) reported only those patients who see specific health professionals are screened. The most common screening technique was clinical conversation (55%) by a member of the treating team (e.g. nurse or oncologist) during a standard consultation, followed by a two-stage survey screen and interview assessment (29%). The common survey used was the Distress Thermometer and Problem Checklist.
Treatment: When asked what occurs to distressed cancer outpatients, representatives indicated support materials (63%) and a range of referrals were offered including community-based Cancer Council services (48%), pastoral care (30%), and mental health professionals (82%) of which the majority were social workers (53%). The results were also reviewed by the treating team as part of a standard consultation (36%). For the majority of services, representatives reported referrals were generated by health professionals without an available formalised protocol (66%).
Reassessment: Just over half (54%) of representatives reported rescreening cancer outpatients. Most service representatives reported completing this second screen at various times throughout the patient journey (69%).
Barriers to implementing or improving distress screening
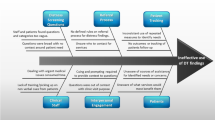
All service representatives were asked to indicate the minor and major barriers to implementing or improving distress screening practices (Fig. 1). Minor barriers were defined as those that would pose a challenge, whereas major barriers would prevent implementation. The lack of resources to action the screening results was the most commonly reported barrier (38% major barrier; 36% minor barrier) followed by lack of time to screen patients (24% major barrier; 39% minor barrier) and inadequate staff training to complete distress screening (19% major barrier; 44% minor barrier). The least common barriers were lack of evidence about the value of distress screening (5% major barrier; 27% minor barrier) and inability to record results in medical records (4% major barrier; 29% minor barrier).
Preferred strategies to implement or improve screening practices
Of the 122 service representatives, 78 (64%) indicated interest in improving or implementing screening practices. Commonly preferred implementation strategies among those 78 service representatives included workshops or educational meetings (85%); written educational materials (69%); computerised support (51%); additional financial resources (50%); audit or feedback (45%); reminders or prompts (41%); academic detailing (35%); and rewards or incentives (29%). Furthermore, 59% preferred to use multiple concurrent strategies as opposed to a single implementation strategy. There were no significant associations between respondents’ preferred implementation strategies or interest in improvement and the services’ current screening frequency (e.g. never, rarely, usually, or always).
Discussion
This cross-sectional survey study summarised distress screening and management practices reported by a range of cancer services representatives across Australia. Across the guideline components, there are key areas to improve the comprehensiveness and consistency of this practice for Australian cancer outpatients. At the most basic level, there were 22 services who have not yet started distress screening and a further 22 services who acknowledge only rarely screening patients—this represented 38% of participating service representatives. However, the results indicate that there is general support for screening by service representatives, with a small proportion of services regularly engaging in screening, referring patients who are distressed, and rescreening at least once during the patient journey. Additionally, the majority of service representatives reported that processes had been implemented as an internal service initiative at least a year prior to survey completion suggesting that distress screening is both a service priority and has been sustained. Reassuringly, in recognising the gaps across guideline components and with many representatives acknowledging the benefits of distress screening, a high proportion of service representatives reported interest in improving current practices.
Comparison of reported screening practices to other international audits
Other audits demonstrate similar opportunities for improvement [14, 31, 32]. Zebrack et al. [14] reviewed the electronic medical records of seven multidisciplinary cancer clinics to determine adherence to previously established screening and referral protocols. While adherence differed across clinics, approximately 48–73% of patients had screening results scanned into their records. Lazenby et al. [31] reported only 41% of cancer centres applying to be in an education program were screening prior to participating. A similar screening rate of 49% in the US Quality Oncology Practice Initiative within outpatient cancer services was reported by Chiang et al. [33].
First-stage screening with a patient self-report distress tool is needed
Our study results suggest that of the Australian services who screen with a survey tool, the majority use valid distress measures particularly the Distress Thermometer (90%). However, more than 50% of service representatives reported distress was identified via clinical interview by a member of the treating team without use of a standardised measure. This proportion is higher than that reported by 15 NCCN institutions in 2007 (38%) and 2013 (14%) [32, 34]. Reliance upon professionals’ ability to correctly identify distress is problematic with evidence of many distressed patients falling ‘between the cracks’ under this approach. Efforts to promote use of a standardised tool is an important first step in the distress management process [7,8,9,10]. When selecting a standardised tool, services must consider the clinical purpose of the tool beyond tokenistic screening and review the applicability to the service population including any culturally and linguistically diverse groups. As Australian communities become increasingly diverse, guidance on how to adapt screening processes to minority groups, who are often the most vulnerable to adverse health outcomes and experiences, is essential [35]. While this is beyond the scope of this study, inclusive cancer services are the focus of emerging Australian policy and research [35,36,37].
Consistent referral protocols with comprehensive training are required
In line with this study’s results, previous research also suggests that one of the greatest barriers to screening was the availability of referral options for those who screen positively [38]. Approximately 29% of distressed Australian cancer patients identified via the Distress Thermometer may require subsequent assessment and referrals [39]. While it is beyond the scope of this study to comment on whether improved screening practices would increase the number of referrals within participating services, two important issues must be noted.
Firstly, with increasing financial constraints, the availability and value of supportive care services such as social work, psychology, or psychiatry support must be carefully evaluated and demonstrated if ongoing or additional investment is required. Secondly, structured referral protocols are an ideal tool to ensure limited and costly services (typically higher intensity interventions such as counselling) are consistently and equitably targeted to those patients who would benefit the most. Unfortunately, the current study revealed a sub-optimal proportion of service representatives (28%) reported having an established referral protocol and this is a much-needed area of improvement requiring urgent intervention.
Through qualitative interviews with 12 Australian health professionals, Rankin et al. [40] outlined the barriers that are likely to be encountered when implementing a referral pathway for cancer-related anxiety and depression within Australian cancer services. Five implementation themes emerged in this qualitative work, of which three overlapped with the most common barriers reported by service representatives in our study. Firstly, the referral pathway must be owned and acceptable to the entirety of the team. Approximately 45% of service representatives indicated lack of staff commitment to distress screening was a minor or major implementation barrier. Additionally, 35% indicated that screening tasks were not specifically dedicated to any one member of the health care team with only 18% of physicians responsible for screening. These data suggest that screening and referral for emotional wellbeing and needs may be perceived as outside the remit of physicians with little team ownership.
Secondly, resources and responsibility for screening and management must be made explicit. Within this study, the two greatest barriers were lack of referral resources (74%) and time with patients (67%). Although executive support was not frequently perceived to be a barrier, these two barriers must be overcome through service redesign and commitment to expend additional resources on this aspect of patient care.
Thirdly, education and training was reported as an essential component to referral pathway implementation. This finding was echoed by service representatives of whom 64% indicated a lack of staff training was a barrier and 85% wished to attend a workshop. A systematic review of the effectiveness of clinical guideline implementation strategies reported passive educational strategies such as single didactic or group workshops were ineffective [41]. However, highly interactive educational strategies with opportunities to apply educational content to unique service contexts (i.e. role-playing) were effective with improvements in practice outcomes ranging from 1 to 39%. This more intensive approach will incur greater cost than passive information provision but, with greater effect, may provide a cost-benefit over a longer time period. Other strategies preferred by participants which were associated with some degree of effectiveness were computerised support and a reminder process. As multifaceted interventions were preferred by the majority of participants, a comprehensive implementation program should include interactive training paired with computerised support and feedback. Such programs are currently being tested in Australian settings [42, 43].
Practice implication: screening and management processes must be evaluated and continually re-enforced as best practice
This study found that very few representatives reported their service (22%) had evaluated distress practices and processes. Evaluation can assist to identify gaps in practice or issues that prevent distress screening from being implemented; similarly, evaluation can serve to streamline additional activities, such as distress screening, into routine workflows of all staff. Furthermore, evaluation of the patient and professional outcomes associated with distress screening can reinforce the value of this clinical activity to stakeholders including the individuals conducting the screening and administrators who have committed resources. Ongoing data demonstrating the benefits of this practice along with a training program may be essential to the maintenance of distress screening processes.
Limitations
This study provides a broadly scoped summary of health services’ practices and relied upon one individual to respond on behalf of the service. The data collection may have missed some of the complexities or knowledge of other team members. It is important to acknowledge that screening practices will likely differ within a service, and respondents may not be aware of their colleagues’ diverse screening practices. However, we tried to address this by designing the survey to enable participants to comment on how their personal practice or other health professionals’ practice differed within the service. Ten individuals commented on this, with the majority noting that screening is largely at the discretion of the health professional and is not yet formalised. Qualitative interviews with respondents who indicated variation or sub-optimal screening rates would be ideal to further elucidate the barriers to improving or implementing distress screening in their specific context.
This study was also subject to selection bias. There is limited or inconsistent information publically available on the type of services and representatives who did not participate in the study (i.e. funding structure, patient volume), and we cannot confidently compare these characteristics to respondents in order to assess generalisability. However, the 51% response rate is high for online surveys of health professionals and this is the first audit of this kind in Australia [44]. It is also possible that only those service representatives interested in distress screening were willing to participate, and therefore, the results may provide an overly positive description of current practices. Although it is not possible to assess the degree to which this selection bias occurred within the study, all services received an invitation to participate (as opposed to passive recruitment) and 38% of respondents indicated screening was never or rarely conducted.
The study recruitment approach was structured in a way to ensure only one contact per clinic service was approached to participate. There were 30 instances whereby two individuals from the same hospital responded but on behalf of different internal services; for example, one participant replied on behalf of the medical oncology service and one on behalf of the radiation oncology service within a large metropolitan cancer centre. However, in following ethical procedures, respondents could choose to omit any identifying service information. Therefore, 21 individuals did not directly specify their hospital and 9 individuals may have provided duplicated information (i.e. same clinic in a large hospital). Six respondents did not provide any identifying service information.
Conclusions
Improving integration of distress screening, referral and management into all Australian cancer services is an opportunity to improve patient outcomes [29]. This national cross-sectional survey study provided information on the degree to which distress screening is currently integrated in clinical practice. Although gaps existed across all guideline components, many services have started to screen outpatients. Potential areas of improvement were multifactorial and included additional services to manage problems identified by screening, more staff time for screening, additional staff training, and use of patient-report measures. However, despite these barriers, the majority of service representatives indicated a desire to improve current practice and portrays a positive climate for distress screening implementation in Australia.
References
National Comprehensive Cancer Network (2014) NCCN clinical practice guidelines in oncology: distress management (V2.2014)
Reilly CM, Bruner DW, Mitchell SA, Minasian LM, Basch E, Dueck AC et al (2013) A literature synthesis of symptom prevalence and severity in persons receiving active cancer treatment. Supportive care in cancer: official journal of the Multinational Association of. Supportive Care in Cancer 21(6):1525–1550
Skarstein J, Aass N, Fosså SD, Skovlund E, Dahl AA (2000) Anxiety and depression in cancer patients: relation between the HADS and the EORTC-Q. Journal of psychosomatic research 49(1):27–34
Fann JR, Thomas-Rich AM, Katon WJ, Cowley D, Pepping M, McGregor BA et al (2008) Major depression after breast cancer: a review of epidemiology and treatment. General hospital psychiatry. 30(2):112–126
Snyder CF, Garrett-Mayer E, Brahmer JR, Carducci MA, Pili R, Stearns V et al (2008) Symptoms, supportive care needs, and function in cancer patients: how are they related? Quality of Life Research. 17(5):665–677
Russ TC, Stamatakis E, Hamer M, Starr JM, Kivimäki M, Batty GD (2012) Association between psychological distress and mortality: individual participant pooled analysis of 10 prospective cohort studies. BMJ 345:e4933
Jacobsen PB, Lee M (2015) Integrating psychosocial care into routine cancer care. Cancer Control 22(4):442–449
Newell S, Sanson-Fisher RW, Girgis A, Bonaventura A (1998) How well do medical oncologists’ perceptions reflect their patients’ reported physical and psychosocial problems? Data from a survey of five oncologists. Cancer 83(8):1640–1651
Okuyama T, Akechi T, Yamashita H, Toyama T, Nakaguchi T, Uchida M et al (2011) Oncologists’ recognition of supportive care needs and symptoms of their patients in a breast cancer outpatient consultation. Jpn J Clin Oncol 41(11):1251–1258
Buxton D, Lazenby M, Daugherty A, Kennedy V, Wagner L, Fann JR et al (2014) Distress screening for oncology patients: practical steps for developing and implementing a comprehensive distress screening program. Oncology Issues 29(1):48–52
Gouveia L, Lelorain S, Brédart A, Dolbeault S, Bonnaud-Antignac A, Cousson-Gélie F et al (2015) Oncologists’ perception of depressive symptoms in patients with advanced cancer: accuracy and relational correlates. BMC Psychology 3(1):6
Werner A, Stenner C, Schuz J (2012) Patient versus clinician symptom reporting: how accurate is the detection of distress in the oncologic after-care? Psychooncology 21(8):818–826
McCarter K, Britton B, Baker A, Halpin S, Beck A, Carter G et al (2015) Interventions to improve screening and appropriate referral of patients with cancer for distress: systematic review protocol. BMJ Open 5(9)
Zebrack B, Kayser K, Sundstrom L, Savas SA, Henrickson C, Acquati C et al (2015) Psychosocial distress screening implementation in cancer care: an analysis of adherence, responsiveness, and acceptability. J Clin Oncol 33(10):1165–1170
Carlson LE, Waller A, Groff SL, Bultz BD (2013) Screening for distress, the sixth vital sign, in lung cancer patients: effects on pain, fatigue, and common problems--secondary outcomes of a randomized controlled trial. Psychooncology 22(8):1880–1888
Carlson LE, Groff SL, Maciejewski O, Bultz BD (2010) Screening for distress in lung and breast cancer outpatients: a randomized controlled trial. Journal of Clinical Oncology. 3698
Mustafa M, Carson-Stevens A, Gillespie D, Edwards AG (2013) Psychological interventions for women with metastatic breast cancer. The Cochrane Library.
Boyes A, Newell S, Girgis A, McElduff P, SANSON-FISHER R (2006) Does routine assessment and real-time feedback improve cancer patients’ psychosocial well-being? European journal of cancer care. 15(2):163–171
Mitchell AJ (2013) Screening for cancer-related distress: When is implementation successful and when is it unsuccessful? Acta Oncologica. 52(2):216–224
Cancer Australia (2014) Clinical guidance for responding to suffering in adults with cancer. Australian Government. https://guidelines.canceraustralia.gov.au/guidelines/guideline_22.pdf
Butow P, Price MA, Shaw JM, Turner J, Clayton JM, Grimison P et al (2015) Clinical pathway for the screening, assessment and management of anxiety and depression in adult cancer patients: Australian guidelines. Psychooncology 24(9):987–1001
Network NCC (2003) Distress management. Clinical practice guidelines. Journal of the National Comprehensive Cancer Network 1(3):344
Howell D, Keshavarz H, Esplen M, Hack T, Hamel M, Howes J et al (2015) Pan-Canadian Practice guideline: screening, assessment and management of psychosocial distress, major depression and anxiety in adults with cancer. Oncology
Andersen BL, DeRubeis RJ, Berman BS, Gruman J, Champion VL, Massie MJ et al (2014) Screening, assessment, and care of anxiety and depressive symptoms in adults with cancer: an American Society of Clinical Oncology Guideline Adaptation. Journal of Clinical Oncology 32(15):1605–1619
Lazenby M, Ercolano E, Grant M, Holland JC, Jacobsen PB, McCorkle R (2015) Supporting commission on cancer–mandated psychosocial distress screening with implementation strategies. J Oncol Pract 11(3):413–420
Knies AK, Jutagir DR, Ercolano E, Pasacreta N, Lazenby M, McCorkle R (2018) Barriers and facilitators to implementing the commission on cancer’s distress screening program standard. Palliat Support Care 1–9. https://doi.org/10.1017/S1478951518000378
Mitchell AJ (2013) Screening for cancer-related distress: when is implementation successful and when is it unsuccessful? Acta Oncol 52(2):216–224
Zucca A, Sanson-Fisher R, Waller A, Carey M, Fradgley E, Regan T (2015) Medical oncology patients: are they offered help and does it provide relief? J Pain Symptom Manag 50(4):436–444
Dzidowska M, Price M, Butow P (2010) Identifying research priorities and research needs among health and research professionals in psycho-oncology. Asia Pac J Clin Oncol 6(3):165–172
Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP et al (2014) The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. International Journal of Surgery. 12(12):1495–1499
Lazenby M, Ercolano E, Grant M, Holland JC, Jacobsen PB, McCorkle R (2015) Supporting commission on cancer–mandated psychosocial distress screening with implementation strategies. Journal of Oncology Practice. 11(3):e413–ee20
Jacobsen PB, Ransom S (2007) Implementation of NCCN distress management guidelines by member institutions. Journal of the National Comprehensive Cancer Network 5(1):99–103
Chiang AC, Buia Amport S, Corjulo D, Harvey KL, McCorkle R (2015) Incorporating patient-reported outcomes to improve emotional distress screening and assessment in an ambulatory oncology clinic. J Oncol Pract. 11(3):219–222
Donovan KA, Jacobsen PB (2013) Progress in the implementation of NCCN guidelines for distress management by member institutions. Journal of the National Comprehensive Cancer Network 11(2):223–226
Butow P (2015) Communicating with culturally and linguistically diverse patients in cancer care. Communicating Qual Saf Health Care 11:245
Green A, Jerzmanowska N, Thristiawati S, Green M, Lobb EA (2018) Culturally and linguistically diverse palliative care patients’ journeys at the end-of-life. Palliative & supportive Care:1–7
Gibson A, Lee C, Crabb S (2016) Representations of women on Australian breast cancer websites: Cultural ‘inclusivity’ and marginalisation. Journal of Sociology 52(2):433–452
Watts KJ, Good LH, McKiernan S, Miller L, O’Connor M, Kane R et al (2016) “Undressing” distress among cancer patients living in urban, regional, and remote locations in Western Australia. Supportive Care in Cancer 24(5):1963–1973
Clover K, Carter GL, Mackinnon A, Adams C (2009) Is my patient suffering clinically significant emotional distress? Demonstration of a probabilities approach to evaluating algorithms for screening for distress. Supportive care in cancer: official journal of the Multinational Association of. Supportive Care in Cancer 17(12):1455–1462
Rankin NM, Butow PN, Thein T, Robinson T, Shaw JM, Price MA et al (2015) Everybody wants it done but nobody wants to do it: an exploration of the barrier and enablers of critical components towards creating a clinical pathway for anxiety and depression in cancer. BMC Health Services Research 15:28
Prior M, Guerin M, Grimmer-Somers K (2008) The effectiveness of clinical guideline implementation strategies--a synthesis of systematic review findings. Journal of Evaluation in Clinical Practice 14(5):888–897
Turner J, Kelly B, Clarke D, Yates P, Aranda S, Jolley D et al (2017) A tiered multidisciplinary approach to the psychosocial care of adult cancer patients integrated into routine care: the PROMPT study (a cluster-randomised controlled trial). Supportive care in cancer: official journal of the Multinational Association of. Supportive Care in Cancer 25(1):17–26
Girgis A, Durcinoska I, Levesque JV, Gerges M, Sandell T, Arnold A et al (2017) eHealth System for collecting and utilizing Patient Reported Outcome Measures for Personalized Treatment and Care (PROMPT-Care) among cancer patients: mixed methods approach to evaluate feasibility and acceptability. Journal of Medical Internet Research 19(10):e330
Cho YI, Johnson TP, Vangeest JB (2013) Enhancing surveys of health care professionals: a meta-analysis of techniques to improve response. Evaluation & the Health Professions 36(3):382–407
Acknowledgements
Dr. Fradgley is a Career Advancement Fellow in Cancer Research supported by the Hunter Cancer Research Alliance.
Funding
Project funding was received by the Hunter Cancer Research Alliance with infrastructure support provided by the Hunter Medical Research Institute. Professor Christine Paul is supported by an NHMRC fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authorship team has no conflict of interests to declare. The authorship team has full control of all primary data and would allow the journal to review the de-identified data if requested.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fradgley, E.A., Byrnes, E., McCarter, K. et al. A cross-sectional audit of current practices and areas for improvement of distress screening and management in Australian cancer services: is there a will and a way to improve?. Support Care Cancer 28, 249–259 (2020). https://doi.org/10.1007/s00520-019-04801-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-019-04801-5