Abstract
Purpose
The objective of this study was to describe the incidence of grade 3/4 neutropenia, patterns of chemotherapy treatment, and granulocyte colony-stimulating factor (G-CSF) use patterns among patients with non-Hodgkin’s lymphoma (NHL) <65 and ≥65 years.
Methods
This retrospective, observational study included adult patients with NHL who received cyclophosphamide, doxorubicin, vincristine, and prednisone ± rituximab (CHOP ± R) from January 2006 to June 2010.
Results
A total of 1,579 patients were included, with 54.1 % <65 years and 45.9 % ≥65 years. Most received CHOP-R on a Q3W schedule. Among patients <65 years, the incidence of grade 3/4 neutropenia was 52.3 %, the mean relative dose intensity (RDI) was 80.4 %, and the incidences of dose delays and reductions were 26.5 and 9.6 %, respectively. Among patients ≥65 years, the incidence of grade 3/4 neutropenia was 63.2 %, the mean RDI was 73.9 %, and the incidences of dose delays and reductions were 24.6 and 24.9 %, respectively. Most patients (86.9 %) received G-CSF. Among patients <65 years, 71.9, 17.4, and 10.7 % first received G-CSF as primary prophylaxis, secondary prophylaxis, or treatment, respectively. Among patients ≥65 years, 80.1, 11.6, and 8.3 % first received G-CSF as primary prophylaxis, secondary prophylaxis, or treatment, respectively.
Conclusions
Chemotherapy regimens and schedules were similar among age groups. Grade 3/4 neutropenia, reduced RDI, and dose delays were common in both age groups, though patients ≥65 years had a higher incidence of dose reductions. In spite of these similarities, patients <65 years were less likely to receive primary prophylactic G-CSF. Thus, careful assessment of neutropenia risk factors is needed across age groups to determine appropriate G-CSF use and support planned chemotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Non-Hodgkin’s lymphoma (NHL) is the seventh most common cancer in the USA [1] and was estimated to result in more than 20,000 deaths in 2010 [2]. Approximately half of patients newly diagnosed with NHL are ≥65 years [1, 3], and disease stage at diagnosis is similar among age groups [1]. Across age groups, the current standard of care for aggressive NHL is cyclophosphamide, doxorubicin, vincristine, and prednisone with or without rituximab (CHOP ± R) [4].
Neutropenia is a common, dose-limiting toxicity associated with CHOP-based chemotherapy regimens [5–7]. The risk of infection increases with the duration and severity of neutropenia [8]. Fever is often the first sign of infection in cancer patients with neutropenia, and febrile neutropenia (FN) can be a life-threatening complication that often requires immediate hospitalization. FN is associated with increased rates of mortality [9] and decreased survival [10–12]. For patients hospitalized with FN, the overall inpatient mortality has been reported to range from 6.8 to 10.6 % [13–15]. Additionally, FN is a common cause of dose delays and dose reductions, which result in reductions in relative dose intensity (RDI) [10–12] and worse overall therapeutic outcomes [10–12].
Maintaining adequate RDI of CHOP-based regimens has been shown to improve overall survival in patients with aggressive NHL [10–12]. In a study of 78 patients with NHL who received CHOP, RDI >70 % was associated with improved overall survival [11]. In an analysis of 115 patients with diffuse large B cell lymphoma (DLBCL) who received CHOP, RDI ≥75 % was identified as the single most significant predictor of overall survival [12]. Similarly, in an analysis of 210 patients with DLBCL who received CHOP, RDI >90 % was associated with significantly improved overall survival compared to patients who received an RDI ≤90 % [10].
Prophylactic use of granulocyte colony-stimulating factors (G-CSFs) has been shown to reduce the incidence and duration of neutropenia and neutropenic complications, thereby facilitating administration of full-dose and dose-dense chemotherapy [7, 16–23]. Current American Society of Clinical Oncology (ASCO) and National Comprehensive Cancer Network (NCCN) guidelines recommend the use of primary prophylactic G-CSF following myelosuppressive chemotherapy when the overall risk of FN is ≥20 % [24, 25]. Individual patient risk of FN depends on disease-related [24, 26–28], treatment-related [24, 27, 29], and patient-related factors [26–30]. Among patients with NHL who received CHOP-based chemotherapy without prophylactic G-CSF, the reported rates of FN in clinical trials range from 21 to 44 % [6, 16, 18, 19].
Age is an established risk factor for FN, and the incidence of neutropenia and related complications in patients ≥65 years with NHL has been well studied [31, 32]. However, few studies have focused on patients <65 years [1]. The objectives of this retrospective observational cohort study were to describe the incidence of grade 3/4 neutropenia, patterns of G-CSF use, chemotherapy regimens and dosing schedule, and the incidences of chemotherapy dose delays, dose reductions, and reduced RDI among patients <65 and ≥65 years who received CHOP-based chemotherapy for NHL.
Patients and methods
Patient selection
The study population included adults ≥18 years at the start of chemotherapy who were diagnosed with DLBCL or advanced follicular lymphoma and received standard CHOP ± R on a once every 2-, 3-, or 4-week schedule per NCCN treatment guidelines [4] between January 1, 2006 and June 30, 2010. All patients completed at least the first cycle of chemotherapy and had follow-up data, including at least one absolute neutrophil count (ANC) value, available for at least 4 weeks following the first dose of chemotherapy. Patients were excluded if they had prior or concurrent stem cell transplantation, had received granulocyte-macrophage (GM)-CSF or radiation therapy, had participated in a clinical trial requiring the use of CSFs, or had received other investigational agents.
Study design
This was a retrospective, observational cohort study of a database containing oncology-specific electronic medical record (EMR) data from 46 oncology/hematology practices in 26 states for various payer types (commercial, Medicare, Medicaid, and others) and cash-paying patients. Data were abstracted electronically. Patient data were de-identified and assigned a longitudinally stable identifier; all data were compliant with the Health Insurance Portability and Accountability Act (HIPAA).
Study end points
The primary end point was the incidence of grade 3/4 neutropenia (defined as ANC <1.0 × 109/L) during treatment with CHOP ± R. The original planned study primary end point was the incidence of FN. However, a preplanned feasibility study determined that the incidence of reported FN was ∼3 %, far lower than the reported rates of FN in patients with NHL who had received G-CSF prophylaxis (13–23 %) or who had not received G-CSF prophylaxis (21–44 %) [18, 19, 33], suggesting that the incidence of FN may not be adequately captured in the EMR. Therefore, the primary end point of the study was amended to estimate the incidence of grade 3/4 neutropenia, a laboratory-based end point more likely to be consistently captured in the EMR database.
The secondary end point was to evaluate patterns of G-CSF use, including type of G-CSF used, timing of G-CSF administration, and duration of G-CSF use. G-CSF prophylaxis was defined as initial G-CSF use occurring within 5 days of chemotherapy completion; primary prophylaxis was defined as initial G-CSF use occurring during the first cycle, and secondary prophylaxis was defined as initial G-CSF use occurring during the second or subsequent cycles. G-CSF treatment was defined as G-CSF use occurring more than 5 days after completion of chemotherapy in any cycle.
Exploratory end points were RDI and the incidences of chemotherapy dose delays and reductions. RDI was defined as the average ratio of the delivered dose intensity to the NCCN standard dose intensity for doxorubicin and cyclophosphamide [4]. RDI was calculated by cycle and across the chemotherapy course. Based on current treatment recommendations [4], a course was defined as 6 cycles. If a patient did not complete 6 cycles, then a dose of zero was assigned for each missed cycle and the time was the sum of the observed time for the cycles taken plus the standard time required for the missed cycles. A more granular analysis of planned versus actual RDI was not possible as planned cycles of chemotherapy were frequently not available in the EMRs.
A dose delay was defined as a ≥7-day delay in administration of doxorubicin or cyclophosphamide. Planned dosing intervals were not available from this database; therefore, the dosing schedule was determined based on the minimum duration between cycles. A dose reduction was defined as a ≥15 % reduction relative to NCCN standards. If a dose was reduced and maintained at that same reduced dose for subsequent cycles, this was only counted as a single dose reduction.
Study variables
Patient and disease characteristics collected were age at the initiation of chemotherapy, sex, performance status, medical history, laboratory values (e.g., complete blood count [CBC]), and comorbidities of interest (e.g., autoimmune disease, pulmonary disease, and diabetes).
Statistical methods
End points were analyzed by cycle, across cycles, and by age group (<65 and ≥65 years) at diagnosis. For continuous variables, the mean, median, range, and standard deviation were calculated. For categorical variables, the number of patients and incidence in each category were presented. For incidence of grade 3/4 neutropenia, point estimates were accompanied by two-sided 95 % confidence intervals (CIs). The study period was from the first dose of chemotherapy to 6 weeks following the last dose of chemotherapy or loss to follow-up.
Results
Patient characteristics
This study included 1,579 unique patients; 854 (54.1 %) were <65 years and 725 (45.9 %) were ≥65 years. Because of the retrospective nature of this analysis, EMR data were not available for each analysis and some data points are missing. Thus, the number of patients who could be included in each analysis group varies.
Baseline patient demographics were well balanced between age groups (Table 1), and the proportion of patients with one or more comorbidities was similar among patients < 65 years (n = 139; 16.3 %) and patients ≥ 65 years (n = 166; 22.9 %).
Chemotherapy regimen and schedule were very similar among age groups (Table 2). Among the 1,522 patients who had identifiable chemotherapy regimens, 86.7 % of patients <65 years and 89.5 % of patients ≥65 years received CHOP-R, and 91.1 % of patients <65 years and 94.2 % of patients ≥ 65 years received chemotherapy on a once every 3-week (Q3W) schedule. Both age groups received a mean (SD) of 5 (1.6) cycles of chemotherapy. Nearly all patients received at least 3 cycles of chemotherapy; however, many patients appeared to discontinue chemotherapy after cycle 3 with 49.4 % of patients ≥65 years (n = 347) and 60.6 % of patients <65 years (n = 497) receiving the NCCN-recommended 6 cycles of chemotherapy (Table 2).
Grade 3/4 neutropenia
Across all cycles, patients had a mean of 11.0 ANC values. The mean number of ANC values available was very similar among patients <65 years (10.9; SD = 5.8) and patients ≥65 years (11.2; SD = 6.3). ANC values were recorded in the EMR; 37.4 % of the patients had documented ANC values on the first day of the chemotherapy cycle, 42.1 % had documented ANC values in the first week following initiation of the chemotherapy cycle, 27.7 % in the second week following initiation of the chemotherapy cycle, 19.5 % in the third week following initiation of the chemotherapy cycle, and 10.8 % after the third week following initiation of the chemotherapy cycle.
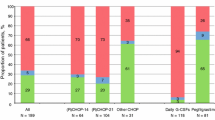
Overall, 873 patients (57.4 %) had documented grade 3/4 neutropenia (95 % CI 54.8–59.9), with 52.3 % (48.8–55.8) of patients <65 years and 63.2 % (59.6–66.8) of patients ≥65 years having documented grade 3/4 neutropenia (Fig. 1). In both age groups, grade 3/4 neutropenia occurred more frequently in cycle 1 (<65 years = 34.4 % [31.1–37.8]; ≥65 years = 41.7 % [38.1–45.5]) than in any other cycle. Overall, 641 patients (42.1 %) had documented grade 4 neutropenia (95 % CI 39.6–44.6), with 36.1 % (32.8–39.5) of patients <65 years and 49.1 % (45.4–52.9) of patients ≥65 years having documented grade 4 neutropenia.
Patterns of granulocyte colony-stimulating factor use
There were 1,522 patients who had data available on G-CSF use. Overall, 1,322 patients (86.9 %) received G-CSF at some point during their chemotherapy, with 84.6 % of patients <65 years (n = 694) and 89.5 % of patients ≥65 years (n = 628) receiving G-CSF (Table 3). Among patients who received G-CSF, 1,002 patients (75.8 %) first received primary prophylaxis, 194 (14.7 %) first received secondary prophylaxis, and 126 (9.5 %) first received treatment. Fewer patients <65 years (n = 499; 71.9 %) than patients ≥65 years (n = 503; 80.1 %) first received G-CSF as primary prophylaxis, while a greater proportion of patients <65 years (n = 121; 17.4 %) than patients ≥65 years (n = 73; 11.6 %) first received G-CSF as secondary prophylaxis. Treatment with G-CSF was similar among patients <65 years (n = 74; 10.7 %) and patients ≥65 years (n = 52; 8.3 %).
Pegfilgrastim was first administered most frequently as primary prophylaxis (n = 975; 77.9 %), whereas filgrastim was first administered predominantly as treatment (n = 154; 79.8 %) (Table 3). At first use, the mean (SD) duration of filgrastim use was 3.2 (2.4) days. In subsequent cycles, the mean (SD) duration of filgrastim use was 4.3 (3.0) days. At first use, the duration of filgrastim use was similar among patients <65 years (mean = 3.0 days; SD = 2.1) and patients ≥65 years (mean = 3.6 days; SD = 2.7). For patients <65 years, the duration of filgrastim use changed little in subsequent cycles (mean = 3.4 days; SD = 2.6). Patients ≥65 years received slightly longer durations of filgrastim in subsequent cycles (5.2 days; SD = 3.1).
Relative dose intensity and chemotherapy dose delays and reductions
There were 1,516 patients who could be evaluated for RDI. Overall, the mean (95 % CI) RDI across 6 cycles was 77.4 % (76.3, 78.5). Mean (95 % CI) RDI was greater among patients <65 years (80.4 % [79.0, 81.9]) than among patients ≥65 years (73.9 % [72.2, 75.6]). RDI remained high through cycle 3 for both age groups (Fig. 2), and differences among patients <65 years and ≥65 years were apparent as early as cycle 3 (Fig. 2).
Overall, 47.0 % of the patients (n = 712) received an RDI ≥85 %, and 41.5 % of the patients (n = 629) received an RDI ≥90 %. The proportion of patients that received an RDI ≥85 % was greater among patients <65 years (n = 429; 52.6 %) than among patients ≥65 years (n = 283; 40.4 %). Similarly, more patients <65 years received an RDI ≥90 % (n = 379; 46.4 %) than patients ≥65 years (n = 250; 35.7 %).
There were 1,522 patients who could be evaluated for dose delays and dose reductions. Overall, 390 patients (25.6 %) had documented chemotherapy dose delays lasting ≥7 days. The incidence of dose delays ≥7 days was similar among patients <65 years (n = 217; 26.5 %) and patients ≥65 years (n = 173; 24.6 %), and the incidence of dose delays was relatively constant across the cycles analyzed (Fig. 3a). Overall, 252 patients (16.6 %) had documented chemotherapy dose reductions ≥15 %. The incidence of dose reductions was lower among patients <65 years (n = 78; 9.6 %) than among patients ≥65 years (n = 174; 24.9 %). The incidence of dose reductions was highest in cycle 1 and declined over subsequent cycles (Fig. 3b).
Among 873 patients with grade 3/4 neutropenia, 251 (28.8 %) had a documented dose delay ≥7 days and 171 (19.6 %) had a documented dose reduction ≥15 %. In this subgroup, no differences in dose delays were seen between age groups; however, patients <65 years with grade 3/4 neutropenia had fewer dose reductions (n = 51; 11.9 %) than patients ≥65 years with grade 3/4 neutropenia (n = 120; 27.0 %). The incidences of dose delays and dose reductions were similar among patients who had grade 3/4 neutropenia and the overall study population. These data suggest that neutropenia was not the major cause of dose delays or dose reductions in this study.
Discussion
The risk of chemotherapy-induced neutropenia and associated complications is well recognized in patients with aggressive NHL who are ≥65 years [4, 24, 25, 31, 32]. However, less attention has been focused on the incidence of neutropenia among younger patients. Here, we found that more than half of patients in both age groups had at least one documented episode of grade 3/4 neutropenia. The rates of grade 3/4 neutropenia in this study may be underestimated due to sparse sampling of ANC values, but the frequency of sampling was similar in patients <65 years and in patients ≥65 years. Thus, the incidence of grade 3/4 neutropenia in this study should not be biased by increased sampling in older patients.
Primary prophylaxis with a single dose of pegfilgrastim per cycle or 9–14 days of filgrastim per cycle has been shown to reduce the duration and severity of neutropenia and the incidence of FN [34–37]. But the use of G-CSF is a complex clinical decision based on an individual patient’s risk of FN. Age is a well-established risk factor for FN that is readily available in most studies; however, other risk factors for FN can be difficult to determine in a retrospective study. For example, the Eastern Cooperative Oncology Group (ECOG) performance status is an established risk factor for FN, but 77 % of patients in this study were lacking ECOG performance status values. Though most physicians calculate the ECOG performance status, these data are rarely included in the EMR.
Primary prophylactic G-CSF is recommended in patients ≥65 years who are receiving CHOP-based chemotherapy; secondary prophylaxis in response to a previous neutropenic event is not a recommended strategy in these patients [24]. Regardless of patient age and other risk factors, G-CSF is not approved for treatment once a patient has developed FN, and guidelines generally recommend against treatment with G-CSF except in special circumstances [24, 25]. In spite of these recommendations, in this study, 11.6 % of patients ≥65 years and 17.4 % of patients <65 years received secondary prophylaxis with G-CSF, and 8.3 % of patients ≥65 years and 10.7 % of patients <65 years received treatment with G-CSF. Furthermore, the mean duration of daily filgrastim use in this study was markedly shorter than that established as clinically beneficial [34, 37].
Historically, myelotoxicity is a major cause of chemotherapy dose delays, dose reductions, and reduced RDI. Here, the incidences of dose delays and dose reductions were similar among patients who had grade 3/4 neutropenia and those who did not have grade 3/4 neutropenia, suggesting that neutropenia was not a major cause of dose delays and dose reductions. Further research is needed to identify and address other potential causes of dose delays and dose reductions, such as patient and physician preference.
Primary prophylactic G-CSF is associated with higher RDI in patients with aggressive NHL [31], and multiple studies have shown that maintaining high RDI is important to maximize treatment outcome when treating patients with aggressive NHL [10–12]. In this study, only 61 % of patients <65 years and 49 % of patients ≥65 years completed the NCCN-recommended 6 cycles of chemotherapy [4]. Because planned number of cycles was not available in the database, the physician’s intended treatment plan could not be discerned. However, RDI was much higher when calculated over the completed number of cycles (data not shown). These data suggest that missed cycles have a greater impact on RDI than dose reductions or dose delays, but the reasons why patients received abbreviated treatment cycles are unclear.
The proportions of patients with NHL achieving RDIs ≥85 % in this study were similar to those achieved over a decade ago [31]. Multiple methods to calculate RDI exist in the literature; however, these differences among calculation methods can significantly impact the results. Thus, differences in RDI among studies must be interpreted cautiously, and methods of calculation should always be reported.
When this study was originally planned, FN was the primary end point. In initial feasibility analyses, the FN incidence captured in the EMR database used here was substantially lower than that reported in the published literature for a similar patient population receiving CHOP-based chemotherapy, suggesting that FN episodes were not reliably captured in the outpatient EMR system. We hypothesized that these data gaps were largely due to treatment of FN in this population in an inpatient setting and that the ANC and temperature data that define FN were not reliably transferred from the hospital records to the EMR. As more clinical records move to EMRs, mechanisms are needed to ensure that diverse data systems are properly integrated to capture important clinical events such as FN. Better data integration between EMRs and hospital databases can facilitate information sharing among different care providers and improve efficiency, patient safety, and patient care [38].
Approximately 173,000 cancer patients (59,000 chemotherapy patients with 448,000 chemotherapy administrations) from 47 oncology/hematology practices in 26 states were captured in the large EMR database used in this study. Thus, the data presented here are likely reflective of the US population of patients with NHL. Additionally, patient eligibility criteria in this study were relatively nonrestrictive, limiting the potential for selection bias. Together, the data here provide a description of clinical practice and may be a more accurate illustration of patient management than are clinical trials, which have strict protocol-based requirements for treatment, supportive care, monitoring, and patient follow-up. However, as with all retrospective chart reviews, the study was limited to the data available in the database. Chemotherapy schedule; planned number of cycles; reasons for dose reductions, dose delays, and missed cycles; and long-term patient outcomes were not available. Additionally, FN rates were likely underreported, and G-CSF use was based on standard definitions of treatment and prophylaxis supported by the literature, rather than physician intent.
In conclusion, chemotherapy regimens and dosing schedules were similar among age groups. Grade 3/4 neutropenia, dose delays, and reduced RDI were common across all ages of patients with NHL receiving CHOP-based chemotherapy, and patients ≥65 years had a higher incidence of dose reductions. However, patients <65 years were less likely to receive primary G-CSF prophylaxis. Thus, careful evaluation of risk factors for grade 3/4 neutropenia and related complications is needed for all patients with NHL, regardless of age.
References
Howlader N, Noone A, Krapcho M, Neyman N, Aminou R, Waldron W, Altekruse S, Kosary C, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Eisner M, Lewis D, Chen H, Feuer E, Cronin K, Edwards B (2011) SEER cancer statistics review, 1975–2008. National Cancer Institute, Bethesda
Altekruse SF, Kosary CL, Krapcho M, Neyman N, Aminou R, Waldron W, Ruhl J, Howlader N, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Cronin K, Chen HS, Feuer EJ, Stinchcomb DG, Edwards BK (2010) SEER cancer statistics review, 1975–2007. National Cancer Institute, Bethesda
Armitage JO, Weisenburger DD (1998) New approach to classifying non-Hodgkin's lymphomas: clinical features of the major histologic subtypes. Non-Hodgkin's Lymphoma Classification Project. J Clin Oncol 16(8):2780–2795
National Comprehensive Cancer Network (2011) NCCN Clinical Practice Guidelines in Oncology: Non-Hodgkin's Lymphomas version 2.2011. National Comprehensive Cancer Network, Fort Washington, PA
Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, Morel P, Van Den Neste E, Salles G, Gaulard P, Reyes F, Lederlin P, Gisselbrecht C (2002) CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med 346(4):235–242. doi:10.1056/NEJMoa011795
Feld R, Bodey GP (1977) Infections in patients with malignant lymphoma treated with combination chemotherapy. Cancer 39(3):1018–1025
Lyman GH, Morrison VA, Dale DC, Crawford J, Delgado DJ, Fridman M (2003) Risk of febrile neutropenia among patients with intermediate-grade non-Hodgkin's lymphoma receiving CHOP chemotherapy. Leuk Lymphoma 44(12):2069–2076. doi:10.1080/1042819031000119262
Bodey GP, Buckley M, Sathe YS, Freireich EJ (1966) Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Ann Intern Med 64(2):328–340
Crawford J, Dale DC, Lyman GH (2004) Chemotherapy-induced neutropenia: risks, consequences, and new directions for its management. Cancer 100(2):228–237
Bosly A, Bron D, Van Hoof A, De Bock R, Berneman Z, Ferrant A, Kaufman L, Dauwe M, Verhoef G (2008) Achievement of optimal average relative dose intensity and correlation with survival in diffuse large B-cell lymphoma patients treated with CHOP. Ann Hematol 87(4):277–283
Epelbaum R, Haim N, Ben-Shahar M, Ron Y, Cohen Y (1988) Dose-intensity analysis for CHOP chemotherapy in diffuse aggressive large cell lymphoma. Isr J Med Sci 24(9–10):533–538
Kwak LW, Halpern J, Olshen RA, Horning SJ (1990) Prognostic significance of actual dose intensity in diffuse large-cell lymphoma: results of a tree-structured survival analysis. J Clin Oncol 8(6):963–977
Caggiano V, Weiss RV, Rickert TS, Linde-Zwirble WT (2005) Incidence, cost, and mortality of neutropenia hospitalization associated with chemotherapy. Cancer 103(9):1916–1924. doi:10.1002/cncr.20983
Dulisse B, Li X, Gayle JA, Barron RL, Ernst FR, Rothman KJ, Legg JC, Kaye JA (2013) A retrospective study of the clinical and economic burden during hospitalizations among cancer patients with febrile neutropenia. J Med Econ. doi:10.3111/13696998.2013.782034
Kuderer NM, Dale DC, Crawford J, Cosler LE, Lyman GH (2006) Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer 106(10):2258–2266. doi:10.1002/cncr.21847
Gisselbrecht C, Haioun C, Lepage E, Bastion Y, Tilly H, Bosly A, Dupriez B, Marit G, Herbrecht R, Deconinck E, Marolleau JP, Yver A, Dabouz-Harrouche F, Coiffier B, Reyes F (1997) Placebo-controlled phase III study of lenograstim (glycosylated recombinant human granulocyte colony-stimulating factor) in aggressive non-Hodgkin's lymphoma: factors influencing chemotherapy administration. Groupe d'Etude des Lymphomes de l'Adulte. Leuk Lymphoma 25(3–4):289–300
Lyman GH, Kuderer NM, Djulbegovic B (2002) Prophylactic granulocyte colony-stimulating factor in patients receiving dose-intensive cancer chemotherapy: a meta-analysis. Am J Med 112(5):406–411
Pettengell R, Gurney H, Radford JA, Deakin DP, James R, Wilkinson PM, Kane K, Bentley J, Crowther D (1992) Granulocyte colony-stimulating factor to prevent dose-limiting neutropenia in non-Hodgkin's lymphoma: a randomized controlled trial. Blood 80(6):1430–1436
Zinzani PL, Pavone E, Storti S, Moretti L, Fattori PP, Guardigni L, Falini B, Gobbi M, Gentilini P, Lauta VM, Bendandi M, Gherlinzoni F, Magagnoli M, Venturi S, Aitini E, Tabanelli M, Leone G, Liso V, Tura S (1997) Randomized trial with or without granulocyte colony-stimulating factor as adjunct to induction VNCOP-B treatment of elderly high-grade non-Hodgkin's lymphoma. Blood 89(11):3974–3979
Scott SD, Chrischilles EA, Link BK, Delgado DJ, Fridman M, Stolshek BS (2003) Days of prophylactic filgrastim use to reduce febrile neutropenia in patients with non-Hodgkin's lymphoma treated with chemotherapy. J Manag Care Pharm 9(2 Suppl):15–21
Jacobson JO, Grossbard M, Shulman LN, Neuberg D (2000) CHOP chemotherapy with preemptive granulocyte colony-stimulating factor in elderly patients with aggressive non-Hodgkin's lymphoma: a dose-intensity analysis. Clin Lymphoma 1(3):211–217, discussion 218
Peters FP, Fickers MM, Erdkamp FL, Wals J, Wils JA, Schouten HC (2001) The effect of optimal treatment on elderly patients with aggressive non-Hodgkin's lymphoma: more patients treated with unaffected response rates. Ann Hematol 80(7):406–410
Pfreundschuh M, Trumper L, Kloess M, Schmits R, Feller AC, Rube C, Rudolph C, Reiser M, Hossfeld DK, Eimermacher H, Hasenclever D, Schmitz N, Loeffler M (2004) Two-weekly or 3-weekly CHOP chemotherapy with or without etoposide for the treatment of elderly patients with aggressive lymphomas: results of the NHL-B2 trial of the DSHNHL. Blood 104(3):634–641. doi:10.1182/blood-2003-06-2095
National Comprehensive Cancer Network (2011) NCCN Clinical Practice Guidelines in Oncology: Myeloid Growth Factors, version 1.2011. National Comprehensive Cancer Network, Fort Washington, PA
Smith TJ, Khatcheressian J, Lyman GH, Ozer H, Armitage JO, Balducci L, Bennett CL, Cantor SB, Crawford J, Cross SJ, Demetri G, Desch CE, Pizzo PA, Schiffer CA, Schwartzberg L, Somerfield MR, Somlo G, Wade JC, Wade JL, Winn RJ, Wozniak AJ, Wolff AC (2006) 2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol 24(19):3187–3205. doi:10.1200/JCO.2006.06.4451
Lyman GH, Lyman CH, Agboola O (2005) Risk models for predicting chemotherapy-induced neutropenia. Oncologist 10(6):427–437. doi:10.1634/theoncologist.10-6-427
Pettengell R, Bosly A, Szucs TD, Jackisch C, Leonard R, Paridaens R, Constenla M, Schwenkglenks M (2009) Multivariate analysis of febrile neutropenia occurrence in patients with non-Hodgkin lymphoma: data from the INC-EU Prospective Observational European Neutropenia Study. Br J Haematol 144(5):677–685. doi:10.1111/j.1365-2141.2008.07514.x
von Minckwitz G, Schwenkglenks M, Skacel T, Lyman GH, Pousa AL, Bacon P, Easton V, Aapro MS (2009) Febrile neutropenia and related complications in breast cancer patients receiving pegfilgrastim primary prophylaxis versus current practice neutropaenia management: results from an integrated analysis. Eur J Cancer 45(4):608–617. doi:10.1016/j.ejca.2008.11.021
Crawford J (2006) Risk assessment and guidelines for first-cycle colony-stimulating factor use in the management of chemotherapy-induced neutropenia. Oncology (Williston Park) 20(5 Suppl 4):22–28
Crawford J, Glaspy JA, Stoller RG, Tomita DK, Vincent ME, McGuire BW, Ozer H (2005) Final results of a placebo-controlled study of filgrastim in small-cell lung cancer: exploration of risk factors for febrile neutropenia. Support Cancer Ther 3(1):36–46. doi:10.3816/SCT.2005.n.023
Lyman GH, Dale DC, Friedberg J, Crawford J, Fisher RI (2004) Incidence and predictors of low chemotherapy dose-intensity in aggressive non-Hodgkin's lymphoma: a nationwide study. J Clin Oncol 22(21):4302–4311. doi:10.1200/JCO.2004.03.213
Morrison VA, Picozzi V, Scott S, Pohlman B, Dickman E, Lee M, Lawless G, Kerr R, Caggiano V, Delgado D, Fridman M, Ford J, Carter WB, Oncology Practice Pattern Study Working Group (2001) The impact of age on delivered dose intensity and hospitalizations for febrile neutropenia in patients with intermediate-grade non-Hodgkin's lymphoma receiving initial CHOP chemotherapy: a risk factor analysis. Clin Lymphoma 2(1):47–56
Balducci L, Al-Halawani H, Charu V, Tam J, Shahin S, Dreiling L, Ershler WB (2007) Elderly cancer patients receiving chemotherapy benefit from first-cycle pegfilgrastim. Oncologist 12(12):1416–1424. doi:10.1634/theoncologist.12-12-1416
Crawford J, Ozer H, Stoller R, Johnson D, Lyman G, Tabbara I, Kris M, Grous J, Picozzi V, Rausch G et al (1991) Reduction by granulocyte colony-stimulating factor of fever and neutropenia induced by chemotherapy in patients with small-cell lung cancer. N Engl J Med 325(3):164–170. doi:10.1056/NEJM199107183250305
Green MD, Koelbl H, Baselga J, Galid A, Guillem V, Gascon P, Siena S, Lalisang RI, Samonigg H, Clemens MR, Zani V, Liang BC, Renwick J, Piccart MJ (2003) A randomized double-blind multicenter phase III study of fixed-dose single-administration pegfilgrastim versus daily filgrastim in patients receiving myelosuppressive chemotherapy. Ann Oncol 14(1):29–35
Holmes FA, O'Shaughnessy JA, Vukelja S, Jones SE, Shogan J, Savin M, Glaspy J, Moore M, Meza L, Wiznitzer I, Neumann TA, Hill LR, Liang BC (2002) Blinded, randomized, multicenter study to evaluate single administration pegfilgrastim once per cycle versus daily filgrastim as an adjunct to chemotherapy in patients with high-risk stage II or stage III/IV breast cancer. J Clin Oncol 20(3):727–731
Trillet-Lenoir V, Green J, Manegold C, Von Pawel J, Gatzemeier U, Lebeau B, Depierre A, Johnson P, Decoster G, Tomita D et al (1993) Recombinant granulocyte colony stimulating factor reduces the infectious complications of cytotoxic chemotherapy. Eur J Cancer 29A(3):319–324
Hillestad R, Bigelow J, Bower A, Girosi F, Meili R, Scoville R, Taylor R (2005) Can electronic medical record systems transform health care? Potential health benefits, savings, and costs. Health Aff (Millwood) 24(5):1103–1117. doi:10.1377/hlthaff.24.5.1103
Acknowledgments
The authors thank Sharon Hunter, Sejal Badre, and Wayne Sheldon for the biostatistical support. Kerri Hebard-Massey (Amgen Inc.) provided medical writing support.
Conflict of interest
This study was sponsored by Amgen Inc. SW is an employee of and stockholder in Amgen Inc. EA was an employee of Amgen Inc. at the time the study was performed. LS has a consultancy or advisory role with Amgen Inc. MS has no conflicts to declare. Authors had full control of the primary data and agree to allow the journal to review the data if requested.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schwartzberg, L.S., Saleh, M., Whittaker, S. et al. Severe neutropenia and relative dose intensity among patients <65 and ≥65 years with non-Hodgkin’s lymphoma receiving CHOP-based chemotherapy. Support Care Cancer 22, 1833–1841 (2014). https://doi.org/10.1007/s00520-014-2157-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-014-2157-8