Abstract
Objective
This study aims to examine the associations between musculoskeletal pain and health-related quality of life (HR-QOL) among breast cancer patients on aromatase inhibitors (AIs) and women without a history of breast cancer.
Methods
A cross-sectional study was conducted among 68 breast cancer patients on AIs for an average of 3.5 years and 137 postmenopausal women without a history of cancer. Musculoskeletal pain was assessed using a 10-cm visual analog scale; HR-QOL was examined using the Medical Outcomes Study Short Form (SF-36) health survey. Linear regression was used to estimate the associations between pain and HR-QOL in both groups.
Results
Approximately 64 % of the breast cancer patients and women in the comparison group reported musculoskeletal pain. Among women with breast cancer, those with pain had significantly lower HR-QOL scores in the physical (52.2 vs. 42.6; p < 0.001) and mental (52.7 vs. 45.5; p = 0.01) component summary scores compared with those without pain. In the comparison group, pain was associated with significantly lower scores in the physical (55.4 vs. 46.0; p < 0.001), but not the mental, component summary score (52.1 vs. 52.4; p = 0.82). The significant associations between pain and HR-QOL persisted after confounder adjustment in both groups. Among women with similar severity of pain, breast cancer patients reported significantly lower HR-QOL in the mental summary component compared with the women in the comparison group.
Conclusions
Among breast cancer patients, musculoskeletal pain adversely affects both mental and physical components of HR-QOL. Preventing or treating AI-associated musculoskeletal pain may improve overall HR-QOL among breast cancer patients treated with AIs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The majority of women with breast cancer are candidates for therapeutic hormonal interventions [1]. Tamoxifen, a selective estrogen receptor modulator (SERM), was the gold standard for treating women with estrogen receptor positive (ER+) forms of breast cancer for many decades until the introduction of aromatase inhibitors (AIs) [2–4]. Proven to be superior to tamoxifen in reducing recurrence and prolonging disease-free survival [5–7], AIs are now first line therapy for postmenopausal women with hormone responsive breast cancer [8, 9]. SERMs and AIs have different mechanisms of action and adverse side effect profiles. Serious adverse effects such as endometrial cancer and venous thromboembolism are less likely to be reported in women on AIs compared with tamoxifen users [10–12]. However, women on AIs are more likely than women on tamoxifen to report musculoskeletal pain [13–15].
The etiology of the musculoskeletal pain remains uncertain, and there is no consensus on the treatment and management of the women who report pain. Randomized clinical trials report that 5–35 % of women taking AIs experience musculoskeletal symptoms, depending on how the symptoms were assessed and categorized [15]. Outside of the randomized clinical trial setting, the prevalence of musculoskeletal symptoms is higher, with studies reporting up to 50 % of women on AIs experiencing symptoms; some of these symptoms are so severe that they require rheumatologic referral [16–21].
A major challenge with the proper assessment of these musculoskeletal symptoms is separating symptoms attributable to AI use from other forms of arthritis, as there is no way to distinguish the drug-induced musculoskeletal aches and pains from other causes of the symptoms. Musculoskeletal symptoms such as joint pain increase with age, especially after the menopausal transition [22–25]. A recent study conducted in women on AIs and a comparison group of postmenopausal women found that the AI-associated symptoms usually present as symmetric pain with a predominant location in the hands, wrists, and feet [26].
Prior studies in noncancer populations have shown that the adverse effect of musculoskeletal pain goes beyond its physical effect on reduced function and activity limitation as it may also negatively affect an individual’s emotional and social well being [27]. Furthermore, development of musculoskeletal pain in women with breast cancer could have a more exaggerated impact on health-related quality of life (HR-QOL) relative to women without a history of breast cancer due to potential distress from a cancer diagnosis. Although randomized clinical trials have evaluated the impact of AI therapy on HR-QOL [28–33], because the musculoskeletal symptoms were an unexpected adverse effect, the studies were not designed to assess the specific association between these musculoskeletal symptoms and HR-QOL.
The objective of this cross-sectional study was to examine the associations between musculoskeletal symptoms and HR-QOL (physical and mental components) in women with breast cancer treated with AIs and a comparison group of women without breast cancer.
Methods
Study population
This cross-sectional study was conducted in a cohort of women with and without breast cancer enrolled in a study at Mercy Medical Center, Baltimore MD between September 2006 and November 2009. The prospective cohort study assessed the incidence of musculoskeletal symptoms in women with breast cancer on AIs within the first 6 months of initiating therapy. Details of the study design and the original cohort have been previously described [26]. Briefly, women with breast cancer were enrolled from the Hoffberger Breast Center and the Medical Oncology practice prior to initiating treatment with AIs. Study participants were postmenopausal, had nonmetastatic (stages I–III) breast cancer, and had completed their surgical treatment and, if indicated, chemotherapy and/or radiation therapy for breast cancer. The participants in the comparison group were women receiving routine screening mammography or gynecologic care and were enrolled through the Weinberg Center for Women’s Health and Medicine. In both groups, women with a history of rheumatoid arthritis or any other cancer except nonmelanoma skin cancer or cervical cancer in situ were ineligible. Written informed consent was obtained from each study participant. The Mercy Medical Center Institutional Review Board approved the study protocol.
A follow-up of the original study cohort was conducted in September 2011, an average of 3.5 years from participant enrollment in the parent study. Women were mailed a self-administered questionnaire to obtain data on changes to their medical history as well as to assess musculoskeletal symptoms and HR-QOL. Of the 289 participants (95 with breast cancer and 194 in the comparison group) who completed the baseline assessment, 12 women (ten with breast cancer and two in the comparison group) were deceased at the time of the follow-up. Six of the women with breast cancer died from breast cancer-related deaths (metastatic disease); the other deaths among the breast cancer patients were noncancer related. Causes of death in the two women in the comparison group were unknown. Thus, 277 follow-up questionnaires were mailed, with ten undeliverable because the participants had moved and had no forwarding address. A repeat mailing was sent to nonrespondents with follow-up telephone calls to women who did not return completed questionnaires within 3 weeks of the mailing. Of the 267 women who received the follow-up questionnaire, 211 women (69 with breast cancer and 142 in the comparison group) agreed to participate in the follow-up study by returning completed questionnaires, for a response rate of 82.1 and 76.7 % in women with breast cancer and the comparison group, respectively.
In the breast cancer group, responders and nonresponders were similar in demographics, including age, race, marital status, and education. There were also no statistically significant differences in musculoskeletal pain including proportion reporting pain and reported severity of musculoskeletal pain at the last study assessment (6 months). In the comparison group, nonresponders were more likely to be nonwhite (26.8 % vs. 11.6 %; p = 0.009) and to report joint pain at the last study assessment (79.3 vs. 48.8 %; p = 0.003) compared with nonresponders.
Five women in the comparison group who had been recently diagnosed with cancer (breast and ovarian) and one woman in the breast cancer group whose disease had progressed to metastatic breast disease were excluded from the analysis. Thus, the final sample size for the cross-sectional study was 68 women with breast cancer and 137 postmenopausal women without a history of breast cancer. Of the 68 women with breast cancer who responded to the follow-up questionnaire, 16 (23.5 %) had discontinued AI therapy at the time of follow-up.
Study variables
Musculoskeletal pain
The questionnaire included the same detailed assessment of musculoskeletal symptoms (muscle, joint, and bone) that was used in the parent study [26]. Participants were queried on the presence of muscle, joint, or bone pain over the previous 4 weeks, including an assessment of frequency, duration, and severity. Average severity of pain (in the past 4 weeks) was assessed using a 10-cm visual analogue scale (VAS). Respondents were classified as having musculoskeletal pain (bone, muscle, and joint) if they indicated a >0 pain on the VAS. The highest score reported for any of the pain types (joint, muscle, and bone) was used as a measure of the severity of overall (any) musculoskeletal pain for each study participant.
Health-related quality of life
Study participants completed the Medical Outcomes Study short form 36 questionnaires (SF-36) [34]. The SF-36 is a widely used nondisease-specific instrument for assessing HR-QOL, designed for self-completion or for administration by an interviewer. The questionnaire has been validated in the cancer population as well as in the general population [35–37]. High internal consistency of the SF-36 subscales has been reported with Cronbach’s alpha in the range of 0.68 to 0.94 [37]. Questions in the SF-36 refer to the 4-week time period prior to the completion of the questionnaire. The instrument captures both the physical (physical functioning, role limitations due to physical health problems, bodily pain, and general health perceptions) and mental (vitality-energy/fatigue, social functioning, role limitations because of emotional problems, and general mental health) aspects of health in eight subscales [34]. Responses for each domain were transformed into a score of 0–100, with higher scores indicating better HR-QOL. Summary scores (physical and mental) were computed from the responses to the different HR-QOL subscales for each study participant using a weighted algorithm through a computer-based program provided by Quality Metrics.
Other covariates
Self-reported information was obtained on demographics including age (years), race (white vs. nonwhite), education (years), and marital status (married vs. single, divorced, or widowed) at the baseline visit. Body mass index (BMI) was calculated based on self-reported height and weight obtained from each study participant and further classified as normal/ underweight (<25 kg/m2), over weight (≥25 to <30) or obese (≥30). Study participants were also queried on their health history including past and current medical diagnoses. For the purposes of this study, a score was calculated by summing up the total number of health conditions reported by each participant (other than breast cancer) with each health condition contributing equal weight. All women with breast cancer ever treated with an AI were grouped together in the analysis regardless of treatment switches or therapy discontinuation.
Data analyses
Analyses were conducted within each group (cancer /non-cancer) to examine the association between HR-QOL and presence of musculoskeletal pain. To assess the impact of current AI use, we also conducted the analysis within the breast cancer group stratified by current AI use (currently treated/discontinued AI). Within each group, comparisons were made between those with and without pain. Demographic and clinical characteristics (age, race, marital status, education, BMI, and health conditions) by musculoskeletal pain status among the women with breast cancer and those in the comparison group were compared using t tests for continuous variables and Chi-square and Fisher exact tests for categorical variables.
Musculoskeletal pain was analyzed as a dichotomous (yes/no) variable and as a continuous variable (0–10), as reported on the VAS. Analyses were conducted separately for each of the three pain types (joint, muscle, and bone). An overall measure of musculoskeletal pain severity (any pain) was also derived using the highest score reported for any of the pain types (joint, muscle, and bone) for each study participant. Additionally, musculoskeletal pain severity was classified into mild (VAS 0–3), moderate (VAS >3–6), and severe pain (VAS >6–10); these categories have been shown to be valid and to yield reproducible assessments in patients with cancer and in the noncancer population [38–39].
Analysis of variance (ANOVA) was used to evaluate differences in the mean HR-QOL score by severity (categorical) of musculoskeletal pain (any, muscle, joint, and bone). All analyses were conducted for the two summary component scores (physical and mental) and subsequently for each of the eight subscales. Linear regression models were used to estimate the independent associations of any musculoskeletal pain (continuous) with HR-QOL adjusting for factors associated with both musculoskeletal pain and HR-QOL. Separate regression models were developed for the two composite dimensions of HR-QOL (physical component summary (PCS) and mental component summary (MCS)) in women with and those without breast cancer. A stepwise method was used to add each potential confounding factor to the model. Age, race, marital status, education, and clinical factors, including BMI and number of health conditions, were adjusted for in the analysis. Regression models in women with breast cancer also adjusted for cancer treatment variables—prior chemotherapy, prior radiation, and current use of AI therapy.
An alpha level of p ≤ 0.05 (two tailed) was established for significance in all statistical analyses, which were conducted using SAS statistical software v. 9.2.
Results
Patient characteristics
In the analytic sample, musculoskeletal pain was reported in 64.7 and 63.5 % of the women with breast cancer and the comparison group, respectively. Characteristics of study participants, stratified by musculoskeletal pain status within the breast cancer and the comparison groups, are presented in Table 1. There were no statistically significant differences in age, race, marital status, and education among those with musculoskeletal pain compared with those without pain among the women with breast cancer. Among women with breast cancer, women who discontinued treatment were more likely to report pain compared with those currently treated (68.8 vs. 63.5 %), although the difference was not statistically significant (data not shown). Similarly, within the comparison group, no demographic variables were significantly associated with musculoskeletal pain. In both groups, subjects with musculoskeletal pain tended to report a higher number of health conditions compared with those without pain, although the difference was not statistically significant. In the breast cancer group, the mean time since diagnosis was approximately 4 years. Time since breast cancer diagnosis and time since chemotherapy and/or radiation treatment were not significantly associated with presence of musculoskeletal pain.
Health-related quality of life
Table 2 shows the associations between musculoskeletal pain and HR-QOL subscales according to breast cancer status. Patterns of association varied by cancer status; among women with breast cancer, women with musculoskeletal symptoms reported statistically significantly lower scores in all HR-QOL subscales assessed compared with women without pain. Women who were currently taking AI had a slightly higher mean score both in the PCS score (48.3 vs. 46.6) and in the MCS score (47.1 vs. 43.8) compared with women that discontinued AI treatment, although the difference was not statistically significant. In the noncancer group, all physical component subscales (physical functioning, role-physical, general health, and bodily pain) and two mental health subscales (vitality and social functioning) were lower in women with musculoskeletal pain compared with women without pain. Role emotional, mental health, and the composite mental health summary score were not significantly associated with musculoskeletal pain among women in the comparison group.
The diagnosis of breast cancer, even in the absence of musculoskeletal pain, may affect HR-QOL. To assess this, analyses were also conducted stratifying by presence or absence of pain and then comparing those with and without breast cancer (Table 3). Among women with musculoskeletal pain, women with breast cancer reported significantly lower HR-QOL in all subscales assessed except bodily pain (54.4 vs. 59.7; p = 0.18) and the PCS (42.6 vs. 46.0; p = 0.06) compared with the women without breast cancer. By contrast, in the group without musculoskeletal pain, there were no statistically significant differences in the HR-QOL subscales except for physical functioning comparing women with and without breast cancer.
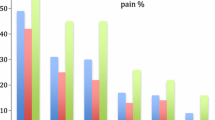
For the association between pain severity and HRQOL, analyses were conducted according to categories of pain severity (low, moderate, and severe), stratified by breast cancer status. The results are displayed in Fig. 1. Both the breast cancer and the noncancer groups had evidence of a dose–response trend in the physical component of the HR-QOL assessment; specifically, increasing severity of pain was associated with poorer physical HR-QOL. However, the patterns were different between women with breast cancer and the comparison group for the mental component. A trend of poorer mental component with increasing severity of pain was noted among breast cancer patients but not among women in the comparison group.
Health-related quality of life (physical component summary and mental component summary) by severity of any musculoskeletal pain (low, 0– < 3; moderate, 3– < 6; and severe, 6–10) in women with breast cancer and comparison group. Values reported are means. *p < 0.05, significant difference of the HR-QOL score by severity of musculoskeletal pain among women with breast cancer and in the comparison group
Potential differences in HR-QOL among subjects with similar severity of musculoskeletal pain between the cancer and noncancer groups were also assessed. Among women with similar severity of musculoskeletal pain, breast cancer patients with moderate and severe pain also reported significantly lower HR-QOL in the MCS but not PCS relative to the comparison group (Fig. 2).
Health-related quality of life (physical component summary and mental component summary) by severity of any musculoskeletal pain (low, 0– < 3; moderate, 3– < 6; and severe, 6–10) in breast cancer and comparison group. *p < 0.05, significant difference of the HR-QOL score among women with breast cancer and the comparison group by severity of musculoskeletal pain
The association between musculoskeletal pain and HR-QOL was also examined adjusting for confounders among both the women with breast cancer and the comparison group. Table 4 shows the unadjusted and adjusted associations between musculoskeletal pain and the physical and mental components of HR-QOL, stratified by group. In both the unadjusted and adjusted analyses, musculoskeletal pain was significantly associated with both the physical (−2.1; p < 0.001) and mental (−1.8; p < 0.001) dimensions of HR-QOL among women with breast cancer. We observed that race was independently associated with both the PCS and MCS in women with breast cancer. Sensitivity analysis conducted limiting the analysis to only subjects that were still on AI (n = 42) yielded similar results. A significant association was only observed for the physical health domain (−2.2; p < 0.001) in the comparison group.
Discussion
The study identified differences in the association of musculoskeletal pain on HR-QOL in women with breast cancer compared with the women without a history of breast cancer. Among those with breast cancer, women with musculoskeletal pain reported significantly lower HR-QOL in all subscales assessed, including the physical and mental summary components. By contrast, in the comparison group, the impact of musculoskeletal pain was only observed in the physical but not the mental health summary component of HR-QOL.
Furthermore, increasing pain severity had an incremental adverse effect on physical HR-QOL that was similar among women with breast cancer and women in the comparison group. However, the association between increasing pain severity and lower mental health HR-QOL was only observed among the women with breast cancer. Comparing women with breast cancer to the women without a history of breast cancer, among those with similar severity of musculoskeletal pain, women with breast cancer reported lower mental HR-QOL. This is the first study, to our knowledge, that has examined the specific association between AI-associated musculoskeletal pain and HR-QOL in women with breast cancer.
The incremental adverse effect of musculoskeletal symptoms on the physical HR-QOL subscales such as physical functioning and bodily pain found in both groups is not surprising, particularly among women with breast cancer. Women have likened the musculoskeletal symptoms experienced while taking AIs to “feeling like they aged suddenly” [40]. The results of this study provide an objective measure of the association between this side effect and well-being.
Musculoskeletal symptoms were significantly associated with the mental HR-QOL of women with breast cancer experiencing musculoskeletal pain but not among the cancer-free women with pain. One can only speculate that for women with breast cancer, musculoskeletal pain may raise concern about metastatic disease, thus causing more anxiety and distress than among women without a history of cancer. Prior studies have reported that up to 30 % of women with breast cancer experience psychological distress many years after the completion of their primary treatment [41], and pain may be a factor in this distress. The findings from the study suggest that musculoskeletal pain rather than the diagnosis of cancer may be a source of distress, as mental HR-QOL in the absence of pain was similar to the comparison group. The fear of cancer recurrence has been shown to be associated with psychological distress [42, 43] and lower HR-QOL [42]. van den Beuken-van Everdingen and colleagues, in their study of 136 women with breast cancer, also found that pain was a strong predictor of overall fear and of fear of cancer recurrence [43]. Thus, musculoskeletal pain in women with breast cancer may further exacerbate persistent concerns about cancer recurrence.
Certain limitations must be taken into consideration when interpreting the study findings, including the cross-sectional design, which precludes the determination of cause and effect, and the small sample size. It is possible that women with lower mental component HR-QOL scores may be more likely to report musculoskeletal pain; thus, the temporality of these associations remains to be explored. Furthermore, the results may also not be generalizable to women of other race/ethnic groups because we had a high percentage of Caucasian women and there may potentially be racial/ethnic differences in the association, between musculoskeletal pain and the HR-QOL outcomes [44–46]. We observed that race was independently associated with both the PCS and MCS in women with breast cancer. The finding is consistent with prior studies that have also reported race and ethnic disparities in quality of life among women with breast cancer, with Caucasian women reporting higher HR-QOL [47, 48]. A potential reason for difference in HR-QOL by racial status is that race may be a surrogate for other unmeasured variables such as social support that was not assessed in this study. We did not observe race as an independent factor in the comparison group of women without a history of cancer. However, it must also be noted that in our study the sample size is very limited for the minority population and these findings should be interpreted with caution.
Notwithstanding these limitations, the study broadens our understanding of the effect of musculoskeletal pain on HR-QOL in women with breast cancer on AIs. Future prospective studies might prospectively evaluate the effect in a larger, more diverse setting. In addition, we did not assess psychological distress or fear of cancer recurrence, which may be the mediator in the association and could be further explored in future studies. The issue of fear of cancer recurrence in particular needs to be better addressed in women with musculoskeletal pain.
AI-associated musculoskeletal symptoms impact both physical and mental well-being of breast cancer patients. Preventing this side effect or alleviating the pain should be an important aim with therapy. The study findings can assist physicians in their approach to discussing musculoskeletal symptoms with women initiating AI therapy and developing interventions to reducing anxiety and fear and improving HR-QOL in these women.
References
Anderson WF, Chatterjee N, Ershler WB, Brawley OW (2002) Estrogen receptor breast cancer phenotypes in the Surveillance, Epidemiology, and End Results database. Breast Cancer Res Treat 76(1):27–36
Grana G (2004) Shifting paradigms in hormonal therapy for breast cancer. Cancer Biol Ther 3(9):797–805
Narashimamurthy J, Rao AR, Sastry GN (2004) Aromatase inhibitors: a new paradigm in breast cancer treatment. Curr Med Chem Anticancer Agents 4(6):523–534
Howell A (2005) Anastrozole: a new gold standard of hormonal treatment for breast cancer? Womens Health (Lond Engl) 1(3):309–322
Dowsett M, Cuzick J, Ingle J, Coates A, Forbes J, Bliss J et al (2010) Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J Clin Oncol 28:509–518
Regan MM, Neven P, Giobbie-Hurder A, Goldhirsch A, Ejlertsen B, Mauriac L et al (2011) Assessment of letrozole and tamoxifen alone and in sequence for postmenopausal women with steroid hormone receptor-positive breast cancer: the BIG 1–98 randomised clinical trial at 8 · 1 years median follow-up. Lancet Oncol 12(12):1101–1108
Cuzick J, Sestak I, Baum M, Buzdar A, Howell A, Dowsett M et al (2010) Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol 11(12):1135–1141
Aiello EJ, Buist DS, Wagner EH, Tuzzio L, Greene SM, Lamerato LE et al (2008) Diffusion of aromatase inhibitors for breast cancer therapy between 1996 and 2003 in the Cancer Research Network. Breast Cancer Res Treat 107(3):397–403
Shen Y, Dong W, Feig BW, Ravdin P, Theriault RL, Giordano SH (2009) Patterns of treatment for early stage breast cancers at the M. D. Anderson Cancer Center from 1997 to 2004. Cancer 115(10):2041–2051
Amir E, Seruga B, Niraula S, Carlsson L, Ocaña A (2011) Toxicity of adjuvant endocrine therapy in postmenopausal breast cancer patients: a systematic review and meta-analysis. J Natl Cancer Inst 103(17):1299–1309
Duffy SR, Distler W, Howell A, Cuzick J, Baum M (2009) A lower incidence of gynecologic adverse events and interventions with anastrozole than with tamoxifen in the ATAC trial. Am J Obstet Gynecol 200(1):80.e1–e7
Hernandez RK, Sørensen HT, Pedersen L, Jacobsen J, Lash TL (2009) Tamoxifen treatment and risk of deep venous thrombosis and pulmonary embolism: a Danish population-based cohort study. Cancer 115(19):4442–4449
Khan QJ, O'Dea AP, Sharma P (2010) Musculoskeletal adverse events associated with adjuvant aromatase inhibitors. J Oncol pii: 654348
Tomao F, Spinelli G, Vici P, Pisanelli GC, Cascialli G, Frati L et al (2011) Current role and safety profile of aromatase inhibitors in early breast cancer. Expert Rev Anticancer Ther 11:1253–1263
Gaillard S, Stearns V (2011) Aromatase inhibitor-associated bone and musculoskeletal effects: new evidence defining etiology and strategies for management. Breast Cancer Res 13(2):205
Henry NL, Giles JT, Ang D, Mohan M, Dadabhoy D, Robarge J et al (2008) Prospective characterization of musculoskeletal symptoms in early stage breast cancer patients treated with aromatase inhibitors. Breast Cancer Res Treat 111:365–372
Park JY, Lee SK, Bae SY, Kim J, Kim MK, Kil WH et al (2013) Aromatase inhibitor-associated musculoskeletal symptoms: incidence and associated factors. J Korean Surg Soc 85(5):205–211
Singer O, Cigler T, Moore AB, Levine AB, Hentel K, Belfi L et al (2012) Defining the aromatase inhibitor musculoskeletal syndrome: a prospective study. Arthritis Care Res (Hoboken) 4(12):1910–1918
Robidoux A, Rich E, Bureau NJ, Mader S, Laperrière D, Bail M et al (2011) A prospective pilot study investigating the musculoskeletal pain in postmenopausal breast cancer patients receiving aromatase inhibitor therapy. Curr Oncol 18(6):285–294
Moxley G (2010) Rheumatic disorders and functional disability with aromatase inhibitor therapy. Clin Breast Cancer 10(2):144–147
Kidwell KM, Harte SE, Hayes DF, Storniolo AM, Carpenter J, Flockhart DA et al (2014) Patient-reported symptoms and discontinuation of adjuvant aromatase inhibitor therapy. Cancer. doi:10.1037/ a0028240
Sueblinvong T, Taechakraichana N, Phupong V (2001) Prevalence of climacteric symptoms according to years after menopause. J Med Assoc Thai 84(12):1681–1691
Bairy L, Adiga S, Bhat P, Bhat R (2009) Prevalence of menopausal symptoms and quality of life after menopause in women from South India. Aust N Z J Obstet Gynaecol 49(1):106–109
Dugan SA, Powell LH, Kravitz HM, Everson Rose SA, Karavolos K, Luborsky J (2006) Musculoskeletal pain and menopausal status. Clin J Pain 22(4):325–331
Freeman EW, Sammel MD, Lin H, Gracia CR, Pien GW, Nelson DB, et al., Symptoms associated with menopausal transition and reproductive hormones in midlife women. Obstet Gynecol 110:230–240
Helzlsouer KJ, Gallicchio L, MacDonald R, Wood B, Rushovich E (2012) A prospective study of aromatase inhibitor therapy, vitamin D, C-reactive protein and musculoskeletal symptoms. Breast Cancer Res Treat 131(1):277–285
Tüzün EH (2007) Quality of life in chronic musculoskeletal pain.Best. Pract Res Clin Rheumatol 21(3):567–579
Takei H, Ohsumi S, Shimozuma K, Takehara M, Suemasu K, Ohashi Y et al (2012) Health-related quality of life, psychological distress, and adverse events in postmenopausal women with breast cancer who receive tamoxifen, exemestane, or anastrozole as adjuvant endocrine therapy: National Surgical Adjuvant Study of Breast Cancer 04 (N-SAS BC 04). Breast Cancer Res Treat 133(1):227–236
Ohsumi S, Shimozuma K, Ohashi Y, Shinji M, Hozumi Y, Mukai H et al (2011) Health-related quality of life and psychological distress of breast cancer patients after surgery during a phase III randomized trial comparing continuation of tamoxifen with switching to anastrozole after adjuvant tamoxifen for 1–4 years: N-SAS BC 03. Breast Cancer Res Treat 127:143–152
Buijs C, de Vries EG, Mourits MJ, Willemse PH (2008) The influence of endocrine treatments for breast cancer on health-related quality of life. Cancer Treat Rev 34:640–655
Fallowfield LJ, Kilburn LS, Langridge C, Snowdon CF, Bliss JM, Coombes RC, Trial Steering Committee IES (2012) Long-term assessment of quality of life in the Intergroup Exemestane Study: 5 years post-randomisation. Br J Cancer 106:1062–1067
Cellar D, Fallowfield L, Barker P, Cuzick J, Locker G, Howell A (2006) Quality of life of postmenopausal women in the ATAC ("Arimidex", tamoxifen, alone or in combination) trial after completion of 5 years' adjuvant treatment for early breast cancer. Breast Cancer Res Treat 100:273–284
van Nes JG, Fontein DB, Hille ET, Voskuil DW, van Leeuwen FE, de Haes JC et al (2012) Quality of life in relation to tamoxifen or exemestane treatment in postmenopausal breast cancer patients: a Tamoxifen Exemestane Adjuvant Multinational (TEAM) Trial side study. Breast Cancer Res Treat 34:267–276
Ware JE, Sherbourne CD (1992) The MOS 36-item Short-Form Health Survey (SF-36): I. Conceptual framework and item selection. Med Care 30:473–483
Stewart AL, Hays RD, Aare JE Jr (1988) The MOS Short-Form General Health Survey: reliability and validity in a patient population. Med Care 26:724–732
Okamoto T, Shimozuma K, Katsumata N, Koike M, Hisashige A, Tanaka K et al (2003) Task Force of the Japanese Breast Cancer Society for 'The Development of Guidelines for Quality of Life Assessment Studies of Breast Cancer Patients'. Measuring quality of life in patients with breast cancer: a systematic review of reliable and valid instruments available in Japan. Breast Cancer 10:204–213
Pinar R (2005) Reliability and construct validity of the SF-36 in Turkish cancer patients. Qual Life Res 14:259–264
Paul SM, Zelman DC, Smith M, Miaskowski C (2005) Categorizing the severity of cancer pain: further exploration of the establishment of cutpoints. Pain 113:37–44
Jones KR, Vojir CP, Hutt E, Fink R (2007) Determining mild, moderate, and severe pain equivalency across pain-intensity tools in nursing home residents. J Rehabil Res Dev 44:305–314
Winters L, Habin K, Flanagan J, Cashavelly BJ (2010) "I feel like I am 100 years old!" managing arthralgias from aromatase inhibitors. Clin J Oncol Nurs 14:379–382
Vickberg SJ (2003) The Concerns About Recurrence Scale (CARS): a systematic measure of women’s fears about the possibility of breast cancer recurrence. Ann Behave Med 25:16–24
Lebel S, Rosberger Z, Edgar L, Devins GM (2009) Emotional distress impacts fear of the future among breast cancer survivors not the reverse. J Cancer Surviv 3:117–127
van den Beuken-van Everdingen MH, Peters ML, de Rijke JM, Schouten HC, van Kleef M, Patijn J (2008) Concerns of former breast cancer patients about disease recurrence: a validation and prevalence study. Psychooncology 17:1137–1145
Green CR, Hart-Johnson T, Loeffler DR (2011) Cancer-related chronic pain: examining quality of life in diverse cancer survivors. 117:1994–2003
Ashing Giwa KT, Lim JW (2011) Examining emotional outcomes among a multiethnic cohort of breast cancer survivors. Oncol Nurs Forum 38:279–288
Janz NK, Mujahid MS, Hawley ST, Griggs JJ, Alderman A, Hamilton AS, Graff J, Katz SJ (2009) Racial/ethnic differences in quality of life after diagnosis of breast cancer. J Cancer Surviv 3:212–222
Morrow PK, Broxson AC, Munsell MF, Basen-Enquist K, Rosenblum CK, Schover LR et al (2014) Effect of age and race on quality of life in young breast cancer survivors. Clin Breast Cancer 14(2):e21–e31
Bowen DJ, Alfano CM, McGregor BA, Kuniyuki A, Bernstein L, Meeske K et al (2007) Possible socioeconomic and ethnic disparities in quality of life in a cohort of breast cancer survivors. Breast Cancer Res Treat 106(1):85–95
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Olufade, T., Gallicchio, L., MacDonald, R. et al. Musculoskeletal pain and health-related quality of life among breast cancer patients treated with aromatase inhibitors. Support Care Cancer 23, 447–455 (2015). https://doi.org/10.1007/s00520-014-2364-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-014-2364-3