Abstract
Purpose
The purpose of this study was to assess whether incorporation of an original reproductive health assessment and algorithm into breast cancer care helps providers appropriately manage patient reproductive health goals and to follow laboratory markers for fertility and correlate these with menstruation.
Methods
This prospective observational pilot study was set in an urban, public hospital. Newly diagnosed premenopausal breast cancer patients between 18 and 49 years old were recruited for this study prior to chemotherapy initiation. As the intervention, these patients received a reproductive health assessment and care per the study algorithm at 3-month intervals for 24 months. Blood samples were also collected at the same time intervals. The main outcome measures were to assess if the reproductive health management was consistent with patient goals and to track any follicle-stimulating hormone (FSH) and thyroid-stimulating hormone (TSH) level changes throughout treatment and post-treatment period.
Results
Two patients were pregnant at study initiation. They received obstetric consultations, opted to continue pregnancies, and postpone treatment; both delivered at term without complications. One woman desired future childbearing and received fertility preservation counseling. All women received family planning consultations and received/continued effective contraceptive methods. Seventy-three percent used long-term contraception, 18 % remained abstinent, and 9 % used condoms. During chemotherapy, FSH rose to menopausal levels in 82 % of patients and TSH rose significantly in 9 %. While 82 % of women experienced amenorrhea, 44 % of these women resumed menstruation after chemotherapy.
Conclusions
The assessment and algorithm were useful in managing patients’ reproductive health needs. Chemotherapy-induced endocrine disruption impacted reproductive health.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most common cancer diagnosed in women of childbearing age. According to the American Cancer Society, 288,130 women were newly diagnosed with breast cancer in 2011 [1]. Approximately one quarter of these women were within the reproductive age range. As survival rates for this malignancy continue to improve, quality of life issues have assumed paramount importance. In pursuit of survival, reproductive health issues, including fertility conservation and contraception, are often overlooked. Several studies indicate that the reproductive health needs of women with cancer are inadequately assessed by providers [2–5].
The reproductive health challenges in breast cancer survivors include endocrine disruption, iatrogenic infertility, and teratogenicity. These issues are further complicated by limitations in patient and provider knowledge and preventive management as well as the unreliability of endocrine markers to assess fertility. A case series from our institution documented the exclusion of primary elements of reproductive health care from cancer management [6]. A subsequent survey further highlighted the disconnection between patient reproductive interests and management plans. Approximately half the women surveyed were interested in future childbearing [2]. Of those who had completed childbearing, many were not utilizing contraception [2] and of those who were, lower efficacy barrier methods were most often used [7].
We developed a health assessment and algorithm to incorporate reproductive health into cancer care. We hypothesize that use of this reproductive health assessment and algorithm would better align reproductive health goals and management within the context of breast cancer care.
Materials and methods
Study design and patient population
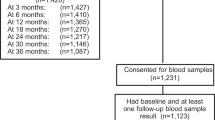
This was an Institutional Review Board-approved, prospective observational pilot study conducted within the Family Planning Division and the Minority-Based Community Clinical Oncology Program (SHCC MBCCOP) of John H. Stroger, Jr. Hospital of Cook County. This study was funded by the Chicagoland Area Affiliate of Susan G. Komen for the Cure. Women were eligible for the study if they were within 3 months of their breast cancer diagnosis, were between the ages of 18 and 49 years at diagnosis, were receiving cancer care at Stroger Hospital, had not initiated chemotherapy or radiation therapy, and had evidence of ovarian function. Pregnant women were eligible. A total of 48 women who presented to the medical oncology clinic were screened for study eligibility by SHCC MBCCOP staff. Twenty-nine women (60 % of total women presenting) were deemed ineligible: 10 (34 %) for having non-malignant breast tumors, 9 (31 %) for prior initiation of chemotherapy or radiation therapy, 1 (3 %) was postmenopausal, 2 (7 %) had prior hysterectomy, 2 (7 %) planned to receive treatment at outside institutions, and 5 (17 %) were more than 3 months post-diagnosis. Three (6 % of total women presenting) patients were not recruited for participation due to physician disinterest in the study. Of the remaining 16 (33 % of total women presenting) eligible patients, 5 (31 %) declined participation. All participants signed informed consent documents prior to their inclusion in the study. Participants were recruited from March 2008 through April 2009 and followed up for 24 months.
Questionnaire
We designed a 30-question reproductive health assessment to determine perceptions and choices regarding basic sexuality, contraception, and oncofertility. The types of questions that were addressed included the following: diagnosis, stage of disease, age of diagnosis, cancer history, treatment plan, contraceptive history and current usage, menses, sexuality, childbearing desires, concern for child health during cancer treatment, fertility details, and plans for unintended pregnancy during treatment. The survey instrument utilized was not validated; however, it may serve as a base for future patient assessments among reproductive age women with a diagnosis of breast cancer. This assessment was administered to study participants upon enrollment and at 3-month intervals for 24 months.
Algorithm
An algorithm (Fig. 1) was designed and followed to promote care consistent with each patient’s reproductive health assessment. The algorithm initially stratified subjects into pregnant and non-pregnant groups. Pregnant women were referred for obstetrics and gynecology consultations to determine a pregnancy plan that was consistent with the patient’s planned cancer treatment. Women who were not pregnant were referred for a family planning consultation to discuss contraception and future childbearing interests. Further stratification along the algorithm was based on desire for future childbearing. Those who had not completed childbearing were referred for a reproductive endocrinology consultation to discuss fertility preservation options.
Biochemical markers
Assessments of follicle-stimulating hormone (FSH) and thyroid-stimulating hormone (TSH) levels were scheduled at baseline, prior to each chemotherapy cycle and every 3 months post-treatment. Women who were pregnant at study enrollment did not receive laboratory assessments until postpartum.
Objectives and statistics
Study objectives were to (1) perform periodic reproductive health assessments and to follow a reproductive health algorithm for each patient, (2) implement reproductive health management plans in accordance with reproductive health goals derived from the reproductive health assessment, (3) follow laboratory markers for fertility status and assess prevalence of endocrine disruption, and (4) correlate laboratory markers with menstruation.
The primary endpoint for objectives 1 and 2 was adoption of appropriate reproductive health management consistent with reproductive health goals as stated at baseline. Management was considered appropriate if (1) pregnant women were referred to an obstetrician and were counseled about pregnancy options, (2) women interested in future childbearing were counseled and provided a referral for fertility preservation counseling, or (3) women not interested in immediate pregnancy, independent of desire for future childbearing, were provided a referral for family planning to receive contraception consistent with plans for sexual activity and future childbearing.
For objectives 3 and 4, FSH level was considered to have reached the menopausal range at >23.0 mIU/mL. TSH level below 0.20 and above 4.50 mIU/L was considered abnormal and an indication of endocrine disruption. Menstruation information was collected in the reproductive health assessment. Women were asked the date of their last menstrual period, if they were menstruating regularly, and whether they believed they had stopped menstruating.
Statistical analyses were performed using SAS 9.2. Descriptive statistics were used to analyze the study population. Pearson correlation coefficient was used to evaluate the association between menstruation and laboratory markers; student t tests were used to evaluate age differences between those who experienced endocrine disruption and those who did not.
Staff training
Referral lines to appropriate members of the Obstetric and Gynecologic faculty were developed to operate within the MBCCOP/medical oncology service.
Results
Demographics
The 11 study patients ranged in age from 23 to 48 years (mean, 39; SD, 7.4). Of these, 27 % (3/11) had no children, 9 % (1/11) had one child, and 64 % (7/11) had two or more children. The majority of participants were English-speaking Hispanics, 64 % (7/11); the remaining 36 % (4/11) identified themselves as Black/African American. Upon enrollment, 27 % (3/11) of women were single, 64 % (7/11) married, and 9 % (1/11) divorced. Education level varied: 27 % (3/11) completed primary school, 9 % (1/11) attended some high school, 46 % (5/11) graduated from high school, and 18 % (2/11) attended some college (Table 1).
Reproductive health assessment/algorithm navigation
Of the 11 women, two (18 %) were pregnant at the time of recruitment and nine (82 %) were not. Both pregnant women had become pregnant after or during cancer staging but prior to initiation of chemotherapy. Each had an obstetric consultation and chose to continue their pregnancies. Each delivered at term without complication. One woman began chemotherapy in the second trimester; the other began postpartum. After completion of their pregnancies, these two women passed over into the “Not Pregnant” cohort of the algorithm.
Of the 11 women in the newly constituted “Not Pregnant” cohort, 9 % (1/11) had not yet completed childbearing, whereas 91 % (10/11) had. The one woman interested in future childbearing received fertility preservation counseling. All 11 women, independent of future childbearing interest, received a family planning consultation.
At the time of cancer diagnosis, 18 % (2/11) of women had already undergone permanent sterilization and 18 % (2/11) had an intrauterine device (IUD) in place. After receiving family planning consultations, an additional 37 % (4/11) of women selected an IUD. Throughout the study period, 73 % (8/11) of women continued or started long-term contraception, whereas 18 % (2/11) remained abstinent and 9 % (1/11) selected condoms. Both women who were pregnant at study initiation received IUDs after delivery. Of note, the one woman who had not yet completed childbearing selected an IUD for interim contraception.
By study definition, 100 % of the women received appropriate referrals consistent with their initial and continued reproductive health goals. The two pregnant women were referred to an obstetrician before delivery and to a family planning specialist after delivery. The one woman interested in future childbearing had both a reproductive endocrinology as well as a family planning referral. The remaining eight women who had completed childbearing received family planning consultations.
Reproductive health biomarkers and menstrual findings
Of the nine non-pregnant participants at study initiation, seven reported cessation of menses at an average of 3.3 months (SD, 1.6) after initiation of chemotherapy. Of the two remaining women, one continued to menstruate regularly, although she did not adhere to her chemotherapy regimen. The other indicated irregular bleeding but was not able to assess whether menstruation had ceased. Of the seven patients who reported cessation of menstruation, three confirmed resumption at an average of 12.6 months (SD, 2.9) after the initiation of chemotherapy. The two pregnant women did not resume menstruation between delivery and initiation of chemotherapy, and thus cessation information was not available. One of these women resumed menstruation 8.9 months after initiation of chemotherapy.
In total, five women indicated they were menstruating after chemotherapy, five indicated they were not, and one was unsure. The mean age of those who reported menstruating after chemotherapy was 36.8 years (SD, 4.86) which differed significantly from those who did not report menstruating after chemotherapy, mean age of 44 years (SD, 3.39) (p = 0.0265).
Of the 11 women, 10 were followed up for laboratory assessments of fertility, examining FSH and TSH. Changes in FSH and TSH were noteworthy. FSH rose to menopausal levels during chemotherapy treatment in 90 % (9/10) of study participants. Forty-four percent (4/9) of these women returned to premenopausal levels after treatment within the 24-month time period (Fig. 2). The woman whose FSH levels did not reach menopausal levels was not adherent to chemotherapy regimens. Mean age of the five women who had a FSH level within the premenopausal range at study completion was 35 years (SD, 7.84), whereas the mean age of those whose FSH levels remained elevated was 44 years (SD, 3.39). This difference was statistically significant (p = 0.0463).
For the nine individuals where FSH levels and menstruation status was known, four indicated that they were menstruating after chemotherapy. FSH returned to premenopausal levels after chemotherapy in these four individuals. Similarly, FSH levels among the five individuals who failed to resume menstruation after chemotherapy remained within menopausal levels throughout the remainder of the study. For these nine individuals, FSH levels and menstrual status after treatment correlated perfectly (p < 0.0001).
One of the 10 patients had a mildly elevated level of TSH (4.56 uIU/mL) at study entry which returned to normal levels by her next blood draw, 2.8 months after chemotherapy initiation. A second patient experienced a slight drop in TSH (0.143 uIU/mL) 2.8 months after chemotherapy initiation. These levels returned to normal at the next blood draw, 10.5 months after chemotherapy initiation. A third patient experienced a profound and unexpected change in TSH (137 uIU/mL) 8.4 months after chemotherapy initiation (Fig. 3).
Discussion
The use of the reproductive health assessment and algorithm assisted providers to ensure that all study participants received management consistent with their personal reproductive health goals.
Inconsistencies between reproductive health desires and management are problematic. Breast cancer survivors are advised to defer future childbearing for up to 3 years post-treatment due to the threat of cancer progression and teratogenic therapies [8]. Regardless, one study of 114,165 patients showed that 6 % of pregnancies occurred in women prescribed category D and X medications [9, 10]. In our study, the two patients who were pregnant at study initiation became pregnant after their diagnosis of cancer. These pregnancies were unintended and may have been prevented by earlier contraceptive counseling on the part of the diagnostic team. While some physicians may find it difficult to initiate conversations regarding reproductive health following initial diagnosis, this is a critical timeframe to prevent unintended pregnancy and/or early infertility.
Contraceptive provision is essential for breast cancer survivors who may temporarily or permanently wish to defer childbearing. Unintended pregnancy accounts for 50 % of all pregnancies in the USA [11], and this rate is higher among women with chronic diseases [12–14]. Women with chronic disease are more likely to terminate an unintended pregnancy [15, 16] compared to matched control subjects. However, contraceptive provision to women with breast cancer is complicated by the fact that estrogen and progestin are largely contraindicated, thereby limiting options [17]. Lack of provider knowledge of appropriate and effective, non-hormonal contraceptives (copper IUD) may impede patient access.
The fact that most patients had completed childbearing at study entry may not reflect the general population. With one study reporting that 56 % of young breast cancer survivors desire future childbearing at the time of their diagnosis [18], it is imperative that physicians assess patient’s future childbearing plans to ensure referrals for fertility preservation when appropriate.
Some cancer therapy modalities induce amenorrhea. After a short period of chemotherapy-induced amenorrhea, 50 % of women younger than 35 years resume menstruation, whereas in older women, the risk of amenorrhea is increased due to reduced follicular reserve [19]. Our results were consistent with this observation that older age was associated with continued amenorrhea. The absence of menstruation, however, does not necessarily indicate lack of ovarian function and fertility [20]. Additionally, the possibility of spontaneous recovery of ovarian function has been observed [20]. Literature suggests that fertility rates may decrease between 10 to 50 % post-chemotherapy [21–23].
For patients who undergo chemotherapy, ovarian function should be reassessed periodically. This reassessment may serve dual purposes, guiding those who wish to maintain fertility as well as those who do not. Since menstruation is not a reliable index of ovarian function, various tests assessing FSH, inhibin A or B or antimullerian hormone (AMH) levels and vaginal ultrasonography assessment for number of antral follicles can be used [20]. Our study demonstrated significant alterations in FSH levels and menstruation. Although the sample size was limited, age was associated with endocrine disruption. Evaluation of a large sample of patients will be necessary to develop correlations between laboratory and physical findings and long-term fertility status. Recent literature indicates the best biochemical indicators of ovarian reserve may be serum FSH and AMH levels [24, 25]. The knowledge of functional ovarian reserve may benefit patients prior to making important decisions regarding treatment, fertility preservation, and contraception [26]. Additionally, while pregnancy may be a future goal, contraception, specifically long-term reversible contraception, should be offered even if pregnancy is deferred for only 1 year.
An important finding of this study relates to the alteration in TSH level observed in 3 of the 10 women who completed the laboratory portion of the study. Alterations in thyroid function have been noted in women with breast cancer with a baseline rate of autoimmune thyroid disease 2–3 times that of the general population [27]. Thyroid function may be affected by chemotherapy and radiation treatment, particularly when treatment is localized to the vicinity of the thyroid [28]. At this point, however, the literature does not support guidelines regarding screening for thyroid disease in cancer care.
The major strength of this investigation was its ability to pilot the use of a previously untested algorithm to better guide assessment of and compliance with reproductive health needs of patients presenting with breast cancer. The unexpected detection of a serious abnormality in TSH levels warrants further investigation in a large sample. Indeed, our small sample size was a limitation in obtaining a greater appreciation of laboratory abnormalities in this population. It also inhibited us from correlating chemotherapy and menstrual cessation. Both areas need further study. Future studies should also include investigations of AMH. Additionally, no quality of life indicators were collected in the survey to gauge whether reproductive health referrals impacted patient well-being. As the study was implemented in a safety net institution, participants may not be generalizable to the greater population of women diagnosed with cancer during reproductive age. Finally, because of its pilot nature, this study did not include controls.
In summary, this study demonstrates that the utilization of our novel reproductive health assessment and algorithm may significantly improve reproductive health management within the context of cancer care. The second finding of the study demonstrates women with breast cancer undergoing chemotherapy experience significant endocrine disruptions affecting the ovaries and possibly thyroid. Such disruptions may lead to symptoms that affect quality of life indicators related to reproductive health. Implications of both tenants of this study remain the important aspects of research within oncology care. A large-scale, multicenter trial has been developed based on the findings of this important pilot study.
References
American Cancer Society. Breast cancer facts & figures 2011–2012. Atlanta: American Cancer Society, Inc
Patel A, Sreedevi M, Malapati R, Sutaria R, Schoenhage MB, Patel AR et al (2009) Reproductive health assessment for women with cancer: a pilot study. Am J Obstet Gynecol 201(2):190
Duffy CM, Allen SM, Clark MA (2005) Discussions regarding reproductive health for young women with breast cancer undergoing chemotherapy. J Clin Oncol 23(4):766–73
Thewes B, Meiser B, Rickard J, Friedlander M (2003) The fertility- and menopause-related information needs of younger women with a diagnosis of breast cancer: a qualitative study. Psychooncology 12(5):500–11
Peate M, Meiser B, Hickey M, Friedlander M (2009) The fertility-related concerns, needs and preferences of younger women with breast cancer: a systematic review. Breast Cancer Res Treat 116(2):215–23
Patel AA, Mini S, Sutaria RP, Schoenhage MB, Patel AR, Radeke EK et al (2008) Reproductive health issues in women with cancer. J Oncol Pract 4(2):101–5
Steiner MJ, Trussell J, Johnson S (2007) Communicating contraceptive effectiveness: an updated counseling chart. Am J Obstet Gynecol 197(1):118
Helewa M, Levesque P, Provencher D, Lea RH, Rosolowich V, Shapiro HM (2002) Breast cancer, pregnancy, and breastfeeding. J Obstet Gynaecol Can 24(2):164–80, quiz 81-4
Steinkellner A, Chen W, Denison SE (2010) Adherence to oral contraception in women on category X medications. Am J Med 123(10):929–34 e1
Andrade SE, Raebel MA, Morse AN, Davis RL, Chan KA, Finkelstein JA et al (2006) Use of prescription medications with a potential for fetal harm among pregnant women. Pharmacoepidemiol Drug Saf 15(8):546–54
Finer LB, Zolna MR (2011) Unintended pregnancy in the United States: incidence and disparities, 2006. Contraception 84(5):478–85
St James PJ, Younger MD, Hamilton BD, Waisbren SE (1993) Unplanned pregnancies in young women with diabetes. An analysis of psychosocial factors. Diabetes Care 16(12):1572–8
Holing EV, Beyer CS, Brown ZA, Connell FA (1998) Why don't women with diabetes plan their pregnancies? Diabetes Care 21(6):889–95
Chor J, Rankin K, Harwood B, Handler A (2011) Unintended pregnancy and postpartum contraceptive use in women with and without chronic medical disease who experienced a live birth. Contraception 84(1):57–63
Green DM, Whitton JA, Stovall M, Mertens AC, Donaldson SS, Ruymann FB et al (2002) Pregnancy outcome of female survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Am J Obstet Gynecol 187(4):1070–80
Winther JF, Boice JD Jr, Svendsen AL, Frederiksen K, Olsen JH (2009) Induced abortions in Danish cancer survivors: a population-based cohort study. J Natl Cancer Inst 101(9):687–9
Mitwally MF (2008) Management of reproductive needs in cancer patients: clinical perspectives. Expert Rev Anticancer Ther 8(10):1589–95
Partridge AH, Gelber S, Peppercorn J, Sampson E, Knudsen K, Laufer M et al (2004) Web-based survey of fertility issues in young women with breast cancer. J Clin Oncol 22(20):4174–83
Chasle S, How CC (2003) The effect of cytotoxic chemotherapy on female fertility. Eur J Oncol Nurs 7(2):91–8
Del Mastro L, Catzeddu T, Venturini M (2006) Infertility and pregnancy after breast cancer: current knowledge and future perspectives. Cancer Treat Rev 32(6):417–22
Schwarz EB, Hess R, Trussell J (2009) Contraception for cancer survivors. J Gen Intern Med 24(Suppl 2):S401–6
Byrne J, Mulvihill JJ, Myers MH, Connelly RR, Naughton MD, Krauss MR et al (1987) Effects of treatment on fertility in long-term survivors of childhood or adolescent cancer. N Engl J Med 317(21):1315–21
Cvancarova M, Samuelsen SO, Magelssen H, Fossa SD (2009) Reproduction rates after cancer treatment: experience from the Norwegian radium hospital. J Clin Oncol 27(3):334–43
Gleicher N, Weghofer A, Barad DH (2010) Anti-Mullerian hormone (AMH) defines, independent of age, low versus good live-birth chances in women with severely diminished ovarian reserve. Fertil Steril 94(7):2824–7
Su HI, Sammel MD, Green J, Velders L, Stankiewicz C, Matro J et al (2010) Antimullerian hormone and inhibin B are hormone measures of ovarian function in late reproductive-aged breast cancer survivors. Cancer 116(3):592–9
Rosendahl M, Andersen CY, la Cour Freiesleben N, Juul A, Lossl K, Andersen AN (2010) Dynamics and mechanisms of chemotherapy-induced ovarian follicular depletion in women of fertile age. Fertil Steril 94(1):156–66
Reinertsen KV, Cvancarova M, Wist E, Bjoro T, Dahl AA, Danielsen T et al (2009) Thyroid function in women after multimodal treatment for breast cancer stage II/III: comparison with controls from a population sample. Int J Radiat Oncol, Biol, Phys 75(3):764–70
Giani C, Fierabracci P, Bonacci R, Gigliotti A, Campani D, De Negri F et al (1996) Relationship between breast cancer and thyroid disease: relevance of autoimmune thyroid disorders in breast malignancy. J Clin Endocrinol Metab 81(3):990–4
Acknowledgments
The authors would like to thank Vanessa Barrera for her contributions of data collection and Kelly Stempinski for her contributions of manuscript editing.
Financial disclosures
Funding for this research was provided by The Chicagoland Affiliate of Susan G. Komen for the Cure, Hillside, IL, Grant No. 3010.
Conflict of interest
None of the coauthors have a conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Patel, A., Roston, A., Uy, A. et al. Reproductive health and endocrine disruption in women with breast cancer: a pilot study. Support Care Cancer 23, 411–418 (2015). https://doi.org/10.1007/s00520-014-2381-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-014-2381-2