Abstract
Purpose
To examine benefit of sulindac for relief of musculoskeletal symptoms (MSS) in patients stable on aromatase inhibitors (AIs).
Methods
Sulindac was evaluated at 150 mg twice daily for effects on MSS at 3, 6, 9, and 12 months in 50 postmenopausal women stable on AI therapy for a median of 12.5 months for hormone receptor-positive breast cancer. A separate, non-randomized group of 50 similar patients was observed for change in MSS over 12 months. MSS severity was assessed using the Western Ontario and McMaster Universities Osteoarthritis (WOMAC) Index and Brief Pain Inventory Short Form (BPI-SF). The Functional Assessment of Cancer Therapy—General form (FACT-G) measured quality of life (QOL). Change in MSS and QOL across time was assessed in each group using linear mixed effects models.
Results
Stiffness, not pain, was the main complaint at baseline. At 12 months, sulindac patients reported decreases (improvements) in mean (95% CI) Total WOMAC score [− 5.85 (− 9.73, − 1.96)] and WOMAC pain [− 5.40 (− 10.64, − 0 .18)], Stiffness [− 9.53 (− 14.98, − 4.08)] and Physical Function [− 5.61 (− 9.62, − 1.60)] subscales, but not BPI-SF worst pain. Among sulindac patients with higher baseline MSS severity, 35% experienced ≥ 50% improvement in Total WOMAC and Total FACT-G scores [6.18 (2.08, 10.27); P = 0.003]. For the observation group, MSS and QOL did not improve over 12 months, even among those with higher baseline MSS severity.
Conclusions
Sulindac may relieve MSS in AI patients, especially physical function and stiffness. Randomized controlled trials should further evaluate NSAIDs on AI-MSS and AI adherence.
Trial registration number and date of registration
NCT01761877, December, 2012.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Five or more years of adjuvant endocrine therapy (ET) with an aromatase inhibitor (AI) is superior to selective estrogen modulator therapy for reducing deaths due to postmenopausal hormone receptor-positive (HR +) breast cancer [1]. High rates of discontinuation and higher recurrence rates in non-adherent patients, however, have emerged as a challenge in clinical practice [2,3,4,5,6,7,8].
ET side effects are reported by more than one-third of patients as the main cause for early discontinuation [9]. For AIs, which act by suppressing peripheral estrogen synthesis, new onset or worsening of musculoskeletal symptoms (MSS) (e.g., arthralgia) is a common reason given by patients for non-adherence [10]. Currently, there is no consensus definition for AI-MSS, though symmetrical joint pains characterize AI-associated MSS (AI-MSS) and involve the wrists, hands, hips, and knees along with morning stiffness and general myalgia [11, 12]. Onset and intensity of AI-MSS are variable, typically appearing within the first 2 months and peaking between 3 and 6 months, though the natural history thereafter remains poorly characterized [13,14,15]. Pooled incidence estimates of 46% using “joint pain without objective pathology” [12], and findings that symptoms are relieved by discontinuing AI use, demonstrate unequivocally that AI-MSS is a drug-induced disorder.
AI-induced estrogen depletion has been suggested to increase inflammation, mediated by bradykinin and pro-inflammatory prostaglandins [16]. However, the exact mechanism(s) that underlie AI-MSS are unknown. Experimental evidence suggests that AIs enhance sensitivity to pain signals (i.e., nociception) [17]. This includes findings that AI drugs react chemically with the transient receptor potential ankyrin 1 (TRPA1), a ‘pain-transducing’ cation channel expressed on primary sensory neurons of the dorsal root ganglia, to promote neurogenic inflammation and AI-MSS in mice [18, 19]. Such nociception mechanisms may explain benefits of the selective serotonin and norepinephrine reuptake inhibitor (SSNRI) duloxetine (Cymbalta) [20] and acupuncture [21, 22] in patients with moderate-to-severe pain.
Clinically, current practice recommendations [23] are directed at managing symptoms based on their severity. Initial therapy often includes use of non-steroidal anti-inflammatory drugs (NSAIDs) based on anecdotal evidence and on efficacy in osteoarthritis/joint symptoms in postmenopausal women [24]. For the prevention and treatment of mild-to-moderate symptomology, emphasis is now increasingly placed on weight loss and on the benefits of aerobic and strengthening exercises, including yoga, on moderate-level evidence [25] and the other associated health benefits of exercise. Use of an alternate AI or switching to tamoxifen and introduction of an SSNRI show benefit in patients with more severe symptoms and are recommended [23].
As part of our investigation of the NSAID sulindac for anti-cancer effects on breast density after 12 months of use in patients on AI therapy [26], we included longitudinal measures of MSS severity using validated patient-reported outcome (PRO) questionnaires. Sulindac, marketed as Clinoril™, is a non-selective NSAID approved for use for the treatment of osteoarthritis and rheumatoid arthritis. Its activity is attributed to the non-selective inhibition of cyclooxygenase 2 and synthesis of pro-inflammatory prostaglandin E2. MSS severity over 12 months was also evaluated in a separate, non-randomized control group of patients on AI therapy with similar eligibility criteria to patients receiving sulindac.
Methods
Study design and patient eligibility
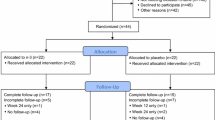
We conducted an open-label phase II study of sulindac (Clinoril®) 150 mg given orally, twice daily for 12 months in patients on AI therapy for HR + postmenopausal breast cancer for effects on breast density [26]. A total of 58 patients enrolled and initiated a 4-month NSAID washout, of which 50 started sulindac treatment and 43 completed to 12 months. Separately, a non-randomized control arm of 56 postmenopausal breast cancer patients on AI therapy was enrolled to observation only, and 40 completed to 12 months. Eligible patients included postmenopausal women age ≤ 75 years stable on AI therapy for at least 3 months for treatment of early-stage HR + breast cancer. Patients were enrolled at The University of Arizona Cancer Center or the Stony Brook Cancer Center. For the sulindac arm, additional inclusion criteria included willingness to refrain from NSAID use (low-dose aspirin [≤ 81 mg/day] was permitted), adequate renal function, normal or controlled blood pressure, and no contraindications to NSAIDs. There was no pre-specified baseline pain or arthralgia level requirement. The study was registered with ClinicalTrials.gov (NCT01761877) and approved by the institutional review boards (IRB) at both study sites. All patients provided written informed consent.
Outcome assessment
For arthralgia symptom severity and pain, patients completed the WOMAC (version 3.1) and other questionnaires at baseline (prior to initiation of sulindac) and after 3, 6, and 12 months. The WOMAC is a 24-item instrument validated to assess lower-extremity joint symptoms in the past 7 days using 3 subscales: pain (5 items), stiffness (2 items), and physical function (17 items) [27]. All questions use a 5-point rating scale (0 = none to 4 = extreme). As discussed in Bellamy [27], for convenience and for comparison purposes to previous studies, total scores and each subscale were normalized to a range of 0–100. Higher scores indicate greater symptom severity. In the case of missing data, subscales were considered valid as long as ≤ 3 core items were missing for function, ≤ 1 for pain, and ≤ 1 for stiffness.
The Brief Pain Inventory Short Form (BPI-SF) is a 15-item questionnaire developed and validated for use in cancer [28] and osteoarthritis [29] patients to assess worst pain, pain severity, and pain interference (range from no symptoms to worst, 0–10) over the past 24 h. Pain severity was derived as the average of responses to 4 questions on worst pain, average pain, least pain, and pain right now (complete data required). In the case of the interference score, 4 of 7 responses were required, and the average interference score of the completed items was imputed for missing values, as previously described [28].
The 28-item Functional Assessment of Cancer Therapy—General form (FACT-G) uses a 5-point Likert scale [0 (not at all) to 4 (very much)] that measures functional and physical well-being (score range 0–108) comprising 4 subscales: physical well-being (7 items, score range 0–28), social/family well-being (7 items, score range 0–28), emotional well-being (6 items, score range 0–24), and functional well-being (7 items, score range 0–28). For missing data, if ≥ 50% of the items within a subscale were answered, a subscale score was computed as the prorated sum of the item responses for that subscale as described [30]. The FACT-G total score was computed as the sum of the 4 subscale scores for all participants with an overall item response of at least 80% (i.e., at least 22 of the 27 items answered). Negatively worded items (all 7 physical well-being questions, and 5 of the 6 emotional well-being questions) were reverse scored prior to summing so that higher subscale and total scores indicate better QOL [31].
Toxicity assessment
Toxicity data were collected prospectively for the sulindac group and are detailed in Thompson et al. [26].
Statistical analyses
Spearman correlation coefficients measured associations between baseline patient characteristics (age, BMI, time on AI, anastrozole, letrozole, exemestane) and each summary pain/QOL score. Change in each outcome at 3 (sulindac group only), 6, 9 (sulindac group only), and 12 months relative to baseline (after washout for sulindac) was estimated in each group separately using linear mixed effects models with an indicator variable for each time point, adjusted for age at baseline, body mass index (BMI), time on AI, and use of non-NSAID pain medication. To assess the importance of baseline symptoms to change, a sensitivity analysis was conducted for the subset of subjects whose baseline total WOMAC was greater than the sulindac group–specific median of 19.2 points (sulindac group, n = 25; observation group, n = 14). No corrections were made for multiple comparisons.
Results
Study population
Participants who started sulindac or observation had a respective median age of 61.9 and 63.0 years, median BMI of 26.9 and 28.2 kg/m2, and were primarily non-Hispanic White with some college education. Patients enrolled to both arms had a similar median time on AI therapy prior to enrollment of ~ 1 year (Table 1). Anastrozole was the most prescribed AI therapy. Patients who enrolled to sulindac reported a higher use of exemestane than those enrolling to observation, likely a reflection of switching from one of the non-steroidal AIs (letrozole or anastrozole) due to intolerance. In the sulindac intervention group, 13 (26%) participants reported taking low-dose aspirin, and 8 (16%) reported any use of non-NSAID medication for pain (e.g., tramadol, duloxetine), of which 4 were also taking low-dose aspirin. For patients enrolled to observation, 15 (30%) reported taking an NSAID or aspirin at any dose, of which 13 (26%) reported using low-dose aspirin. Of 10 (20%) who reported any use of non-NSAID medication as needed for pain, 5 were also taking low-dose aspirin.
Baseline pain in each study group
For patients enrolled to sulindac, baseline measures occurred after a 4-month NSAID washout. Mean WOMAC subscale scores (Table 2) were in the mild range of severity: Total (22.9), Pain (23.5), Stiffness (34.0), and Physical Function Limitation (21.4). Forty percent of patients reported moderate or higher symptom severity in at least one WOMAC subscale with average stiffness severity scores higher than the other subscales. The individual question ‘how severe has your stiffness been after you first woke up in the morning?’ yielded the highest group mean score (37.0). Consistent with previous reports [12], pain in the joints, particularly the hands and wrists, were more common (data not shown). Mean BPI worst pain was mild at 3.34, with 42% reporting no or minimal joint pain (BPI worst pain score ≤ 2), 26% reporting mild joint pain (BPI worst pain score 3–4), and 30% reporting moderate-to-severe pain (BPI worst pain score 5–10). Overall, the study population reported high QOL (mean total FACT-G, 89.7 of 108 points).
For patients enrolled to observation, the baseline mean WOMAC subscale scores ranged from no to mild symptom intensity: Total (12.5), pain (13.5), stiffness (22.2) and physical function limitation (11.0) (Supplemental Table 1). Only 22% of participants reported moderate symptom severity in at least one WOMAC subscale, with stiffness contributing more than pain or physical function limitations. Baseline mean pain measured by BPI worst pain was low at 2.3, with 50% reporting no or minimal joint pain (BPI worst pain score ≤ 2), 34% reporting mild joint pain (BPI worst pain score 3–4), and 16% reporting moderate-to-severe pain (BPI worst pain score 5–10).
Change in MSS over 12 months in each study group
From baseline to end of treatment at 12 months, patients enrolled to sulindac reported small, but significant, improvements in each of the WOMAC subscales (Table 2). Decreases in total WOMAC (− 5.8, P = 0.003), pain (− 5 .4, P = 0.043), stiffness (− 9.5, P < 0.001), and function (− 5.6, P = 0.006) were observed. No change was observed in BPI worst pain, severity, or interference. For QOL measures, a small but significant improvement in Total FACT-G (+ 3.0, P = 0.028) was observed, with non-significant changes in physical, social/family, emotional, or functional subscores. Responses to the FACT-G question, “I am bothered by side effects of my treatment” significantly improved (0.65–0.22, P = 0.008) over the 12-month intervention. The most clinically relevant MSS improvements are illustrated in Fig. 1A–C and included reduction of moderate-level complaints related to functional limitations (getting out of bed, doing heavy chores) and stiffness, as opposed to pain.
When restricted to patients enrolled to sulindac whose baseline Total WOMAC score was above the group median of 19.2 points (n = 25), significant improvements were observed in all WOMAC subscales at 3, 6, 9 and 12 months (Table 3). By 12 months, 37% of patients with higher baseline symptoms reported ≥ 50% improvement in Total WOMAC (i.e., meaningful clinical improvement [32, 33]) and a respective 39% and 42% for WOMAC pain and physical function limitation subscales. However, only 16% of patients in this subgroup experienced ≥ 50% decrease in stiffness symptom severity by 12 months. BPI worst pain also decreased by 1.4 points, from 4.8 to 3.4 (P = 0.015) with decreases in BPI severity (0.82 points, P = 0.033) and interference (0.98 points, P = 0.008). Overall QOL improved at 12 months (increase in Total FACT-G from 84.1 to 90.3, P = 0.003) with improvements in physical well-being scores from 21.7 to 23.8 (P = 0.002) at 12 months. Underlying this change were significant improvements in specific questions related to energy level, treatment-related side effects, meeting needs of family, and pain level.
For the observation group, there were no significant changes in patient-reported outcomes for WOMAC, BPI-SF, or FACT-G (Supplemental Table 1). Illustrated with a few specific questions in Fig. 1D–F, daily activities, pain, and stiffness levels showed no significant improvement over the 12 months of observation. In patients with complete data whose baseline Total WOMAC scores were above the sulindac baseline median (n = 13), mean WOMAC pain scores declined (35.7–32.0), as did stiffness (50.0–46.9) and function (31.0–29.2), but the decreases were small and not significant (Supplemental Table 2). Furthermore, patients in this subgroup reported significant increases in BPI-Interference (0.9, P = 0.018) and decreases in Physical Well-Being subscale (− 2.4, P = 0.039), along with no significant increase in BPI worst pain (1.3, P = 0.125). The proportion of the 14 observation patients with elevated baseline symptoms who experienced a ≥ 50% improvement in symptoms over 12 months of observation was 12.5% for Total WOMAC, 25% for WOMAC pain, and 12.5% each for WOMAC stiffness and physical function limitation.
Safety and tolerability of sulindac
Overall, sulindac therapy was well tolerated in the study sample, with grade 1 and 2 gastrointestinal adverse events (AE) [nausea, abdominal pain, gastroesophageal reflux disease (GERD)] as the primary side effect [26]. Of 50 participants, 11 experienced a grade 2 AE (n = 9) or serious AE (SAE, n = 2) considered possibly or probably related to study agent. Three AEs, including two SAEs (1 transient pancreatitis and 1 cerebral hemorrhage in a patient with amyloid angiopathy) and one grade 2 gastrointestinal event, resulted in early discontinuation of study drug.
Discussion
In this open-label study of sulindac at doses used to treat arthritis in breast cancer patients on AI therapy, 40% of patients reported moderate-to-high symptom severity in at least one of the WOMAC subscales at baseline. Moderate-to-severe stiffness, rather than pain, was the most common and disabling condition. After 12 months of sulindac intervention, the proportion of patients with moderate or higher symptom severity reduced to 18.6%, with significant declines in Total WOMAC and all three WOMAC subscale scores. Patients enrolled to observation had no improvements in Total WOMAC or any WOMAC subscale over the year of follow-up.
Consistent with the recommended use of NSAIDs for short duration [23], improvements in the sulindac group occurred by 3 months and one-third experienced ≥ 50% improvement in WOMAC pain and function by 12 months. The mean difference of − 15.8 points for function and − 20.6 points for stiffness meet consensus values of absolute change necessary for minimal clinically important improvement (MCII) [32]. However, the 14.3-point decrease for WOMAC pain is less than the consensus 20-point change for MCII.
Improvements in WOMAC physical function and stiffness in the sulindac group were most significant in patients with baseline symptoms above the group median. Notably, baseline arthralgia symptoms were 13.1 points higher in overweight and obese women relative to healthy weight women. In exploratory analyses, statistically significant improvement in Total WOMAC scores at 3, 6, 9, and 12 months was present only among overweight/obese patients in the sulindac group (Supplemental Table 3). No change in Total WOMAC was observed over 12 months in the observation group by BMI (data not shown).
A number of research groups have conducted randomized clinical trials of drug or supplement interventions for AI-MSS using change in BPI worst pain score as the primary outcome (e.g., duloxetine [20], vitamin D [33, 34], omega-3 fatty acids [ω-3 FA] [35, 36]). These studies enrolled patients selected for moderate-to-severe BPI worst pain scores > 3. Patients in the current study were stable on AI therapy with no eligibility criteria based on baseline pain severity. Among all 50 participants who initiated sulindac, 20 (40%) had a baseline BPI worst pain score ≥ 4, and 16 completed 12 months of therapy. Of these 16 patients, 13 (81%) showed any improvement (≥ 1 point), and 9 (56%) improved by ≥ 2 points. Separately, among participants in the observation group, 16 (32%) had a baseline worst pain score ≥ 4, and 11 of these patients were followed for 12 months. Only 5 patients (45%) showed improvement over 12 months without defined therapy, all by ≥ 2 points.
Supporting the potential benefit of sulindac, the change in FACT-G exceeded the 4.0% increase MCII identified from meta-analyses for small but meaningful benefit [37]. This was particularly notable in patients with higher baseline symptoms (9.0% change). The lack of any significant improvements in MSS or QOL in the observation group suggest that for patients on AI therapy with MSS, symptoms likely persist, remain stable, or even worsen, and affect QOL over time.
While the relevance of our findings to AI adherence is unknown, of 14 patients who responded at baseline to the FACT-G item, “I am bothered by side effects of treatment” and who completed 12 months of sulindac, 13 (93%) reported not being bothered at end of study. Of note, only one sulindac patient discontinued AI therapy across 12 months versus four observation patients.
Major limitations are the lack of randomization or a placebo control. As such, the results may be due in part or entirely to placebo effects. Placebo effects in pain and arthritis studies are well documented. For AI-MSS studies, several randomized placebo-controlled trials including testing effects of duloxetine [20] or supplementation with vitamin D [33, 34] or ω-3 FA [35, 36] on AI-associated pain have shown small to large placebo effects up to 50%. These and our findings may reflect differences in the persistence and possibly underlying pathology of MSS in AI patients with mild-to-moderate symptoms.
While the use of NSAIDs is recommended for new onset or worsening arthralgia in patients initiating AI therapy [23], only one randomized study to our knowledge has published on the effect of an NSAID on AI patient–reported outcomes [38]. In secondary analysis of the Celecoxib Anti-Aromatase Neoadjuvant (CAAN) trial, FACT-G QOL among women taking celecoxib + exemestane was higher than in women taking either exemestane or letrozole alone. Neither BPI nor WOMAC were assessed. Separately, in a published abstract from the ETAN Trial, a phase III randomized placebo-controlled trial of etoricoxib (60 mg/day) in breast cancer patients on AI for the prevention of breast cancer recurrence, MSS pain symptoms were reported to improve with intervention compared to placebo (though the trial discontinued early) [39]. With median etoricoxib and placebo treatment durations of 14 and 12 months, respectively, 50 of 73 treated patients (68%) reported decreased pain compared to 16 of 67 (24%) in the placebo group. However, neither the methods for pain assessment nor the magnitude of change in pain were reported.
In the current study, MSS severity reflects profiles seen in clinical practice. Further, improvement in patient-reported stiffness and functional limitations with sulindac and greater benefit in overweight and obese patients are consistent with underlying contribution of inflammation-associated arthralgia symptoms. As with other NSAIDs, GI upset was the most common and expected side effect with sulindac use occurring in 10% of subjects [26]. While long-term use of NSAIDs is discouraged due to rare cardiovascular risk with extended use [40], further examination of the benefit of short course NSAIDs, especially in combination with efforts directed at increasing physical activity and promoting weight loss among overweight and obese patients initiating AI therapy, is warranted.
Data availability
All associated data are available in a data repository and are available upon request.
Code availability
Not Applicable.
References
Early Breast Cancer Trialists’ Collaborative G (2015) Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet 386:1341–1352. https://doi.org/10.1016/S0140-6736(15)61074-1
Chirgwin JH, Giobbie-Hurder A, Coates AS, Price KN, Ejlertsen B, Debled M, Gelber RD, Goldhirsch A, Smith I, Rabaglio M, Forbes JF, Neven P, Lang I, Colleoni M, Thurlimann B (2016) Treatment adherence and its impact on disease-free survival in the breast international group 1–98 trial of tamoxifen and letrozole, alone and in sequence. J Clin Oncol 34:2452–2459. https://doi.org/10.1200/JCO.2015.63.8619
Chlebowski RT, Kim J, Haque R (2014) Adherence to endocrine therapy in breast cancer adjuvant and prevention settings. Cancer Prev Res (Phila) 7:378–387. https://doi.org/10.1158/1940-6207.CAPR-13-0389
Hershman DL, Shao T, Kushi LH, Buono D, Tsai WY, Fehrenbacher L, Kwan M, Gomez SL, Neugut AI (2011) Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat 126:529–537. https://doi.org/10.1007/s10549-010-1132-4
Murphy CC, Bartholomew LK, Carpentier MY, Bluethmann SM, Vernon SW (2012) Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: a systematic review. Breast Cancer Res Treat 134:459–478. https://doi.org/10.1007/s10549-012-2114-5
Partridge AH, LaFountain A, Mayer E, Taylor BS, Winer E, Asnis-Alibozek A (2008) Adherence to initial adjuvant anastrozole therapy among women with early-stage breast cancer. J Clin Oncol 26:556–562. https://doi.org/10.1200/JCO.2007.11.5451
van Hellemond IEG, Geurts SME, Tjan-Heijnen VCG (2018) Current status of extended adjuvant endocrine therapy in early stage breast cancer. Curr Treat Options Oncol 19:26. https://doi.org/10.1007/s11864-018-0541-1
Hershman DL, Kushi LH, Shao T, Buono D, Kershenbaum A, Tsai W-Y, Fehrenbacher L, Lin Gomez S, Miles S, Neugut AI (2010) Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol 28:4120–4128. https://doi.org/10.1200/JCO.2009.25.9655
Condorelli R, Vaz-Luis I (2018) Managing side effects in adjuvant endocrine therapy for breast cancer. Expert Rev Anticancer Ther 18:1101–1112. https://doi.org/10.1080/14737140.2018.1520096
Kwan ML, Roh JM, Laurent CA, Lee J, Tang L, Hershman D, Kushi LH, Yao S (2017) Patterns and reasons for switching classes of hormonal therapy among women with early-stage breast cancer. Cancer Causes Control 28:557–562. https://doi.org/10.1007/s10552-017-0888-9
Crew KD, Greenlee H, Capodice J, Raptis G, Brafman L, Fuentes D, Sierra A, Hershman DL (2007) Prevalence of joint symptoms in postmenopausal women taking aromatase inhibitors for early-stage breast cancer. J Clin Oncol 25:3877–3883. https://doi.org/10.1200/JCO.2007.10.7573
Beckwée D, Leysen L, Meuwis K, Adriaenssens N (2017) Prevalence of aromatase inhibitor-induced arthralgia in breast cancer: a systematic review and meta-analysis. Support Care Cancer 25:1673–1686. https://doi.org/10.1007/s00520-017-3613-z
Henry NL, Giles JT, Ang D, Mohan M, Dadabhoy D, Robarge J, Hayden J, Lemler S, Shahverdi K, Powers P, Li L, Flockhart D, Stearns V, Hayes DF, Storniolo AM, Clauw DJ (2008) Prospective characterization of musculoskeletal symptoms in early stage breast cancer patients treated with aromatase inhibitors. Breast Cancer Res Treat 111:365–372. https://doi.org/10.1007/s10549-007-9774-6
Howell A, Cuzick J, Baum M, Buzdar A, Dowsett M, Forbes JF, Hoctin-Boes G, Houghton J, Locker GY, Tobias JS, ATAC Trialists’ Group (2005) Results of the ATAC (arimidex, tamoxifen, alone or in combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. The Lancet 365:60–62. https://doi.org/10.1016/S0140-6736(04)17666-6
Laroche F, Coste J, Medkour T, Cottu PH, Pierga J-Y, Lotz J-P, Beerblock K, Tournigand C, Declèves X, de Cremoux P, Bouhassira D, Perrot S (2014) Classification of and risk factors for estrogen deprivation pain syndromes related to aromatase inhibitor treatments in women with breast cancer: a prospective multicenter cohort study. J Pain 15:293–303. https://doi.org/10.1016/j.jpain.2013.11.004
Borrie AE, Kim RB (2017) Molecular basis of aromatase inhibitor associated arthralgia: known and potential candidate genes and associated biomarkers. Expert Opin Drug Metab Toxicol 13:149–156. https://doi.org/10.1080/17425255.2017.1234605
Robarge JD, Duarte DB, Shariati B, Wang R, Flockhart DA, Vasko MR (2016) Aromatase inhibitors augment nociceptive behaviors in rats and enhance the excitability of sensory neurons. Exp Neurol 281:53–65. https://doi.org/10.1016/j.expneurol.2016.04.006
Fusi C, Materazzi S, Benemei S, Coppi E, Trevisan G, Marone IM, Minocci D, De Logu F, Tuccinardi T, Di Tommaso MR, Susini T, Moneti G, Pieraccini G, Geppetti P, Nassini R (2014) Steroidal and non-steroidal third-generation aromatase inhibitors induce pain-like symptoms via TRPA1. Nat Commun 5:5736. https://doi.org/10.1038/ncomms6736
De Logu F, Tonello R, Materazzi S, Nassini R, Fusi C, Coppi E, Li Puma S, Marone IM, Sadofsky LR, Morice AH, Susini T, Terreni A, Moneti G, Di Tommaso M, Geppetti P, Benemei S (2016) TRPA1 mediates aromatase inhibitor-evoked pain by the aromatase substrate androstenedione. J Cancer Res 76:7024–7035. https://doi.org/10.1158/0008-5472.CAN-16-1492
Henry NL, Unger JM, Schott AF, Fehrenbacher L, Flynn PJ, Prow DM, Sharer CW, Burton GV, Kuzma CS, Moseley A, Lew DL, Fisch MJ, Moinpour CM, Hershman DL, Wade JL (2017) Randomized, multicenter, placebo-controlled clinical trial of duloxetine versus placebo for aromatase inhibitor-associated arthralgias in early-stage breast cancer: SWOG S1202. J Clin Oncol 36:326–332. https://doi.org/10.1200/JCO.2017.74.6651
Hershman DL, Unger JM, Greenlee H, Capodice JL, Lew DL, Darke AK, Kengla AT, Melnik MK, Jorgensen CW, Kreisle WH, Minasian LM, Fisch MJ, Henry NL, Crew KD (2018) Effect of acupuncture vs sham acupuncture or waitlist control on joint pain related to aromatase inhibitors among women with early-stage breast cancer: a randomized clinical trial. JAMA 320:167–176. https://doi.org/10.1001/jama.2018.8907
Chen L, Lin CC, Huang TW, Kuan YC, Huang YH, Chen HC, Kao CY, Su CM, Tam KW (2017) Effect of acupuncture on aromatase inhibitor-induced arthralgia in patients with breast cancer: a meta-analysis of randomized controlled trials. Breast 33:132–138. https://doi.org/10.1016/j.breast.2017.03.015
Gupta A, Henry NL, Loprinzi CL (2020) Management of aromatase inhibitor-induced musculoskeletal symptoms. JCO Oncol Pract 16:733–739. https://doi.org/10.1200/OP.20.00113
Niravath P (2013) Aromatase inhibitor-induced arthralgia: a review. Ann Oncol 24:1443–1449. https://doi.org/10.1093/annonc/mdt037
Lu G, Zheng J, Zhang L (2020) The effect of exercise on aromatase inhibitor-induced musculoskeletal symptoms in breast cancer survivors :a systematic review and meta-analysis. Support Care Cancer 28:1587–1596. https://doi.org/10.1007/s00520-019-05186-1
Thompson PA, Huang C, Yang J, Wertheim BC, Roe DJ, Zhang X, Ding J, Chalasani P, Preece C, Martinez JA, Chow H-H, Sherry, Stopeck AT (2021) Evidence that the non-selective NSAID sulindac reduces breast density in postmenopausal women on aromatase inhibitors. Clinical Cancer Research in press.
Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW (1988) Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 15:1833–1840
Cleeland CS, Ryan KM (1994) Pain assessment: global use of the brief pain inventory. Ann Acad Med Singap 23:129–138
Mendoza T, Mayne T, Rublee D, Cleeland C (2006) Reliability and validity of a modified Brief Pain Inventory short form in patients with osteoarthritis. Eur J Pain 10:353–353. https://doi.org/10.1016/j.ejpain.2005.06.002
Fairclough DL, Cella DF (1996) Functional assessment of cancer therapy (FACT-G): non-response to individual questions. Qual Life Res 5:321–329. https://doi.org/10.1007/bf00433916
Webster K, Cella D, Yost K (2003) The functional assessment of chronic illness therapy (FACIT) measurement system: properties, applications, and interpretation. Health Qual Life Outcomes 1:79. https://doi.org/10.1186/1477-7525-1-79
Tubach F, Ravaud P, Beaton D, Boers M, Bombardier C, Felson DT, van der Heijde D, Wells G, Dougados M (2007) Minimal clinically important improvement and patient acceptable symptom state for subjective outcome measures in rheumatic disorders. J Rheumatol 34:1188
Shapiro AC, Adlis SA, Robien K, Kirstein MN, Liang S, Richter SA, Lerner RE (2016) Randomized, blinded trial of vitamin D3 for treating aromatase inhibitor-associated musculoskeletal symptoms (AIMSS). Breast Cancer Res Treat 155:501–512. https://doi.org/10.1007/s10549-016-3710-6
Niravath P, Hilsenbeck SG, Wang T, Jiralerspong S, Nangia J, Pavlick A, Ademuyiwa F, Frith A, Ma C, Park H, Rigden C, Suresh R, Ellis M, Kent Osborne C, Rimawi MF (2019) Randomized controlled trial of high-dose versus standard-dose vitamin D3 for prevention of aromatase inhibitor-induced arthralgia. Breast Cancer Res Treat 177:427–435. https://doi.org/10.1007/s10549-019-05319-4
Hershman DL, Unger JM, Crew KD, Awad D, Dakhil SR, Gralow J, Greenlee H, Lew DL, Minasian LM, Till C, Wade JL, Meyskens FL, Moinpour CM (2015) Randomized multicenter placebo-controlled trial of omega-3 fatty acids for the control of aromatase inhibitor-induced musculoskeletal pain: SWOG S0927. J Clin Oncol 33:1910–1917. https://doi.org/10.1200/JCO.2014.59.5595
Lustberg MB, Orchard TS, Reinbolt R, Andridge R, Pan X, Belury M, Cole R, Logan A, Layman R, Ramaswamy B, Wesolowski R, Berger M, Patterson E, Loprinzi C, Shapiro CL, Yee L (2018) Randomized placebo-controlled pilot trial of omega 3 fatty acids for prevention of aromatase inhibitor-induced musculoskeletal pain. Breast Cancer Res Treat 167:709–718. https://doi.org/10.1007/s10549-017-4559-z
Ringash J, O’Sullivan B, Bezjak A, Redelmeier DA (2007) Interpreting clinically significant changes in patient-reported outcomes. Cancer 110:196–202. https://doi.org/10.1002/cncr.22799
Chow LWC, Yip AYS, Chu WP, Loo WTY, Toi M (2011) Bone metabolism and quality-of-life of postmenopausal women with invasive breast cancer receiving neoadjuvant hormonal therapy: Sub-analyses from celecoxib anti-aromatase neoadjuvant (CAAN) trial. J Steroid Biochem Mol Biol 125:112–119. https://doi.org/10.1016/j.jsbmb.2010.12.018
Rosati MS, Di Seri M, Baciarello G, Lo Russo V, Grassi P, Marchetti L, Giovannoni S, Basile ML, Frati L (2011) Etoricoxib and anastrozole in adjuvant early breast cancer: ETAN trial (phase III). J Clin Oncol 29:533–533. https://doi.org/10.1200/jco.2011.29.15_suppl.533
Marcum ZA, Hanlon JT (2010) Recognizing the risks of chronic nonsteroidal anti-inflammatory drug use in older adults. Ann Longterm Care 18:24–27
Acknowledgements
None.
Funding
The work was supported by funding from the National Cancer Institute (Grant Number R01CA161534).
Author information
Authors and Affiliations
Contributions
Conception and design of study (PAT, ATS, and DJR); acquisition of data (PC, JC, and LB); analysis and/or interpretation of data (JAM, BCW, DJR, and PAT); drafting the manuscript (PAT, JAM, and BCW); approval of the version of the manuscript to be published (PAT, JAM, BCW, DJR, PC, JC, LB, HSC, and ATS).
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to disclose.
Ethical approval
The study was approved by the institutional review boards (IRB) at both the University of Arizona, Tucson, and Stony Brook University, New York.
Consent to participate
All patients provided written informed consent.
Consent for publication
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funding agencies had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Martinez, J.A., Wertheim, B.C., Roe, D.J. et al. Sulindac Improves Stiffness and Quality of Life in Women Taking Aromatase Inhibitors for Breast Cancer. Breast Cancer Res Treat 192, 113–122 (2022). https://doi.org/10.1007/s10549-021-06485-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-021-06485-0