Abstract
Purpose
Pegfilgrastim was introduced over a decade ago. Other long-acting granulocyte colony-stimulating factors (G-CSFs) have recently been developed. We systematically reviewed the efficacy, effectiveness and safety of neutropenia prophylaxis with long-acting G-CSFs in cancer patients receiving chemotherapy.
Methods
We performed a systematic literature search of the MEDLINE, EMBASE and Cochrane Library databases, and abstracts from key congresses. Studies of long-acting G-CSFs for prophylaxis of chemotherapy-induced neutropenia (CIN) and febrile neutropenia (FN) were identified by two independent reviewers. Abstracts and full texts were assessed for final inclusion; risk of bias was evaluated using the Cochrane’s tool. Effectiveness and safety results were extracted according to study type and G-CSF used.
Results
Of the 839 articles identified, 41 articles representing different studies met the eligibility criteria. In five randomised controlled trials, 11 clinical trials and 17 observational studies across several tumour types and chemotherapy regimens, pegfilgrastim was used alone or compared with daily G-CSF, no G-CSF, no upfront pegfilgrastim or placebo. Studies generally reported lower incidence of CIN (4/7 studies), FN (11/14 studies), hospitalisations (9/13 studies), antibiotic use (6/7 studies) and adverse events (2/5 studies) with pegfilgrastim than filgrastim, no upfront pegfilgrastim or no G-CSF. Eight studies evaluated other long-acting G-CSFs; most (5/8) were compared to pegfilgrastim and involved patients with breast cancer receiving docetaxel-based therapy. Efficacy and safety profiles of balugrastim and lipegfilgrastim were comparable to pegfilgrastim in phase 3 studies. Efficacy and safety of other long-acting G-CSFs were mixed.
Conclusions
Pegfilgrastim reduced the incidence of FN and CIN compared with no prophylaxis. Most studies showed better efficacy and effectiveness for pegfilgrastim than filgrastim. Efficacy and safety profiles of lipegfilgrastim and balugrastim were similar to pegfilgrastim.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In patients with cancer receiving cytotoxic chemotherapy, chemotherapy-induced neutropenia (CIN) and febrile neutropenia (FN) are frequent complications. CIN is graded according to severity of the reduction of the absolute neutrophil count (ANC) and FN is commonly defined as ANC <0.5 × 109/L with an oral temperature ≥38 °C for more than 1 h [1]. Patients experiencing neutropenic events are more susceptible to subsequent infections [2]. As a consequence of FN, patients often require hospitalisation and antibiotic treatment and frequently have their chemotherapy dose reduced or delayed [3, 4]. Modifications to chemotherapy may decrease its effectiveness, thereby potentially compromising treatment outcomes [4].
Granulocyte colony-stimulating factors (G-CSFs) stimulate the production and maturation of neutrophils during chemotherapy and reduce the incidence and duration of CIN and incidence of FN [5, 6]. Prophylactic G-CSF use from the first cycle of chemotherapy is recommended by the European Organisation for Research and Treatment of Cancer [7] and other international guidelines [1, 8, 9] if the planned chemotherapy regimen is associated with an FN risk of 20 % or more. For chemotherapy regimens with an intermediate FN risk of 10–20 %, guidelines recommend that patient-related and disease-related factors should also be considered when deciding whether or not to give G-CSF support.
Daily G-CSFs are primarily cleared through the kidneys and require dosing until recovery of the neutrophil count. Long-acting G-CSFs are primarily cleared by neutrophils and have significantly reduced renal clearance compared with daily G-CSFs. They therefore require only a single dose per chemotherapy cycle. Pegfilgrastim (Neulasta®; Amgen Inc., CA, USA), consisting of the human recombinant G-CSF filgrastim pegylated at the N terminus with a 20-kDa polyethylene glycol molecule, is administered subcutaneously as a single 6 mg dose [10]. It was approved in both the USA and Europe in 2002. Lipegfilgrastim (Lonquex®; Teva Pharma B. V.), a long-acting filgrastim molecule that is pegylated at a different site from pegfilgrastim, was approved in Europe in 2013 [11]. Other long-acting G-CSFs, such as balugrastim, are in clinical development [12].
The emergence of these recently developed long-acting G-CSFs necessitates a re-evaluation of the evidence. Direct comparative data are limited, and there are no systematic reviews of long-acting G-CSFs that include data from both observational studies and randomised controlled trials (RCTs). Therefore, we conducted a systematic review to capture the available data on the efficacy, safety and effectiveness of long-acting G-CSFs for prophylaxis of CIN and FN in adult patients with cancer.
Methods
Study design
The systematic review was performed according to a pre-specified protocol that was agreed by all authors. We searched the following electronic databases: MEDLINE In-Process & Other Non-Indexed Citations and OVID MEDLINE 1948–present, EMBASE 1980–present and the Cochrane Library. A search of abstract books was also conducted from the annual meetings of the American Society of Clinical Oncology, the American Society of Hematology, the European Hematology Association, the European Society for Medical Oncology, the European Multidisciplinary Cancer Congress, the International Society for Pharmacoeconomics and Outcomes Research and the Multinational Association of Supportive Care in Cancer. Complete search strings are listed in Online Resource 1. The electronic database searches included articles published up to April 2013 and were restricted to English-language studies. Conference abstracts were limited to those published between January 2009 and April 2013. This report follows the PRISMA statement for reporting systematic reviews and meta-analyses [13].
Study selection
Initially, two independent reviewers screened the titles and abstracts of the search results for studies of human adult haematology or oncology patients who were receiving long-acting-G-CSF primary prophylaxis to reduce the risk of CIN during chemotherapy. Studies in which patients received bone marrow transplantation were excluded. Clinical trials and observational studies were included. Editorials, letters, case reports, guidelines, health technology assessment reports, economic evaluations, narrative reviews and research protocols were excluded. Papers were excluded if they did not report neutropenia-related outcomes. Full texts of the remaining articles were then assessed by the reviewers for final inclusion. Additional exclusion criteria were applied at this second stage: studies comparing pegfilgrastim with a daily G-CSF, placebo or no prophylaxis were excluded if fewer than 50 patients received pegfilgrastim; studies with pegfilgrastim alone (which therefore allowed no comparisons) were excluded if fewer than 100 patients received pegfilgrastim. Studies in which pegfilgrastim was used outside of its approved indication were excluded. These additional exclusion criteria were not applied to studies involving new long-acting G-CSFs because we expected to find far fewer papers on these and wanted to ensure that all available data on these other agents were captured. Papers or abstracts reporting results from the same study were indicated as such. If a study included in the form of a congress abstract was published as a peer-reviewed paper after our literature search, we included the paper in place of the congress abstract.
Data extraction
The data collection comprised study and patient characteristics, efficacy (effect of a treatment under controlled, clinical trial conditions), effectiveness (effect of a treatment under uncontrolled, real-world conditions) and safety. Detailed definitions of outcome measures are listed in Online Resource 2. Studies were classified according to their design: ‘RCTs’ where patients were randomised to G-CSFs; clinical trials in which patients were not randomly assigned to neutropenia prophylaxis or no treatment were termed ‘clinical trials’; and studies of routine clinical practice were termed ‘observational studies’. Evidence found in the literature was extracted as presented by the original authors of the study.
Risk of bias assessment
Two independent reviewers assessed risk of bias; disagreements were resolved within the reviewer team by consensus. RCTs were assessed using the Cochrane Collaboration’s assessment tool [14]. Non-randomised studies were assessed using the Methods Guide for Comparative Effectiveness Reviews of the US Agency for Healthcare Research and Quality [15]. Six domains of bias (selection bias, performance bias, detection bias, attrition bias, reporting bias and other bias) were assessed. Based on the reviewers’ judgments, every article was rated as having a ‘low’, ‘high’ or ‘unclear’ risk of bias. Risk of bias was not assessed for conference abstracts.
Results
Eligible trials and study characteristics
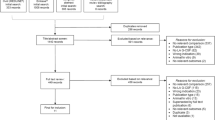
Our search identified 731 full publications and 108 congress abstracts (Fig. 1). After removing duplicates, 700 items were left, of which 482 were excluded on the basis of title and abstract screening, leaving 218 articles (Online Resource 3). Three relevant articles were published after completion of the search: Bondarenko et al. (2013) [16], Almenar-Cubells et al. (2013) [17] and Volovat et al. (2013) [18]; these were included to replace congress abstracts identified by the initial search that described the same studies [19–21]. Finally, 33 publications and 11 congress abstracts representing 41 studies were analysed. Key characteristics of the included studies are presented in Table 1.
Figure 2 illustrates the number of patients exposed to each of the included substances or treatment strategies, the G-CSF interventions used and the study design. The studies included 13 that looked at pegfilgrastim alone, 15 studies in which pegfilgrastim was compared with a daily G-CSF, three studies in which pegfilgrastim was compared with placebo and two studies in which pegfilgrastim primary prophylaxis was compared with no pegfilgrastim primary prophylaxis. We found eight studies that compared other long-acting G-CSFs with daily G-CSFs, pegfilgrastim or placebo. The number of patients who received a long-acting G-CSF was 50,089 (pegfilgrastim = 49,207; lipegfilgrastim = 505; balugrastim = 281; Maxy-G34 = 27; Ro 25-8315 = 28; BCD-017 = 41).
Network diagram. The total number of patients included in randomised controlled trials, clinical trials and observational studies, and in whom a given granulocyte colony-stimulating factor (G-CSF) has been investigated, is stated below the G-CSF’s or comparator’s name. Single-arm studies including pegfilgrastim only are reported in the ‘Pegfilgrastim’ shape. Comparisons between G-CSFs are indicated by arrows specifying the type of study. The arrows point from the investigated G-CSF to the comparator
Pegfilgrastim studies included patients with breast, lung, colorectal or gastro-esophageal cancer, Hodgkin’s lymphoma, non-Hodgkin’s lymphoma, acute myeloid leukaemia and various other solid tumours. These studies included patients taking 12 standard chemotherapy regimens and numerous non-standard regimens. All studies of newer long-acting G-CSFs except one (which looked at lipegfilgrastim in non-small cell lung cancer [22]) were conducted in patients with breast cancer, most of whom were receiving docetaxel and doxorubicin.
Risk of bias assessment
Risk of bias was typically higher in non-randomised trials and observational studies than in RCTs (Fig. 3). Most studies excluded patients receiving concomitant antibiotic prophylaxis or who had previously received chemotherapy; therefore, risk of performance bias was low. Risk of reporting bias was difficult to assess across all types of studies because the study protocols were not published.
Risk of bias assessment of included studies. a Randomised controlled trials (RCTs) are defined as those studies in which patients were randomised to a granulocyte colony-stimulating factor (G-CSF). Adequate sequence generation and allocation concealment refers to selection bias, blinding of participants and staff to performance bias, blinding of outcome assessors to detection bias, incomplete outcome data to attrition bias and selective outcome reporting refers to reporting bias. b Non-randomised clinical trials are those in which patients were not randomised to a G-CSF. The risk of bias assessment includes non-randomised clinical trials and observational studies that included more than one G-CSF. c The risk of bias assessment includes non-randomised clinical trials and observational studies that included pegfilgrastim only
Efficacy and effectiveness of pegfilgrastim
Table 2 shows efficacy and effectiveness endpoints for studies of pegfilgrastim alone or compared with daily G-CSFs, placebo or no treatment.
Incidence of FN
Three RCTs reported a significant reduction in FN for pegfilgrastim versus placebo (1 % vs 17 % [23], 2 % vs 6 % [24, 25] and 2 % vs 8 % [26]) in patients with breast or colorectal cancer receiving chemotherapy regimens associated with various FN risk profiles. One RCT designed to demonstrate non-inferiority in duration of severe neutropenia reported a significant reduction in FN incidence for pegfilgrastim versus filgrastim (9 % vs 18 %) in patients with breast cancer [27]. Another RCT with a similar design found a non-significant trend towards lower FN incidence for pegfilgrastim versus filgrastim (13 % vs 20 %) [28].
Ten clinical trials reported FN incidence across numerous tumour and chemotherapy types, including several dose-dense regimens. In eight of these trials, all patients received pegfilgrastim; FN incidence ranged from 1 to 10 % [29–36]. A study in which FN prophylaxis was changed by protocol amendment in subsequent cohorts of patients with primary breast cancer treated with a high FN-risk regimen (docetaxel, doxorubicin and cyclophosphamide) found a significant reduction in the incidence of FN for pegfilgrastim versus daily G-CSF (7 % vs 18 %) [37]. In contrast, another breast cancer trial in which G-CSF schedules were selected at the physician’s discretion reported a higher FN incidence for pegfilgrastim versus filgrastim (11 % vs 4 %) [38].
Observational studies showed FN incidence was higher among patients with haematological malignancies (14–16 %) [39, 40] than in those with solid tumours (4–12 %) [41–43, 17, 44–46]. Five of these observational studies that reported FN incidence compared neutropenia prophylaxis: two studies across various tumour types reported trends towards reduced FN incidence with pegfilgrastim versus daily G-CSF (11 % vs 24 % and 7 % vs 13 %, respectively) [17, 44], one found a significant reduction (5 % vs 7 %) [45] and two did not find a difference for pegfilgrastim versus filgrastim in non-Hodgkin’s lymphoma (NHL) [39] and breast cancer [47]. Significant reductions in FN incidence for pegfilgrastim primary prophylaxis versus no pegfilgrastim primary prophylaxis were also seen in observational studies of patients with breast cancer (4 % vs 30 %) and in patients with various tumour types (odds ratio [95 % confidence interval (CI)] = 0.49 [0.34–0.68]) [48, 41].
Incidence of CIN
An RCT in patients with colorectal cancer treated with chemotherapy with a low FN risk (FOLFOX, FOLFIRI or FOIL) found pegfilgrastim significantly reduced CIN incidence compared with placebo (13 % vs 43 %) [26]. RCTs comparing pegfilgrastim with filgrastim in a non-inferiority setting reported no significant difference in CIN incidence in patients with breast cancer receiving chemotherapy associated with a high FN risk [27, 28].
In clinical trials investigating dose-dense regimens, CIN incidence with pegfilgrastim was low and ranged from 3 to 11 % in patients with breast cancer [29, 31, 32] and 34 % in gastro-esophageal cancer [34]. In studies of standard-dose chemotherapy regimens across various tumour types, CIN incidence ranged from 22 to 30 % [36, 35]. One trial reported that pegfilgrastim significantly reduced the incidence of CIN compared with daily G-CSF (37 % vs 58 %) in patients with breast cancer [37].
Three observational studies reporting CIN incidence compared neutropenia prophylaxis; a difference was not found between pegfilgrastim and filgrastim in patients with breast cancer [47], but in patients with various tumours or NHL CIN incidence was lower in those receiving pegfilgrastim than those receiving daily G-CSF (28 % vs 49 % and 41 % vs 50 %) [17, 49].
Incidence of hospitalisations due to CIN or FN
One RCT reported a significant reduction in FN-related hospitalisations in patients with breast cancer who received pegfilgrastim versus placebo (1 % vs 14 %) [23], while another in patients with colorectal cancer found no significant difference in CIN-related hospitalisations [26].
In a clinical trial including patients with various tumour types receiving pegfilgrastim primary prophylaxis in community-based practices in the USA, the incidence of FN-related hospitalisations was 4 % [35]. A similar study in elderly patients found the incidence of CIN- or FN-related hospitalisations was 5 % [36]. Two clinical trials of patients with breast cancer found no significant difference in incidence and duration of FN-related hospitalisations between pegfilgrastim and daily G-CSFs [37, 38].
Three retrospective observational studies enrolling patients with various tumour types found trends towards reduced incidence of hospitalisations due to FN for pegfilgrastim versus daily G-CSF (9 % vs 20 %, 3 % vs 11 % and 3 % vs 7 %) [17, 44, 50], whereas another found no significant difference between sargramostim and pegfilgrastim [51]. Two other retrospective observational studies [52, 53] reported significant decreases in the risk of CIN-related hospitalisations for pegfilgrastim compared with filgrastim (1 % vs 4 % and 1 % vs 2 %); findings supported by a study of two US databases that found pegfilgrastim reduced the risk of neutropenia-related hospitalisation compared with filgrastim [54].
Incidence of chemotherapy dose reductions and delays
In one RCT in patients with breast cancer receiving pegfilgrastim or placebo, there was no significant difference in the proportion of patients receiving their full chemotherapy dose on schedule [23]; however, cross-over from the placebo to the pegfilgrastim arm was allowed if FN occurred. Another RCT in colorectal cancer reported a significant decrease in dose reductions (3 % vs 11 %) and delays (4 % vs 20 %) due to neutropenia for pegfilgrastim versus placebo [26].
There was a wide range of incidence of dose delays and reductions in the clinical trials (2–77 % and 2–33 %, respectively), but most papers did not specify whether or not the chemotherapy modifications were due to neutropenia [38, 31, 34, 35]. Only one clinical trial compared the incidence of dose delays (due to FN events and non-haematological toxicity) with pegfilgrastim and filgrastim in patients with breast cancer. It found no significant difference between the two arms [38].
Rates of dose delays and reductions in observational studies also varied considerably between trials (5–55 % and 5–42 %, respectively) [39–41, 43, 17, 44]. One study found a significantly lower incidence of delays for pegfilgrastim primary prophylaxis versus no pegfilgrastim primary prophylaxis in patients with breast cancer (5 % vs 12 %), but found no significant difference in dose reductions [41]. In two studies of patients with various tumour types, fewer dose delays (42 % vs 55 %) [17] and dose reductions (32 % vs 38 % and 7 % vs 21 %) [17, 44] due to neutropenia for pegfilgrastim versus daily G-CSF were observed. In a population of Asian patients with NHL, rates of dose reductions and delays were slightly higher in patients who received pegfilgrastim than in those who received filgrastim [39].
Antibiotic use
In one RCT, a non-significant reduction in antibiotic use was reported for pegfilgrastim versus filgrastim (17 % vs 21 %) in patients with breast cancer [28]. Two RCTs reported a significant reduction in the use of antibiotics due to FN for pegfilgrastim versus placebo, one in breast cancer (2 % vs 10 %) [23] and one in colorectal cancer (2 % vs 7 %) [26].
A clinical trial in breast cancer found no significant difference in the use of antibiotics between patients receiving pegfilgrastim and filgrastim (11 % vs 4 %) [38].
An observational study found a significant reduction in the use of antibiotics for pegfilgrastim primary prophylaxis versus no pegfilgrastim primary prophylaxis (28 % vs 46 %) in patients with breast cancer [41]. Two observational studies in patients with various tumour types found lower rates of FN-related antibiotic use in patients who received pegfilgrastim than those receiving daily G-CSF (4 % vs 11 % and 8 % vs 17 %); in the former study, this difference reached significance [17, 44].
Safety of pegfilgrastim
Table 2 shows safety endpoints for studies of pegfilgrastim alone or compared with daily-G-CSFs, placebo or no treatment.
All G-CSF-related adverse events
Two RCTs in patients with breast cancer reported that G-CSF-related adverse events (AEs) were similar for pegfilgrastim and filgrastim [27, 28]. Another RCT found a non-significant increase in G-CSF-related AEs for pegfilgrastim compared with placebo (11 % vs 1 %) in patients with colorectal cancer, primarily due to increased bone pain [26]. Pegfilgrastim-related serious AEs were also infrequent (0.5 %) in patients with various tumours in a clinical trial [35]. Two observational studies in patients with various tumours reported a non-significant decrease in G-CSF-related AEs for pegfilgrastim versus daily G-CSF (6 % vs 10 % and 1 % vs 5 %) [17, 44]. None of the studies reported any fatal AEs that were attributed to G-CSF prophylaxis.
Musculoskeletal pain
In two placebo-controlled RCTs including patients with breast or colorectal cancer, occurrence of any-grade musculoskeletal pain was higher in the pegfilgrastim arms than the placebo arms (31 % vs 27 % and 11 % vs 1 %) [23, 26]. In two further RCTs of patients with breast cancer randomised to pegfilgrastim or filgrastim, overall rates of bone pain were comparable between arms [27, 28], and severe bone pain appeared reduced for pegfilgrastim versus filgrastim (1 % vs 8 %) [28].
In five non-comparative clinical trials, the incidence of any-grade musculoskeletal pain with pegfilgrastim reported ranged from 7 to 26 % [29, 36] and the incidence of severe musculoskeletal pain ranged from 0 to 9 % [29, 31, 32, 35] across patients with breast cancer and various tumour types.
In general, the reported incidence of musculoskeletal pain was lower in observational studies than in clinical trials. The incidence of any-grade musculoskeletal pain with pegfilgrastim in observational studies varied, from 6 % in one study where all patients received pegfilgrastim (with no patients experiencing serious bone or muscle pain) [46] to 50 % in patients receiving either pegfilgrastim or filgrastim [47]. In two other observational studies of patients with various tumour types that compared pegfilgrastim with daily G-CSF, bone pain was less common in the pegfilgrastim arms (2 % vs 6 % and 1 % vs 3 %) [17, 44].
Other long-acting G-CSFs
Table 3 shows the efficacy and safety endpoints for studies involving other long-acting G-CSFs.
Lipegfilgrastim
Lipegfilgrastim is pegylated at a different site from pegfilgrastim (threonine 134) using a carbohydrate linker involving two enzymatic steps. In a placebo-controlled RCT in patients with lung cancer, there was no statistically significant reduction in the first-cycle incidence of FN compared to placebo (2 % vs 6 %) and a significant reduction in the first-cycle incidence of severe neutropenia (32 % vs 59 %) [22]. G-CSF-related AEs were more common in the lipegfilgrastim arm (14 % vs 10 %) [22]. In a non-inferiority RCT comparing lipegfilgrastim with pegfilgrastim in patients with breast cancer, there was no significant difference in FN incidence (1 % vs 3 %) and a non-significant reduction in severe neutropenia incidence (44 % vs 51 %) [16]. Rates of FN-related hospitalisations and antibiotic use were also comparable between the two study arms (1 % vs 2 % and 1 % vs 3 %, respectively) [16]. AEs, including bone pain (14 % vs 10 %), myalgia (9 % vs 6 %) and arthralgia (5 % vs 2 %), were slightly more common with lipegfilgrastim than with pegfilgrastim, but the difference was not significant [16]. In a second RCT in breast cancer, duration of severe neutropenia for lipegfilgrastim and pegfilgrastim was reported to be similar [55].
Balugrastim
Balugrastim is a non-pegylated recombinant fusion protein composed of human serum albumin and G-CSF harvested from yeast. It has been investigated at a dose of 40 mg in two RCTs in patients with breast cancer treated with doxorubicin and docetaxel. In one, incidence (58 % vs 59 %) and duration (1.1 days vs 1 day) of severe neutropenia in cycle 1 were similar for balugrastim and pegfilgrastim [18]. There was no significant difference in FN incidence in cycle 1 between balugrastim and pegfilgrastim (1 % vs 3 %) [18]. The frequency of treatment-related AEs was similar for balugrastim and pegfilgrastim (20 % vs 19 %) [18]. The second RCT found similar durations of severe neutropenia for balugrastim and pegfilgrastim (1.3 days vs 1.2 days) [19].
BCD-017, Maxy-G34 and Ro 25-8315
BCD-017 (empegfilgrastim), Maxy-G34 and Ro 25-8315 are all covalent conjugates of recombinant human G-CSF and polyethylene glycol. Small RCTs compared BCD-017 and Ro 25-8315 with filgrastim in patients with breast cancer but found that neutropenia-related outcomes, including rates of FN, were generally lower in the filgrastim arms [56, 57]. Safety data were reported in the Ro 25-8315 study and suggest G-CSF-related AEs are more common with Ro 25-8315 than with filgrastim [57]. Maxy-G34 was compared with pegfilgrastim in a clinical trial. The incidence of FN and duration of CIN were similar in the two study arms [58]. No safety data were reported.
Discussion
To our knowledge, this is the only systematic review of long-acting G-CSFs that includes newly developed agents and data from both clinical trials and observational studies. We identified 12 RCTs, 12 clinical trials and 17 observational studies, including 58,342 patients in total. Studies in patients with breast cancer were dominant, partly because these were the registration studies for the G-CSFs.
Pegfilgrastim studies included a range of patient populations, cancer types and stages, and chemotherapy regimens. Efficacy and effectiveness results were generally consistent. Although pegfilgrastim did not uniformly show better efficacy or effectiveness in all studies, the vast majority showed better efficacy or effectiveness compared to daily G-CSF, no upfront pegfilgrastim, no G-CSF or placebo in terms of reduction of the incidence of CIN (4/7 studies), FN (11/14 studies), chemotherapy dose delays and reductions (6/8 studies), antibiotic use (6/7 studies) and neutropenia-related hospitalisations (9/13 studies). The observed variation may be partly explained by differences in patient populations and cancer types, or in the way G-CSF was administered. Thirteen (35 %) studies of pegfilgrastim reported safety data and most of these focused on musculoskeletal pain; only three studies reported other G-CSF-related AEs. This suggests that the safety profile of G-CSFs may be generally accepted and studies now investigate only specific AEs known to be associated with their use. The incidence of G-CSF-related AEs was similar between pegfilgrastim and filgrastim. The incidence of bone pain and severe bone pain was lower or no different for pegfilgrastim than filgrastim in most RCTs and observational studies (4/6 studies).
Previously published systematic reviews and meta-analyses of RCTs comparing pegfilgrastim with daily G-CSF or placebo by Cooper et al. and Pinto et al. found that pegfilgrastim more effectively reduced the incidence of FN [59, 60]. The RCT reported by Decaestecker et al. and Pinter et al. [24, 25], showing better efficacy for pegfilgrastim than placebo in reducing the incidence of neutropenia in colorectal cancer patients, reported in this systematic review was not included in these previous systematic reviews. We additionally included non-randomised clinical trials and observational studies that have not been included in former systematic reviews [59, 60]. Nevertheless, the results of our systematic review are generally consistent with these studies. However, while well-designed RCTs have a low risk of bias, inclusion criteria can be restrictive. The observational studies included in our review indicate an advantage for pegfilgrastim over daily G-CSFs or no treatment, suggesting that the efficacy of pegfilgrastim demonstrated in clinical trials has been translated into clinical practice. In fact, we found a greater magnitude of reduction in CIN incidence with pegfilgrastim versus filgrastim in observational studies than RCTs; this could be due to a shorter duration of G-CSF use in current practice (e.g. 5–6 days in clinical practice vs 10–11 days in clinical trials) [7]. Importantly, the safety data from observational studies were consistent with data from RCTs, suggesting that the pegfilgrastim safety profile can be used to guide treatment in a broad patient population. However, care should be taken when interpreting the results of observational studies, owing to the higher risk of bias and confounding factors.
Almost all the studies including other long-acting G-CSFs were RCTs of patients with breast cancer (7/8 studies) receiving doxorubicin and docetaxel (5/8 studies). Lipegfilgrastim has been the most extensively tested (3/8 studies) and appears to be similar to pegfilgrastim regarding the reduction in duration of severe neutropenia in patients with breast cancer. Efficacy of lipegfilgrastim in reducing the incidence of FN was not statistically superior to placebo in a congress abstract describing an RCT in patients with lung cancer [22]. Lipegfilgrastim has now been approved in Europe for reducing the incidence and duration of FN in adults with cancer who are receiving cytotoxic chemotherapy [11]. Further clinical and observational studies in a wider range of tumour types and chemotherapy regimens will confirm whether its efficacy and safety are maintained across a broader patient population in real-world clinical practice. Balugrastim has also been investigated in two phase 3 RCTs of patients with breast cancer and has an efficacy and safety profile comparable to that of pegfilgrastim. Again, further studies will determine whether this translates to other patient populations. Notably, the incidence of FN in the pegfilgrastim arms of the lipegfilgrastim and balugrastim studies (3 % in cycle 1 for both studies [16, 18]) was lower than in the registrational pegfilgrastim studies (9 % and 7 % in cycle 1 [27, 28]), despite a similar study design and patient population. Maxy-G34 also appears to be non-inferior to pegfilgrastim; however, it was tested in only a very small number of patients (n = 35) [58]. BCD-017 and Ro 25-8315 did not appear to be as effective at reducing the incidence of FN as filgrastim [56, 57].
Because very few studies reported long-term outcomes of G-CSF use and two systematic reviews by Kuderer and Lyman et al. [61, 62] looking at survival have previously been published, we did not include overall survival as an endpoint. In 2007, Kuderer et al. [61] published a systematic review of infection-related and early mortality during chemotherapy by type of G-CSF. They reported that there is insufficient data to draw conclusions. An updated analysis in 2013 by Lyman et al. [62] concluded that all-cause mortality is reduced in patients receiving chemotherapy with primary G-CSF support. However, Lyman et al. did not report results by type of G-CSF. We are still awaiting long-term survival data for the newer long-acting G-CSFs. Future research should examine in more detail the effects of long-acting G-CSFs on survival outcomes.
As is true for all systematic reviews, the validity of our findings is limited by the quality of its underlying studies. Another limitation is that some studies did not report how many patients received primary prophylaxis versus secondary prophylaxis. This may have led to an underestimation of effectiveness. Furthermore, the studies were not all consistent in their definitions of FN and CIN and the number of chemotherapy cycles over which they reported data. Finally, combined measures of effect are missing in our analysis.
It is clear that pegfilgrastim is widely used in clinical practice across a broad patient population. Lipegfilgrastim and balugrastim were similar to pegfilgrastim in reducing the duration and incidence of CIN and FN in five studies. Furthermore, the safety profiles of the recently developed long-acting G-CSFs were comparable to pegfilgrastim based on the phase 3 studies identified by this systematic review. These G-CSFs may prove to be valuable therapeutic options; however, there is a need for further studies in broader patient populations to confirm their effectiveness and safety in real-world clinical practice. New biosimilar G-CSFs and next-generation drugs targeting the G-CSF receptor are also in the early stages of development [12] and should be assessed against the current standard of care.
References
Crawford J, Caserta C, Roila F (2010) Hematopoietic growth factors: ESMO Clinical Practice Guidelines for the applications. Ann Oncol: Off J Eur Soc Med Oncol ESMO 21(Suppl 5):v248–v251. doi:10.1093/annonc/mdq195
Dale DC (2009) Advances in the treatment of neutropenia. Curr Opin Support Palliat Care 3(3):207–212. doi:10.1097/SPC.0b013e32832ea6ae
Rossi L, Tomao F, Lo Russo G, Papa A, Zoratto F, Marzano R, Basso E, Giordani E, Verrico M, Ricci F, Pasciuti G, Francini E, Tomao S (2013) Efficacy and safety analysis of once per cycle pegfilgrastim and daily lenograstim in patients with breast cancer receiving adjuvant myelosuppressive chemotherapy FEC 100: a pilot study. Ther Clin Risk Manag 9:457–462. doi:10.2147/TCRM.S48387
Lyman GH (2009) Impact of chemotherapy dose intensity on cancer patient outcomes. J Natl Compr Cancer Netw: JNCCN 7(1):99–108
Saloustros E, Tryfonidis K, Georgoulias V (2011) Prophylactic and therapeutic strategies in chemotherapy-induced neutropenia. Expert Opin Pharmacother 12(6):851–863. doi:10.1517/14656566.2011.541155
Curran MP, Goa KL (2002) Pegfilgrastim. Drugs 62(8):1207–1213, discussion 1214–1205
Aapro MS, Bohlius J, Cameron DA, Dal Lago L, Donnelly JP, Kearney N, Lyman GH, Pettengell R, Tjan-Heijnen VC, Walewski J, Weber DC, Zielinski C (2011) 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer (Oxford, England: 1990) 47(1):8–32. doi:10.1016/j.ejca.2010.10.013
Crawford J, Allen J, Armitage J, Blayney DW, Cataland SR, Heaney ML, Htoy S, Hudock S, Kloth DD, Kuter DJ, Lyman GH, McMahon B, Steensma DP, Vadhan-Raj S, Westervelt P, Westmoreland M (2011) Myeloid growth factors. J Natl Compr Cancer Netw: JNCCN 9(8):914–932
Smith TJ, Khatcheressian J, Lyman GH, Ozer H, Armitage JO, Balducci L, Bennett CL, Cantor SB, Crawford J, Cross SJ, Demetri G, Desch CE, Pizzo PA, Schiffer CA, Schwartzberg L, Somerfield MR, Somlo G, Wade JC, Wade JL, Winn RJ, Wozniak AJ, Wolff AC (2006) 2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol: Off J Am Soc Clin Oncol 24(19):3187–3205. doi:10.1200/JCO.2006.06.4451
Yang BB, Savin MA, Green M (2012) Prevention of chemotherapy-induced neutropenia with pegfilgrastim: pharmacokinetics and patient outcomes. Chemotherapy 58(5):387–398. doi:10.1159/000345626
European Medicines Agency (2013) European public assessment report for Lonquex. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/002556/WC500148383.pdf. Accessed 4 February 2014
Hoggatt J, Pelus LM (2013) New G-CSF agonists for neutropenia therapy. Expert Opin Investig Drugs. doi:10.1517/13543784.2013.838558
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 62(10):e1–e34. doi:10.1016/j.jclinepi.2009.06.006
Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928. doi:10.1136/bmj.d5928
Viswanathan M, Ansari MT, Berkman ND, Chang S, Hartling L, McPheeters LM, Santaguida PL, Shamliyan T, Singh K, Tsertsvadze A, Treadwell JR (2012) Assessing the risk of bias of individual studies in systematic reviews of health care interventions. Agency for Healthcare Research and Quality Methods Guide for Comparative Effectiveness Reviews AHRQ Publication No. 12-EHC047-EF
Bondarenko I, Gladkov OA, Elaesser R, Buchner A, Bias P (2013) Efficacy and safety of lipegfilgrastim versus pegfilgrastim: a randomized, multicenter, active-control phase 3 trial in patients with breast cancer receiving doxorubicin/docetaxel chemotherapy. BMC Cancer 13(1):386. doi:10.1186/1471-2407-13-386
Almenar Cubells D, Bosch Roig C, Jimenez Orozco E, Alvarez R, Cuervo JM, Diaz Fernandez N, Sanchez Heras AB, Galan Brotons A, Giner Marco V, Codes MDVM (2013) Effectiveness of daily versus non-daily granulocyte colony-stimulating factors in patients with solid tumours undergoing chemotherapy: a multivariate analysis of data from current practice. Eur J Cancer Care 22(3):400–412. doi:10.1111/ecc.12043
Volovat C, Gladkov OA, Bondarenko IM, Barash S, Buchner A, Bias P, Adar L, Avisar N (2013) Efficacy and safety of balugrastim compared with pegfilgrastim in patients with breast cancer receiving chemotherapy. Clin Breast Cancer. doi:10.1016/j.clbc.2013.10.001
Gladkov O, Bondarenko I, Elsaesser R, Buchner A, Bias P (2012) Absolute neutrophil counts in a study of lipegfilgrastim compared with pegfilgrastim in patients with breast cancer who are receiving chemotherapy. Ann Oncol: Off J Eur Soc Med Oncol/ESMO 23:abstr 1548PD
Almenar-Cubells D, Roig CB, Jimenez E, M AC, Cuervo JM, Diaz N, Sanchez AB, Galan A, Giner V, Codes M (2011) Daily G-CSFs versus pegfilgrastim (PEG) in cancer patients (pts) undergoing chemotherapy (CT): a multivariate analysis from clinical practice. J Clin Oncol: Off J Am Soc Clin Oncol 29 (15 suppl. 1):(suppl; abstr e19526)
Volovat CD, Gladkov O, Bondarenko IN, Barajas-Figueroa LJ, Buchner A, Avisar N, Bianchi S (2012) Efficacy and safety of balugrastim compared with pegfilgrastim in patients with breast cancer who are receiving chemotherapy. J Clin Oncol: Off J Am Soc Clin Oncol 30:(suppl; abstr 9125)
Gladkov OA, Volovat C, Bondarenko I, Elaesser R, Buchner A, Bias P, Mueller U (2012) Efficacy and safety of lipegfilgrastim in patients with lung cancer who are receiving chemotherapy. Support Care Cancer: Off J Multinatl Assoc Support Care Cancer 20(Suppl 1):S243
Vogel CL, Wojtukiewicz MZ, Carroll RR, Tjulandin SA, Barajas-Figueroa LJ, Wiens BL, Neumann TA, Schwartzberg LS (2005) First and subsequent cycle use of pegfilgrastim prevents febrile neutropenia in patients with breast cancer: a multicenter, double-blind, placebo-controlled phase III study. J Clin Oncol: Off J Am Soc Clin Oncol 23(6):1178–1184. doi:10.1200/JCO.2005.09.102
Decaestecker J, Cesas A, Hotko Y, Abella E, Mo M, Rogowski W (2013) Regional differences in reported febrile neutropenia (FN), adverse events (AES), and serious AES (SAES) in a multinational phase 3 trial. Support Care Cancer: Off J Multinatl Assoc Support Care Cancer TBC:TBC
Pinter T, Abella S, Cesas A, Croitoru A, Decaestecker J, Gibbs P, Hotko Y, Jassem J, Kurteva GP, Novotny J, O’Reilly S, Salek T, Mo MF, Choi LMR, Blanke CD (2013) Results of a phase III, randomized, double-blind, placebo-controlled trial of pegfilgrastim (PEG) in patients (pts) receiving first-line FOLFOX or FOLFIRI and bevacizumab (B) for colorectal cancer (CRC). J Clin Oncol: Off J Am Soc Clin Oncol 30 (suppl 34; abstr LBA445)
Hecht JR, Pillai M, Gollard R, Heim W, Swan F, Patel R, Dreiling L, Mo M, Malik I (2010) A randomized, placebo-controlled phase II study evaluating the reduction of neutropenia and febrile neutropenia in patients with colorectal cancer receiving pegfilgrastim with every-2-week chemotherapy. Clin Colorectal Cancer 9(2):95–101. doi:10.3816/CCC.2010.n.013
Holmes FA, O’Shaughnessy JA, Vukelja S, Jones SE, Shogan J, Savin M, Glaspy J, Moore M, Meza L, Wiznitzer I, Neumann TA, Hill LR, Liang BC (2002) Blinded, randomized, multicenter study to evaluate single administration pegfilgrastim once per cycle versus daily filgrastim as an adjunct to chemotherapy in patients with high-risk stage II or stage III/IV breast cancer. J Clin Oncol: Off J Am Soc Clin Oncol 20(3):727–731
Green MD, Koelbl H, Baselga J, Galid A, Guillem V, Gascon P, Siena S, Lalisang RI, Samonigg H, Clemens MR, Zani V, Liang BC, Renwick J, Piccart MJ, International Pegfilgrastim 749 Study G (2003) A randomized double-blind multicenter phase III study of fixed-dose single-administration pegfilgrastim versus daily filgrastim in patients receiving myelosuppressive chemotherapy. Ann Oncol: Off J Eur Soc Med Oncol ESMO 14(1):29–35
Burstein HJ, Parker LM, Keshaviah A, Doherty J, Partridge AH, Schapira L, Ryan PD, Younger J, Harris LN, Moy B, Come SE, Schumer ST, Bunnell CA, Haldoupis M, Gelman R, Winer EP (2005) Efficacy of pegfilgrastim and darbepoetin alfa as hematopoietic support for dose-dense every-2-week adjuvant breast cancer chemotherapy. J Clin Oncol: Off J Am Soc Clin Oncol 23(33):8340–8347. doi:10.1200/JCO.2005.02.8621
Loibl S, Mueller V, Von Minckwitz G, Conrad B, Koehne CH, Kremers S, Forstbauer H, Linder M, Nekljudova V, Moebus V (2011) Comparison of pegfilgrastim on day 2 vs. day 4 as primary prophylaxis of intense dose-dense chemotherapy in patients with node-positive primary breast cancer within the prospective, multi-center GAIN study: (GBG 33). Support Care Cancer 19:1789–1795. doi:10.1007/s00520-010-1020-9
Pippen J, Paul D, Vukelja S, Clawson A, Iglesias J (2011) Dose-dense doxorubicin and cyclophosphamide followed by dose-dense albumin-bound paclitaxel plus bevacizumab is safe as adjuvant therapy in patients with early stage breast cancer. Breast Cancer Res Treat 130:825–831. doi:10.1007/s10549-011-1678-9
Yardley DA, Zubkus J, Daniel B, Inhorn R, Lane CM, Vazquez ER, Naot Y, Burris HA, Hainsworth JD 3rd (2010) A phase II trial of dose-dense neoadjuvant gemcitabine, epirubicin, and albumin-bound paclitaxel with pegfilgrastim in the treatment of patients with locally advanced breast cancer. Clin Breast Cancer 10(5):367–372. doi:10.3816/CBC.2010.n.048
Miller AA, Wang XF, Gu L, Hoffman P, Khatri J, Dunphy F, Edelman MJ, Bolger M, Vokes EE, Green MR (2008) Phase II randomized study of dose-dense docetaxel and cisplatin every 2 weeks with pegfilgrastim and darbepoetin alfa with and without the chemoprotector BNP7787 in patients with advanced non-small cell lung cancer (CALGB 30303). J Thorac Oncol 3:1159–1165
Toppo L, Tomasello G, Liguigli W, Ratti M, Poli R, Negri F, Curti A, Vismarra M, Maltese M, Delfrate R, Donini M, Colombi C, Brighenti M, Panni S, Perrucci B, Lazzarelli S, Passalacqua R (2013) Efficacy and safety of dose-dense modified TCF regimen (TCF-dd) in metastatic or locally advanced gastroesophageal cancer (GEC). J Clin Oncol: Off J Am Soc Clin Oncol 31:(suppl; abstr 4112)
Ozer H, Mirtsching B, Rader M, Luedke S, Noga SJ, Ding B, Dreiling L (2007) Neutropenic events in community practices reduced by first and subsequent cycle pegfilgrastim use. Oncologist 12(4):484–494. doi:10.1634/theoncologist.12-4-484
Balducci L, Al-Halawani H, Charu V, Tam J, Shahin S, Dreiling L, Ershler WB (2007) Elderly cancer patients receiving chemotherapy benefit from first-cycle pegfilgrastim. Oncologist 12:1416–1424. doi:10.1634/theoncologist.12-12-1416
von Minckwitz G, Kummel S, du Bois A, Eiermann W, Eidtmann H, Gerber B, Hilfrich J, Huober J, Costa SD, Jackisch C, Grasshoff ST, Vescia S, Skacel T, Loibl S, Mehta KM, Kaufmann M, German Breast G (2008) Pegfilgrastim +/− ciprofloxacin for primary prophylaxis with TAC (docetaxel/doxorubicin/cyclophosphamide) chemotherapy for breast cancer. Results from the GEPARTRIO study. Ann Oncol: Off J Eur Soc Med Oncol ESMO 19(2):292–298
Hendler D, Rizel S, Yerushalmi R, Neiman V, Bonilla L, Braunstein R, Sulkes A, Stemmer SM (2011) Different schedules of granulocyte growth factor support for patients with breast cancer receiving adjuvant dose-dense chemotherapy: a prospective nonrandomized study. Am J Clin Oncol 34(6):619–624. doi:10.1097/COC.0b013e3181f94716
Chan A, Leng XZ, Chiang JY, Tao M, Quek R, Tay K, Lim ST (2011) Comparison of daily filgrastim and pegfilgrastim to prevent febrile neutropenia in Asian lymphoma patients. Asia Pac J Clin Oncol 7:75–81. doi:10.1111/j.1743-7563.2010.01355.x
Ng JH, Ang XY, Tan SH, Tao M, Lim ST, Chan A (2011) Breakthrough febrile neutropenia and associated complications in non-Hodgkin’s lymphoma patients receiving pegfilgrastim. Acta Haematol 125:107–114. doi:10.1159/000321545
Hamilton EP, Topping DL, Peppercorn JM, Marcom PK, Kimmick GG, Duff E, Cirrincione CT, Blackwell KL (2013) Clinical impact of febrile neutropenia (FN) increase among patients receiving adjuvant docetaxel/cyclophosphamide (TC) chemotherapy compared to TC plus pegfilgrastim for breast cancer. J Clin Oncol: Off J Am Soc Clin Oncol 31:(suppl; abstr 1076)
Jenkins P, Scaife J, Freeman S (2012) Validation of a predictive model that identifies patients at high risk of developing febrile neutropaenia following chemotherapy for breast cancer. Ann Oncol: Off J Eur Soc Med Oncol ESMO 23(7):1766–1771. doi:10.1093/annonc/mdr493
Ngamphaiboon N, O’Connor TL, Advani PP, Levine EG, Kossoff EB (2012) Febrile neutropenia in adjuvant docetaxel and cyclophosphamide (TC) with prophylactic pegfilgrastim in breast cancer patients: a retrospective analysis. Med Oncol 29:1495–1501. doi:10.1007/s12032-011-0035-5
Almenar D, Mayans J, Juan O, Bueno JMG, Lopez JIJ, Frau A, Guinot M, Cerezuela P, Buscalla EG, Gasquet JA, Sanchez J (2009) Pegfilgrastim and daily granulocyte colony-stimulating factor: patterns of use and neutropenia-related outcomes in cancer patients in Spain—results of the LEARN Study. Eur J Cancer Care 18:280–286. doi:10.1111/j.1365-2354.2008.00959.x
Morrison VA, Wong M, Hershman D, Campos LT, Ding B, Malin J (2007) Observational study of the prevalence of febrile neutropenia in patients who received filgrastim or pegfilgrastim associated with 3–4 week chemotherapy regimens in community oncology practices. J Manag Care Pharm 13:337–348
Jurczak W, Kalinka-Warzocha E, Chmielowska E, Duchnowska R, Wojciechowska-Lampka E, Wieruszewska K (2013) Multicentre, prospective observational study of pegfilgrastim primary prophylaxis (PP) in patients at high risk of febrile neutropenia (FN) in Poland. Support Care Cancer: Off J Multinatl Assoc Support Care Cancer TBC:TBC
Leung M, Eustaquio J, Kano J, Marr T, Higgins BP, Myers RE, Kim J, Jones G (2012) Pain severity and impairment of activity between pegfilgrastim (P) and fixed-dose filgrastim (F) in women with early-stage breast cancer receiving chemotherapy. J Clin Oncol: Off J Am Soc Clin Oncol 30 (suppl; abstr e19570)
Hershman D, Hurley D, Wong M, Morrison VA, Malin JL (2009) Impact of primary prophylaxis on febrile neutropenia within community practices in the US. J Med Econ 12(3):203–210. doi:10.3111/13696990903238603
Salar A, Lopez A, Pío Torres J, Lopez MD, Caballero MD, Prieto E, Batlle M, Giraldo P, Blasco A, Benedit P, Garrido T (2009) Incidence of chemotherapy-induced neutropenia in lymphoma patients and use of prophylaxis with granulocyte colony-stimulating factors in clinical practice. Haematologica 94:521, abstr 1315
Naeim A, Henk HJ, Becker L, Chia V, Badre S, Li X, Deeter R (2013) Pegfilgrastim prophylaxis is associated with a lower risk of hospitalization of cancer patients than filgrastim prophylaxis: a retrospective United States claims analysis of granulocyte colony-stimulating factors (G-CSF). BMC Cancer 13(11). doi:10.1186/1471-2407-13-11
Heaney ML, Toy EL, Vekeman F, Laliberte F, Dority BL, Perlman D, Barghout V, Duh MS (2009) Comparison of hospitalization risk and associated costs among patients receiving sargramostim, filgrastim, and pegfilgrastim for chemotherapy-induced neutropenia. Cancer 115(20):4839–4848. doi:10.1002/cncr.24535
Tan H, Tomic K, Hurley D, Daniel G, Barron R, Malin J (2011) Comparative effectiveness of colony-stimulating factors for febrile neutropenia: a retrospective study. Curr Med Res Opin 27:79–86. doi:10.1185/03007995.2010.536527
Weycker D, Malin J, Kim J, Barron R, Edelsberg J, Kartashov A, Oster G (2009) Risk of hospitalization for neutropenic complications of chemotherapy in patients with primary solid tumors receiving pegfilgrastim or filgrastim prophylaxis: a retrospective cohort study. Clin Ther 31:1069–1081. doi:10.1016/j.clinthera.2009.05.019
Henk HJ, Becker L, Tan H, Yu J, Kavati A, Naeim A, Deeter R, Barron R (2013) Comparative effectiveness of pegfilgrastim, filgrastim, and sargramostim prophylaxis for neutropenia-related hospitalization: two US retrospective claims analyses. J Med Econ 16:160–168. doi:10.3111/13696998.2012.734885
Buchner A, Bias P, Kaufmann M (2011) A randomized, double-blind, active control, multicenter, dose-finding study of XM22, glycopegfilgrastim, in patients with breast cancer receiving myelosuppressive therapy. J Clin Oncol: Off J Am Soc Clin Oncol 29:(suppl; abstr 9080)
Salafet OV, Chernovskaya TV, Sheveleva LP, Khorinko AV, Prokopenko TI, Nechaeva MP, Burdaeva ON, Matrosova MP, Kovalenko NV, Ovchinnikova EG, Koroleva I, Ivanov RA (2013) Efficacy and safety of BCD-017, a novel pegylated filgrastim: results of open-label controlled phase II study in patients with breast cancer receiving myelosuppressive chemotherapy. J Clin Oncol: Off J Am Soc Clin Oncol 31:(suppl; abstr e20593)
Viens P, Chabannon C, Pouillard P, Janvier M, Brugger W, Blay JY, Oberling F, Capdeville R, Newman C, Meresse V, Xu ZX, Platzer E, Van der Auwera P, Maraninchi D (2002) Randomized, controlled, dose-range study of Ro 25-8315 given before and after a high-dose combination chemotherapy regimen in patients with metastatic or recurrent breast cancer patients. J Clin Oncol: Off J Am Soc Clin Oncol 20(1):24–36
Schwartzberg LS, Sankar SL, Apt D, Goldstein E, Vetticaden SJ, Chitour K, Gilfoyle D, Kim S, Keiholz U, Possibger K (2009) An open-label, dose-escalating study of Maxy-G34, a novel potent, long-acting Pegylated G-CSF, compared with pegfilgrastim (PF) for the treatment of chemotherapy induced neutropenia (CIN). J Clin Oncol: Off J Am Soc Clin Oncol 27:(suppl; abstr e14500)
Cooper KL, Madan J, Whyte S, Stevenson MD, Akehurst RL (2011) Granulocyte colony-stimulating factors for febrile neutropenia prophylaxis following chemotherapy: systematic review and meta-analysis. BMC Cancer 11:404. doi:10.1186/1471-2407-11-404
Pinto L, Liu Z, Doan Q, Bernal M, Dubois R, Lyman G (2007) Comparison of pegfilgrastim with filgrastim on febrile neutropenia, grade IV neutropenia and bone pain: a meta-analysis of randomized controlled trials. Curr Med Res Opin 23(9):2283–2295. doi:10.1185/030079907X219599
Kuderer NM, Dale DC, Crawford J, Lyman GH (2007) Impact of primary prophylaxis with granulocyte colony-stimulating factor on febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: a systematic review. J Clin Oncol: Off J Am Soc Clin Oncol 25(21):3158–3167. doi:10.1200/JCO.2006.08.8823
Lyman GH, Dale DC, Culakova E, Poniewierski MS, Wolff DA, Kuderer NM, Huang M, Crawford J (2013) The impact of the granulocyte colony-stimulating factor on chemotherapy dose intensity and cancer survival: a systematic review and meta-analysis of randomized controlled trials. Ann Oncol: Off J Eur Soc Med Oncol ESMO 24(10):2475–2484. doi:10.1093/annonc/mdt226
Gladkov O, Moiseyenko V, Bondarenko IN, Shparyk JV, Barash S, Herpst JM, Mueller U (2012) A randomized, non-inferiority study of balugrastim and pegfilgrastim in breast cancer patients receiving myelosuppressive therapy. Support Care Cancer 20:S234
Braess J, Spiekermann K, Staib P, Gruneisen A, Wormann B, Ludwig WD, Serve H, Reichle A, Peceny R, Oruzio D, Schmid C, Schiel X, Hentrich M, Sauerland C, Unterhalt M, Fiegl M, Kern W, Buske C, Bohlander S, Heinecke A, Baurmann H, Beelen DW, Berdel WE, Buchner T, Hiddemann W (2009) Dose-dense induction with sequential high-dose cytarabine and mitoxantone (S-HAM) and pegfilgrastim results in a high efficacy and a short duration of critical neutropenia in de novo acute myeloid leukemia: a pilot study of the AMLCG. Blood 113(17):3903–3910. doi:10.1182/blood-2008-07-162842
Noga SJ, Choksi JK, Ding B, Dreiling L, Ozer H (2007) Low incidence of neutropenic events in patients with lymphoma receiving first-cycle pegfilgrastim with chemotherapy: results from a prospective community-based study. Clin Lymphoma Myeloma 7:413–420. doi:10.3816/CLM.2007.n.020
Rader M, Breyer W, Leudke S, Ding B, Dreiling L, Ozer H (2010) Low rate of neutropenia and related events in patients with breast cancer receiving pegfilgrastim from the first cycle of chemotherapy in community practices. Commun Oncol 7:273–280
Acknowledgements
We would like to thank Ryan Bishop and Iain Fotheringham from Oxford PharmaGenesis™ Ltd (UK) for providing assistance with designing and conducting the systematic review. Funding for this support was provided by Amgen (Europe) GmbH. We would also like to acknowledge James O’Kelly of Amgen Ltd for coordinating our research collaboration, for doing the second risk of bias assessment and for his valuable feedback on the manuscript.
Authors’ contributions
All authors were involved in the design of the study. KA was responsible for the first draft of the protocol, which was critically reviewed, further developed and approved by all authors. KA performed the literature search, and collected and extracted the data. AMP was responsible for the risk of bias assessment and the first draft of the manuscript. KA and AMP were responsible for the second draft. All authors contributed to data interpretation, critically reviewed all manuscript versions and approved the final version.
Conflicts of interest
AMP’s institution of employment receives unrestricted scientific/educational grants from Amgen. KA is an employee of Oxford PharmaGenesis™ Ltd, which has received project funding from Amgen. RP received honoraria from Amgen and Roche. GvM receives research funding from Amgen and Teva and served on an advisory board for Amgen. MS’s institution of employment receives unrestricted scientific/educational grants from Amgen and he has served on advisory boards for Amgen. ZS is an employee of Amgen and owns stock and stock options in the company.
Author information
Authors and Affiliations
Corresponding author
Additional information
Schwenkglenks and Zsolt Szabo joint last authorship
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 141 kb)
Rights and permissions
About this article
Cite this article
Pfeil, A.M., Allcott, K., Pettengell, R. et al. Efficacy, effectiveness and safety of long-acting granulocyte colony-stimulating factors for prophylaxis of chemotherapy-induced neutropenia in patients with cancer: a systematic review. Support Care Cancer 23, 525–545 (2015). https://doi.org/10.1007/s00520-014-2457-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-014-2457-z