Abstract
Purpose
Cancer care team attitudes towards distress screening are key to its success and sustainability. Previous qualitative research has interviewed staff mostly around the startup phase. We evaluate oncology teams’ perspectives on psychosocial distress screening, including perceived strengths and challenges, in settings where it has been operational for years.
Methods
We conducted, transcribed, and analyzed semi-structured interviews with 71 cancer care team members (e.g., MDs, RNs, MSWs) at 18 Commission on Cancer-accredited cancer programs including those serving underrepresented populations.
Results
Strengths of distress screening identified by participants included identifying patient needs and testing provider assumptions. Staff indicated it improved patient-provider communication and other aspects of care. Challenges to distress screening included patient barriers (e.g., respondent burden) and lack of electronic system interoperability. Participants expressed the strengths of distress screening (n = 291) more than challenges (n = 86). Suggested improvements included use of technology to collect data, report results, and make referrals; complete screenings prior to appointments; longitudinal assessment; additional staff training; and improve resources to address patient needs.
Conclusion
Cancer care team members’ perspectives on well-established distress screening programs largely replicate findings of previous studies focusing on the startup phase, but there are important differences: team members expressed more strengths than challenges, suggesting a positive attitude. While our sample described many challenges described previously, they did not indicate challenges with scoring and interpreting the distress screening questionnaire. The differences in attitudes expressed in response to mature versus startup implementations provide important insights to inform efforts to sustain and optimize distress screening.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Distress has been defined as an “unpleasant experience of a psychological (i.e., cognitive, behavioral, emotional), social, spiritual, or physical nature that may interfere with one’s ability to cope effectively with cancer, it’s physical symptoms, and its treatment” [1]. Physical symptoms and side effects, psychological issues, and social problems are common among patients with cancer and may cause distress [2]. Patients with cancer who are vulnerable to socioeconomic stressors often experience greater distress from unmet needs [3]. Programs that screen for distress and address unmet needs can effectively improve care quality, especially when staff are well-aligned and supported in this role [4].
Guidance for managing cancer patient distress has been provided in standards developed by the National Comprehensive Cancer Network (NCCN) and the Commission on Cancer (CoC) [1, 5]. These standards suggest screening cancer patients for distress at pivotal visits using patient-reported outcome measures (PROMs) [1, 5]. The PROMs used for distress screening may be a brief measure of distress, like the Patient Health Questionnaire-2 (PHQ-2), or longer, multidimensional instruments such as the NCCN Distress Thermometer (DT) and problem list, which includes distress and a number of cancer-related symptoms and issues. Once issues are identified by screening, they can be addressed by the cancer care team to improve patients’ quality of life, functioning, and treatment adherence.
The practice of distress screening is widespread, due in part to the Commission on Cancer’s (CoC) Standard 3.2 for psychosocial distress screening, which is required at over 1,500 CoC-accredited cancer programs which see approximately 72% of incident cancer cases in the USA [5, 6]. Reflecting this, recent studies of oncology practice staff [7] and cancer programs [8] have found that PROMs are commonly used for distress screening in these settings. Despite widespread and increasing use, research on the real-world use of distress screening and other clinical PROMs in routine practice is limited [1, 9, 10]. Such research could inform efforts to establish, optimize, and maintain their use. Studies are needed to describe provider perspectives on clinical PROM use, including real and perceived barriers to and facilitators of their use [11].
A recent review of qualitative studies of cancer team perspectives on clinical PROM use, including distress screening, found overall sentiment was neutral [12]. Prevalent barriers to distress screening and other clinical PROMs include added demands on staff time and staff’s lack of knowledge and skills to address identified problems [1, 11, 12]. Primary facilitators included staff perception of the benefits of PROM use and training [1, 11, 12]. However, such studies have typically been conducted at single, academic institutions, and the degree to which they care for underserved population is not reported [12, 13] limiting their generalizability to other settings.
Furthermore, most studies have focused on early adoption (e.g., program development, feasibility, or setup) [12, 13]. The issues relevant to early adoption of interventions often differ from those important to sustaining and optimizing established interventions [14]. The CoC distress screening standard requires use of PROMs for distress screening at a pivotal visit and has been operational since 2015 [5], making CoC-accredited facilities good candidates to study cancer team perspectives towards well-established clinical PROM interventions.
This paper reports oncology teams’ perspectives, including perceived barriers and facilitators, on psychosocial distress screening in settings where it has been operational for a sustained time. Further, we oversampled facilities caring for underserved populations.
Methods
Sterling IRB provided ethical review of this study (IRBID: 6308).
Facility eligibility and recruitment
Eligible cancer centers were one of the five most common types of CoC-accredited facilities (Table 1). We used CoC-defined facility types, which vary according to structure, services provided, and the number of cases accessioned each year [5].
An announcement describing the study and soliciting volunteer facilities was emailed to staff occupying five CoC-defined roles [5]—the Cancer Committee Chair, the Cancer Liaison Physician, the Cancer Program Coordinator, the Quality Improvement Coordinator, and the Psychosocial Distress Coordinator—at all CoC-accredited facilities (n = 1253, Table 1). It indicated that we sought facilities with a range of PROM use from basic to sophisticated, as well as those serving underserved populations. Interested facilities were asked to email a brief, written description of their clinical PROMs. Fifty eligible facilities responded to the study invitation. Volunteers were stratified by facility-type, those whose only cancer clinical PROM was distress screening versus those with additional cancer clinical PROMs, and those with a higher proportion of uninsured/Medicaid patients (to represent facilities serving underserved populations). Facilities were randomly selected, oversampling those with underserved populations.
Cancer team recruitment
To be eligible for interviews, facility staff occupied one of the five roles mentioned above or were selected as an alternate with similar professional characteristics (see Appendix 1 for characteristics and rationale). Local site coordinators obtained agreement from four eligible stakeholders at each site, sent an email introducing the study, provided the consent form, and introduced the interviewer. Seventy-one stakeholders completed interviews, one of which was not successfully recorded. Interviewees’ professions were physician (n = 23), nurse (n = 22), mental health professional (n = 13), clerical staff (n = 6), administrator (n = 5), and physical therapist (n = 1).
Interviews
The semi-structured interview guide was developed based on the literature and the expertise of all authors, pre-tested in two interviews, reviewed, and revised. The revised guide (Appendix 2) used for all subsequent interviews had a warm-up followed by questions and prompts about distress screening strengths, challenges, clinicians’ attitudes towards distress screening, and ways to improve distress screening at their institution. Interviews were conducted via telephone and recorded.
Analyses
Each interview recording was transcribed verbatim. Text was coded at the sentence level and counted at the paragraph level. More than one code could be applied to a paragraph. Data were coded using exploratory multiple case study [15] and line-by-line open coding techniques [16]. Codes were added or modified as necessary as new meanings emerged [17]. Using a constant comparison method [16], each piece of text was systematically compared to the codes. Codes and their assignment to text were checked and rechecked to optimize consistency. Once the data were coded, codes were compared to identify themes and topics. Negative case analysis [18] was used to check code affiliation with themes and topics and consider alternative explanations when they did not fit. Interpretations of the themes were checked against the raw data [18]. The coding system was developed by authors AB and TS. AB coded all transcripts. TS reviewed 20% of transcripts (n = 14), all themes and topics. Disagreements were resolved through discussion. NVivo 12 qualitative data analysis software was used [19].
Results
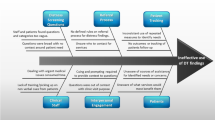
In accordance with CoC standards, all 18 participating facilities used PROMs for psychosocial distress screening. The PROMs used for distress screening by participating facilities were the NCCN Distress Thermometer (n = 23) or a version of the Patient Health Questionnaire (PHQ, n = 9), the most common instruments used by CoC facilities [20]. Results are reported as codes grouped into themes within topics (Fig. 1, Topic II. Challenges). In some cases, no themes emerged within a topic (Fig. 1, VI. Distress screening training).
Topic I. Strengths
Participants described the Strengths (Table 2, Topic I) of distress screening 291 times in 12 separate codes, making it the most expressed topic. Codes were organized into four themes. The first theme, Clinician Awareness of Patient Needs I (I.A. n = 85), indicated that stakeholders believed distress screening allowed clinicians to test their assumptions about and improve awareness of patients’ needs. As one participant stated, “It’s a nice way to make sure that people don’t fall through the cracks. My personal experience with it is that sometimes we assume that someone who looks like they have it all together, and that’s a costly assumption to make.” Participants indicated that distress screening enabled clinicians to Identify Patient Needs (code I.A.1. n = 58) directly, the most common code. Some participants focused specifically on the ability of distress screening to Identify Patient Emotional Needs (I.A.2. n = 13). Participants stated that distress screening can Help Patients Express Needs (I.A.3. n = 14) they might not otherwise feel comfortable conveying, possibly for cultural reasons, which also improves clinician awareness of patients’ needs.
The most frequently expressed theme was Clinical Benefits (I.B. n = 109), which entailed four codes. First, participants felt that distress screening enables clinicians to provide Comprehensive Care (I.B.1. n = 27) by identifying and meeting patients’ physical and other needs such as emotional distress, finances, childcare, transportation, and relationships. This code was also expressed as treating the whole person, not just their cancer or physical problems. Participants indicated that, by identifying potential barriers to cancer care, distress screening enabled them to address those issues and Increase Treatment Adherence (I.B.2. n = 29). Another clinical benefit participants expressed is the ability to Improve Coordination of Care (I.B.3. n = 38) among practitioners from multiple disciplines, which might not otherwise communicate and work collaboratively to meet patients’ needs. Finally, participants thought that distress screening enables clinicians to Detect Problems Early (I.B.4. n = 15) before they potentially worsen.
The third theme was Enhance Patient-Clinician Communication (I.C. n = 49). Participants noted that distress screening improves patient-clinician communication by creating a climate where Patients Open to Voice Concerns (I.C.1. n = 23). Participants also described that clinicians use distress screening results to Guide Conversations and Save Time (I.C.2. n = 16) by allowing them to target each patient’s specific needs. Participants expressed that using distress screening Clinicians Show Caring (I.C.3. n = 10) by eliciting and responding to patient needs in a caring manner, which may create a supportive environment.
The fourth theme involved increased Patient Awareness (I.D. n = 48). Participants indicated that distress screening improves Patient Awareness of Their Own Needs (I.D.1. n = 37). As a result, screening enables the cancer care team to improve Patient Awareness of Resources (I.D.2. n = 11), which are often available at the treating facility for both medical and non-medical needs.
Topic II. Challenges
Challenges (II.) described by participants were organized into two themes. The first theme was Patient Barriers (II.A. n = 58). Participants described that some patients complain of excessive paperwork, either in the context of intake packets, which include the distress screener, or when screening is administered at every visit (II.A.1. n = 16). Participants indicated that, while most patients are willing and interested in completing distress screening instruments, in some cases, Patient Resistance (II.A.2. n = 21) is a challenge. This resistance may take the form of patients simply refusing to complete the form or completing the form but underreporting their distress or problems. Several participants noted that resistance is more common in older patients who seem reticent to discuss their emotions and problems. Participants reported that sometimes patient Cultural, Language and Literacy Barriers (II.A.3. n = 21) posed a challenge in terms of the patient’s willingness to complete the distress screening instrument or language and literacy problems. One participant noted that the absence of non-English versions of the screening instrument was a problem. Others noted that patients with language or literacy barriers might engage family members or staff to help them read and interpret the questions, which may sometimes result in responses that do not reflect the patient’s needs.
When queried about technical barriers to distress screening, many participants indicated their use of paper and pencil forms led to No Challenges (II.B.1. n = 33) with technology. Some participants noted problems with Poor EMR Interoperability (II.B.2. n = 7). Specifically, different electronic medical record systems are often siloed and are not inter-operational, thus posing challenges to users attempting to access or integrate information across systems. Lack of electronic medical record integration can also create workflow inefficiencies, requiring users to register patients in two systems.
Code II.B.1., No Challenges, is not a challenge. Some challenges were identified under other topics. The total number of challenges expressed, 86, comes from summing six codes: II.A.1. Too Much Paperwork, Screening Fatigue; II.A.2. Patient Resistance; II.A. 3. Cultural, Language and Literacy Barriers; II.B.2. Poor Interoperability Between Electronic Medical Record Platforms; III.A.2. Burden on Time; and III.A.3. Lack of Automatic Referrals.
Topic III. Integration into charts and workflow
Participants’ comments about integrating distress screening results into charts and workflow (III. N = 85) had four codes, organized into two themes. While acknowledging that distress screening took some time, many participants indicated that it was Well-Integrated (III.A.1. n = 23) into the workflow and posed minimal to no burden. In contrast, others noted the Burden on Time (III.A.2. n = 11) imposed by distress screening. Examples included the time it takes to administer the instrument and input the information into electronic systems and extra time for patients with high distress or multiple needs. A few participants indicated that Lack of Automatic Referrals (III.A.3. n = 10) led to inefficiencies. Many participants thought there were No Challenges with Scoring and Reporting (III.B.1. n = 41) distress screening. Instead, they felt the distress screening tool was straightforward and easy to score and report.
Topic IV. Clinician attitudes about distress screening
When asked to describe clinician attitudes about distress screening at their facility, participants most often indicated that clinicians had Positive Attitudes (IV.1. n = 41) and understood its value as a tool that provides important information about their patients. However, some comments reflect Mixed Attitudes (IV.2. n = 10) indicating that, while most clinicians valued distress screening, some were less engaged with it or had concerns about time requirements or being sued for failing to follow-up on an identified problem. Finally, some participants stated the reasons some clinicians were Not Supportive (IV.3. n = 11) of distress screening due to time requirements or feeling it is unnecessary.
Topic V. Suggestions for improving distress screening
When asked to suggest improvements to distress screening at their facility, some participants had No Suggestions (V.1. n = 10) because the screening processes were good. The most common suggestion was Integrating Technology (V.2. n = 19) by transitioning from paper to electronic data collection and reporting; this would save staff time, making the process more efficient. (Four of the 18 cancer centers used electronic methods for distress screening.) Patients Completing Screening before Appointments (V.3. n = 7), possibly electronically at home, was suggested to improve workflow and reduce delays in appointments. Participants from facilities that screened only once suggested Longitudinal Assessment (V.4. n = 10), while others suggested standardizing the pattern of longitudinal screening. More Training (V.5. n = 7) was also suggested, not only on the screening instrument but also on resources for providing comprehensive care. Finally, participants suggested hiring More Supportive Care Personnel (V.6. n = 7).
Topic VI. Distress screening training
When asked about distress screening training, some participants described Formal Training (VI.1. n = 26) including coursework in school, certification training, and training offered by clinical cancer committee members. Informal Training (VI.2. n = 44)—including on-the-job training by a predecessor, online webinars, staff meetings, reading literature, and training during employee orientation—was most commonly mentioned. Some participants indicated that they received No Training (VI.3. n = 11) and had to learn about the distress screening on their own.
During stakeholder interviews, the most commonly mentioned reason for patient referral based on distress screening was financial issues (n = 46), followed by emotional concerns (n = 19), and transportation problems (n = 15). Regarding financial issues, stakeholders indicated that patients were concerned about paying for gas and transportation, insurance coverage, out-of-pocket costs, medication costs, and monthly bills while receiving treatment.
Discussion
In this paper, we described cancer care team members’—doctors, nurses, social workers, medical assistants, and others—attitudes towards distress screening at their institution. Participants expressed more strengths (n = 291) than challenges (n = 86) of distress screening, and perceptions of positive clinician attitudes towards screening (n = 41) outweighed mixed (n = 10) and negative (n = 11) ones, suggesting that our sample had an overall positive attitude towards screening. Previous qualitative research found that health providers had an overall neutral sentiment towards clinical PROMs, representing a mix of positive, neutral, and negative attitudes [12]. This discrepancy may be related to participants in our study reporting on well-established implementations, rather than those in early adoption as seen in most other research [12]. Well-established implementations have had time to optimize processes, train staff, and gain acceptance, which could reduce reported challenges. Staff may have experienced benefits, and the CoC standard suggests widespread acceptance, which could increase reported strengths. In contrast, new implementations have challenges and barriers unique to clinical PROMs [12] and, more generally, the tendency of staff to resist any new process as described in the field of change management [21]. Our observation aligns with findings that institutional support increased during the first couple of years of distress screening implementation [11]. Cancer teams’ positive attitude towards and belief in distress screening benefits are important factors in its success and sustainability [1, 11, 12, 14, 21].
The benefits of distress screening described by participants often aligned with its core purpose [1, 5]. Identifying patient needs (Codes I.A.1.–I.A.2.) is a core function of distress screening, which empowers patients to influence their care [1]; participants saw this as a benefit, which included helping patients become aware of and express their needs (Code I.A.3.). Participants thought distress screening enabled comprehensive care (I.B.1.), treating the whole patient, not just the cancer. Furthermore, participants noted that distress management facilitated the engagement of relevant specialists when needed, thus improving care coordination (I.B.3.), a benefit noted elsewhere [12]. Participants indicated that distress screening improves adherence to cancer treatment (I.B.2.), as shown in a study of clinical PROMs [22]. Improved patient-clinician communication was a benefit identified by participants (I.C.1.–I.C.3.), which is fundamental to distress management and well documented in the literature on clinical PROMs [1, 23].
Challenges to distress screening noted by participants in our study included excessive paperwork, in general (II.A.1.); overly frequent distress screening (II.A.1.); and patient resistance to completing screening due to the stigma of mental health issues or cultural and language barriers (II.A.2.–II.A.3). These barriers are noted elsewhere in the literature [1, 12]. Linguistic barriers can be addressed to some degree by using standard measures such as the NCCN DT and the PHQ-9, which are available in many languages. Some barriers expressed in previous studies of attitudes towards clinical PROMs— e.g., concerns about scoring/reporting results, resources for addressing detected problems [12]— were less evident in our sample (III.B.1.). Unlike previous studies that focused on early stages of PROM adoption [12], the well-established distress management protocols reported here may have addressed these concerns during earlier stages of implementation, in part by using measures with well-established clinical cutoffs.
A few participants in our study indicated that distress screening was unnecessary because they already collected this information in other ways (IV.3.), an attitude expressed in a few other studies [12]. However, most participants in our and other studies [12] indicate that distress screening plays a critical role in ensuring important problems experienced by patients are detected (IV.1.). This view of the importance of PROMs in detecting symptoms and problems is supported by research demonstrating that clinician assessment without PROMs often underestimates patients’ symptoms and needs [24, 25].
Suggestions for improving distress screening included patients completing screening at home before the appointment (V.3.), having regularly scheduled longitudinal assessments (V.4.), and adding supportive care staff (V.6.). Another suggestion involved training in screening and follow-up of detected problems or symptoms (V.5.), which is key to optimal implementation [1, 11, 12]. Low-cost approaches to addressing follow-up of positive findings include encouraging clinician use of symptom management guidelines [26], pocket guides [27], and lists of local resources. More formal training or education might match staff responsibilities; clerical staff could receive education on conducting assessments, while physicians develop skills to discuss results with patients and address needs.
The most common suggestion for improvement was transitioning to electronic PROs (ePROs) (V.2.), which can reduce staff and patient burden and enable remote assessment between clinic visits [28,29,30]. Other participants noted ePROs poor integration with electronic health records (EHRs) (II.B.2.) and lack of automatic referrals lowered efficiency (III.A.3.). This suggests that ideal ePRO systems automate data capture and reporting, provide support and referrals for detected problems, and integrate with EHRs. Strategies for supporting ePROs may vary between major cancer centers and smaller, less well-resourced centers.
The rich data we present should be interpreted in light of several strengths and limitations. Oversampling facilities with underserved populations provided a multi-institutional sample of 70 stakeholders from a range of settings and professional backgrounds ensuring representation of diverse perspectives. Our sample size was larger than most qualitative studies [31, 32], improving the likelihood that we captured all pertinent perspectives. Facility implementation of the CoC distress standard provided some uniformity across facilities. Due to possible sampling bias, the degree to which this sample represents all CoC facilities is unclear. Additionally, distress screening occurs at non-CoC facilities not included in this study.
Conclusion
Our rich data suggest that many cancer care team members see the value of distress screening. Now that distress screening has been in place for several years, successful demonstrations of its implementation may have diminished concerns about the feasibility of integrating it effectively into workflow and follow-up of detected symptoms. However, efforts are needed to increase the use of ePROMs, better integrate screening into electronic medical record systems, increase the availability of training in distress screening, and ensure that resources are available to manage identified problems.
Data availability
Research data are not shared.
Code availability
NVivo 12 qualitative data analysis software was used.
References
NCCN Clinical Practice Guidelines in Oncology: Distress Management (version 2). National Comprehensive Cancer Network
American Cancer Society (2019) Cancer treatment & survivorship facts & figures 2019–2021. American Cancer Society
Carlson LE, Waller A, Mitchell AJ (2012) Screening for distress and unmet needs in patients with cancer: review and recommendations. J Clin Oncol 30:1160–1177. https://doi.org/10.1200/JCO.2011.39.5509
Cimino T, Said K, Safier L et al (2020) Psychosocial distress among oncology patients in the safety net. Psychooncology. https://doi.org/10.1002/pon.5525
(2016) Cancer Program Standards: Ensuring Patient-Centered Care. American College of Surgeons Commission on Cancer
Mallin K, Browner A, Palis B et al (2019) Incident cases captured in the national cancer database compared with those in U.S. population based central cancer registries in 2012–2014. Ann Surg Oncol 26:1604–1612. https://doi.org/10.1245/s10434-019-07213-1
Zhang B, Lloyd W, Jahanzeb M, Hassett MJ (2018) Use of patient-reported outcome measures in quality oncology practice initiative-registered practices: results of a national survey. J Oncol Pract 14:e602–e611. https://doi.org/10.1200/JOP.18.00088
Smith TG, Bontemps-Jones J, James TA, McCabe RM Feasibility and utility of abstracting data from policy and procedure documents to describe real world distress screening: a case study of 18 U.S. Cancer Centers. Submitted
Anatchkova M, Donelson SM, Skalicky AM et al (2018) Exploring the implementation of patient-reported outcome measures in cancer care: need for more real-world evidence results in the peer reviewed literature. J Patient Rep Outcomes 2:64. https://doi.org/10.1186/s41687-018-0091-0
Howell D, Molloy S, Wilkinson K et al (2015) Patient-reported outcomes in routine cancer clinical practice: a scoping review of use, impact on health outcomes, and implementation factors. Ann Oncol 26:1846–1858. https://doi.org/10.1093/annonc/mdv181
Knies AK, Jutagir DR, Ercolano E et al (2019) Barriers and facilitators to implementing the commission on cancer’s distress screening program standard. Palliat Support Care 17:253–261. https://doi.org/10.1017/S1478951518000378
NicGiollaEaspaig B, Tran Y, Bierbaum M et al (2020) What are the attitudes of health professionals regarding patient reported outcome measures (PROMs) in oncology practice? A mixed-method synthesis of the qualitative evidence. BMC Health Serv Res 20:102. https://doi.org/10.1186/s12913-020-4939-7
Boyce MB, Browne JP, Greenhalgh J (2014) The experiences of professionals with using information from patient-reported outcome measures to improve the quality of healthcare: a systematic review of qualitative research. BMJ Qual Saf 23:508–518. https://doi.org/10.1136/bmjqs-2013-002524
Chambers DA, Glasgow RE, Stange KC (2013) The dynamic sustainability framework: addressing the paradox of sustainment amid ongoing change. Implement Sci 8:117. https://doi.org/10.1186/1748-5908-8-117
Yin RK (2002) Case study research: design and methods, 3rd edn. SAGE Publications Inc, Thousand Oaks
Glaser BG, Strauss AL (2017) Discovery of grounded theory: strategies for qualitative research. Routledge
Schilling J (2006) On the pragmatics of qualitative assessment: designing the process for content analysis. Eur J Psychol Assess 22:28–37. https://doi.org/10.1027/1015-5759.22.1.28
Lincoln YS, Guba EG Naturalistic Inquiry. SAGE Publications, Newbury Park
(2018) NVivo qualitative data analysis software. QSR International Pty Ltd
Miller NS, McCabe RM, Knutson A (2016) The Commission on Cancer Psychosocial Distress Screening Standard: the first year in review. JCO 34:198–198. https://doi.org/10.1200/jco.2016.34.3_suppl.198
Piderit SK (2000) Rethinking resistance and recognizing ambivalence: a multidimensional view of attitudes toward an organizational change. AMR 25:783–794. https://doi.org/10.5465/amr.2000.3707722
Basch E, Deal AM, Kris MG et al (2016) Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol 34:557–565. https://doi.org/10.1200/JCO.2015.63.0830
Chen J, Ou L, Hollis SJ (2013) A systematic review of the impact of routine collection of patient reported outcome measures on patients, providers and health organisations in an oncologic setting. BMC Health Serv Res 13:211. https://doi.org/10.1186/1472-6963-13-211
Atkinson TM, Ryan SJ, Bennett AV et al (2016) The association between clinician-based common terminology criteria for adverse events (CTCAE) and patient-reported outcomes (PRO): a systematic review. Support Care Cancer 24:3669–3676. https://doi.org/10.1007/s00520-016-3297-9
Newell S, Sanson-Fisher RW, Girgis A, Bonaventura A (1998) How well do medical oncologists’ perceptions reflect their patients’ reported physical and psychosocial problems? Data from a survey of five oncologists. Cancer 83:1640–1651
NCCN Guidelines for Supportive Care. In: National Comprehensive Cancer Network. https://www.nccn.org/professionals/physician_gls/default.aspx#supportive. Accessed 10 Jul 2020
Managing Symptoms, Side Effects & Well-Being. In: Cancer Care Ontario. https://www.cancercareontario.ca/en/symptom-management. Accessed 3 Jul 2020
Jensen RE, Snyder CF, Abernethy AP et al (2014) Review of electronic patient-reported outcomes systems used in cancer clinical care. J Oncol Pract 10:e215-222. https://doi.org/10.1200/JOP.2013.001067
Jensen RE, Rothrock NE, DeWitt EM et al (2015) The role of technical advances in the adoption and integration of patient-reported outcomes in clinical care. Med Care 53:153–159. https://doi.org/10.1097/MLR.0000000000000289
Johansen MA, Henriksen E, Horsch A et al (2012) Electronic symptom reporting between patient and provider for improved health care service quality: a systematic review of randomized controlled trials. part 1: state of the art. J Med Internet Res 14:e118. https://doi.org/10.2196/jmir.2214
Vasileiou K, Barnett J, Thorpe S, Young T (2018) Characterising and justifying sample size sufficiency in interview-based studies: systematic analysis of qualitative health research over a 15-year period. BMC Med Res Methodol 18. https://doi.org/10.1186/s12874-018-0594-7
Marshall B, Cardon P, Poddar A, Fontenot R (2015) Does sample size matter in qualitative research?: a review of qualitative interviews in is research. J Comput Inform Syst 54:11–22. https://doi.org/10.1080/08874417.2013.11645667
Acknowledgements
We appreciate cancer center staff who participated in this study. The content of this publication does not necessarily reflect the views of the American Cancer Society.
Funding
This study is intramurally funded by the American Cancer Society.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
The Sterling Institutional Review Board determines this study (IRBID: 6308) as exempt from IRB review because participant interview responses gathered during this study would not “reasonably place the subjects at risk of criminal or civil liability or be damaging to the subjects’ financial standing, employability, or reputation” in the unlikely event they were accidentally disclosed outside of research.
Consent to participate
A consent form was emailed to participants and verbal informed consent was obtained prior to the interview.
Consent for publication
The consent form and verbal consent included consent to publish data captured during the interviews.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Smith, T.G., Beckwitt, A.E., van de Poll-Franse, L.V. et al. Oncology team perspectives on distress screening: a multisite study of a well-established use of patient-reported outcomes for clinical assessment. Support Care Cancer 30, 1261–1271 (2022). https://doi.org/10.1007/s00520-021-06458-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-021-06458-5