Abstract
The long-term effects of pegfilgrastim administered in the first cycle of chemotherapy in day-to-day practice remain unclear. We retrospectively identified 114 patients aged ≥ 70 years with diffuse large B-cell lymphoma who received a rituximab-cyclophosphamide-doxorubicin-vincristine-prednisolone (R-CHOP) regimen in our institution. Twenty-six patients received pegfilgrastim (pegfilgrastim group); of the 88 patients scheduled to receive conventional granulocyte-colony stimulating factor (G-CSF) when their neutrophil count decreased (neut-adjusted-G group), conventional G-CSF was ultimately administered to 57. During the first cycle of R-CHOP, the incidence of febrile neutropenia was lower in the pegfilgrastim group than in the neut-adjusted-G group (0% vs. 18%, p = 0.020). Throughout all cycles, a higher proportion of patients exhibited sustained relative dose intensity (≥ 80%) in the pegfilgrastim group than in the neut-adjusted-G group (25% vs. 4.0%, p = 0.008). A lower proportion of patients received a reduced dose in the second cycle in the pegfilgrastim group than in the neut-adjusted-G group (0% vs. 10%, p = 0.116). Although the differences were not significant, the pegfilgrastim group showed higher progression-free survival and overall survival than the neut-adjusted-G group. Adequate prevention of febrile neutropenia using pegfilgrastim during the first cycle of R-CHOP may contribute to avoidance of dose intensity reduction in all cycles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common form of non-Hodgkin’s lymphoma (NHL). The disease is aggressive, and patients typically present with rapidly enlarging lymphadenopathy and constitutional symptoms, necessitating immediate treatment [1]. The incidence of DLBCL has been reported to rise steadily with age; therefore, effective treatment of DLBCL in elderly patients has become an important issue [2]. Rituximab in combination with cyclophosphamide, doxorubicin, vincristine, and prednisolone (R-CHOP) is the standard treatment for patients with DLBCL [3]. Previous studies have suggested that there is a correlation between delivering the full, planned dose of chemotherapy on time and positive disease outcome in and survival of patients with aggressive NHL [4,5,6]. To avoid the unintended reduction of dose intensity, the appropriate prevention of adverse reactions, including febrile neutropenia (FN), is necessary [7, 8]. Moreover, cancer-related neutropenia and infections have been reported to be associated with unintended hospital admission and higher medical costs [9].
Granulocyte-colony stimulating factor (G-CSF) has been widely used for the prophylaxis of FN. The use of G-CSF increases the chemotaxis and migration of neutrophils and ameliorates neutropenia and its complications [10]. There are two types of G-CSF, namely, long-acting G-CSF and conventional G-CSF. Pegfilgrastim, a long-acting G-CSF, is a polyethylene glycol-modified form of filgrastim and its injection is required once per cycle, 1–4 days after chemotherapy. In contrast, conventional G-CSF is first administered 1–4 days after the completion of chemotherapy, followed by daily injections until the absolute neutrophil count (ANC) recovers to normal levels according to National Comprehensive Cancer Network guidelines [11]. However, in many hospitals, including ours, in Japan, conventional G-CSF is initiated when the ANC is less than 500/μL as a standard practice [12].
A clinical trial has shown that a single fixed subcutaneous dose of pegfilgrastim is comparable to daily injections of conventional G-CSF in terms of safety and efficacy [13]. Thus, as a routine procedure, there may be potential benefits of using pegfilgrastim over the daily administration of other G-CSF products, owing to the fewer injections required. Furthermore, we previously evaluated the effect of pegfilgrastim in the first cycle of the R-CHOP regimen and showed that it is advantageous in the prevention of FN, reduces overall health care costs, and decreases the length of hospital stay compared with the conventional G-CSF [14]. However, the long-term effects of pegfilgrastim use during the first cycle remain unclear. Because the risk of FN is the highest in the first cycle, it is possible that the adequate prevention of FN during the first cycle using pegfilgrastim may contribute to safe and effective chemotherapy in day-to-day practice [15,16,17,18,19].
In this study, we evaluated the short- and long-term clinical effects of pegfilgrastim use during the first cycle of the R-CHOP regimen in elderly patients with newly diagnosed DLBCL. We conducted an analysis of patients ≥ 70 years of age because G-CSF prophylaxis was being considered for these aged patients in our institute, and a previous study reported risks of developing FN with this therapy [20].
Materials and methods
Patients and data collection
We retrospectively identified patients diagnosed with DLBCL aged ≥ 70 years who were treated with R-CHOP in the University of Tokyo Hospital between January 2008 and December 2018. All DLBCL cases were diagnosed pathologically by immunostaining. We analyzed all patients with DLBCL, including those with subtypes possibly associated with a poor prognosis, such as CD5-positive, transformation from follicular lymphoma, non-GCB, and MYC-rearrangement (double hit). All patients who received at least one cycle of the R-CHOP regimen were included to evaluate patient baseline characteristics and outcome from the first cycle. Patients were excluded from analysis of the outcome of all cycles if their disease had progressed before the completion of the R-CHOP regimen, they could not continue treatment because of adverse events other than infections, or they were transferred to other hospitals. Clinical charts were reviewed by the investigators. The characteristics evaluated were the patients’ age; sex; performance status; body mass index; and albumin, hemoglobin, and lactate dehydrogenase (LDH) levels; and characteristics of the disease (stage, international prognostic index, presence or absence of bone marrow infiltration, CD5-positivity, and transformation from follicular lymphoma); presence or absence of diabetes mellitus; and medical history. The study protocol was approved by the Ethics Review Board of The University of Tokyo. All procedures were performed following the general ethical principles outlined in the Declaration of Helsinki.
Study drugs and treatment procedures
Rituximab in combination with cyclophosphamide, doxorubicin, vincristine, and prednisolone chemotherapy at a 100% dosage is composed of rituximab (375 mg/m2), cyclophosphamide (750 mg/m2), doxorubicin (50 mg/m2), and vincristine (1.4 mg/m2, up to a maximum dose of 2 mg) on day 1 and prednisone (60 mg/m2) from days 1 to 5. Body surface area was calculated using the Mosteller formula [21]. In our institute, patients aged 70–79 years received the first cycle of R-CHOP at a 67% dose, which was increased to 100% in the second and following cycles according to each physician’s judgment. Patients aged over 80 years started R-CHOP at a 50% dose. The dose of one patient was adjusted further owing to organ dysfunction, the occurrence of FN, and the grade of neutropenia in the previous R-CHOP cycle. In our institute, according to the Common Terminology Criteria for Adverse Events (CTCAE), the policy is to increase the dose in the subsequent cycles when patients received a reduced dose of R-CHOP and experienced no serious adverse events (≥ grade 3 hematological and non-hematological toxicities). On the contrary, dose reduction in the following cycles was recommended when patients showed grade 4 hematological toxicities. When patients did not meet both these criteria, the chemotherapeutic dose was not changed in the following cycles.
Conventional G-CSF administration was initiated when ANC decreased under 500/μL and was continued until ANC reached 1000/μL. There were patients who were scheduled to receive conventional G-CSF during the cycle but did not because their ANC had not decreased to under 500/μL. Therefore, patients were grouped into the pegfilgrastim treatment group and the neutrophil-adjusted daily G-CSF treatment group (neut-adjusted-G group). The neut-adjusted-G group was further subclassified into the actual administration group (actual-G group) and the no G-CSF group (no-G group). Although we made it a rule to use pegfilgrastim for all patients who were over 70 years of age from 2016 to 2018 in our institution, whether patients ultimately received conventional G-CSF or pegfilgrastim was decided on a case-by-case basis by the treating hematologist.
Blood samples were collected before each cycle of chemotherapy, and at least 3 days a week during the first cycle, which was administered under hospitalization. Although subsequent cycles of chemotherapy were administered in an outpatient clinic, patients were followed for the development of neutropenia and its complications, generally at the discretion of the treating hematologist. Treatments were typically repeated up to six cycles. When the chemotherapy dose was decreased, we considered extending it up to eight cycles depending on the occurrence of adverse effects such as hematopoietic recovery.
Clinical outcomes
We first evaluated the clinical efficacy of pegfilgrastim in the first cycle of chemotherapy. The occurrence of FN and initial dose of R-CHOP used in the first and second cycles were assessed. The initial dose was calculated as a ratio of the actual dose of cyclophosphamide and doxorubicin administered to each patient compared with the 100% dose of cyclophosphamide (750 mg/m2) and doxorubicin (50 mg/m2). When the ratio of the actual doses of cyclophosphamide and doxorubicin were different, the initial dose was calculated using the average of each ratio. FN was defined as axillary temperature of more than 37.5 °C and neutropenia by an ANC of < 500/μL.
To evaluate the long-term effects of pegfilgrastim used in the first cycle, we assessed the relative dose intensity (RDI), occurrence of FN, progression-free survival (PFS), overall survival (OS), and total medical costs during all cycles of chemotherapy.
Relative dose intensity was estimated based on the mean dose of cyclophosphamide and doxorubicin. It was calculated as follows: First, the ratio of the actual dose compared with the 100% dose of cyclophosphamide and doxorubicin during all cycles of R-CHOP was calculated. Second, the ratio from day 1 of the first cycle to day 1 of the last cycle of the planned duration of treatment was calculated, according to the following equation: actual duration of treatment days from day 1 of the first cycle to day 1 of the last cycle/21 × (total cycles − 1). Overall, the RDI equation was: (actual dose/full dose)/(actual duration of treatment/planned duration of treatment).
Sustained RDI was defined as greater than 80% RDI. PFS was defined as the number of days from the first day of cycle 1 to the date of lymphoma recurrence. OS was defined as the number of days from the first day of cycle 1 to the date of death. Medical costs were calculated from day 1 of the first cycle to day 21 of the last cycle. Medical costs were evaluated in the following two ways: including only the hematology department cost and including the costs from all departments.
Statistical analysis
We used Mann–Whitney non-parametric test to compare the following baseline characteristics between the groups: age; body mass index; and albumin, hemoglobin, and LDH levels. Fisher’s exact test was used to compare the following baseline characteristics between the groups: male sex, activities of daily living, advanced stage of DLBCL, diabetes, kidney failure, liver failure, administration of antibiotics for prophylaxis, bone marrow infiltration of lymphoma, CD5-positive lymphoma, transformation from follicular lymphoma, use of pegfilgrastim after the first cycle, and the year of diagnosis of lymphoma. To evaluate clinical outcomes, the RDI, total cycles, observation periods, and total medical costs were compared between the groups using Mann–Whitney non-parametric test, and sustained RDI and occurrence of FN were compared between the groups using Fisher’s exact test. For the PFS and OS analyses, the unadjusted log-rank test was used to compare the Kaplan–Meier survival curves between the groups. We used EZR software version 1.52 for the analyses, and results with a two-sided P value of less than 0.05 were considered statistically significant [22].
Results
Patient characteristics
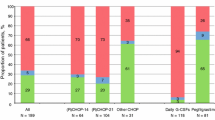
One hundred and fourteen patients who fulfilled the inclusion criteria were enrolled in the study. Among them, 26 patients were classified into the pegfilgrastim group, whereas 88 patients were classified into the neut-adjusted-G group. The neut-adjusted-G group was subclassified with 57 patients in the actual-G group and 31 patients in the no-G group. In the neut-adjusted-G group, 16 patients received pegfilgrastim in the second and subsequent outpatient cycles. Table 1 and Supplementary Table 1 show the clinical baseline characteristics of the patients in the groups. DLBCL was diagnosed significantly more recently in patients in the pegfilgrastim group than in the neut-adjusted-G group, the actual-G group, and the no-G group. The actual-G group had a tendency toward more severe lymphoma stage and kidney dysfunction than the no-G group. Moreover, the patients in the actual-G group presented significantly lower albumin and hemoglobin levels. The median administration date of pegfilgrastim was day 3 (range 2–6). The median initiation date of conventional G-CSF was day 13 (range 6–20) and conventional G-CSF was administered for a median of 2 (range 1–8) days.
Outcomes
The outcome of the first cycle for each group is shown in Table 2 and Supplementary Table 2. There was no significant difference in the initial dose intensity between the groups. Fewer patients in the pegfilgrastim group had their chemotherapy dose downgraded in the second cycle than those in the neut-adjusted-G group or the actual-G group (Table 2a, b), but not significantly. The incidence of FN was significantly lower in the pegfilgrastim group than in the neut-adjusted-G group (0% vs. 18%, p = 0.020) and lower than that in the actual-G group (0% vs. 23%, p = 0.007). There was no significant difference in the occurrence of FN between the pegfilgrastim group and the no-G group (0% vs. 10%, p = 0.242).
Of the 114 patients, 100 patients completed all cycles. The outcome of all cycles were shown in Table 3 and Supplementary Table 3. The median RDI was 64% in the pegfilgrastim group and 59% in the neut-adjusted-G group (p = 0.046). Among patients in the neut-adjusted-G group, 12 patients who received pegfilgrastim after the first cycle showed a median RDI of 48%. The pegfilgrastim group presented a significantly higher RDI than the actual-G group (64% vs. 58%, p = 0.039). The proportion of patients who maintained a sustained RDI was significantly higher in the pegfilgrastim group than in the neut-adjusted-G group (25% vs. 4%, p = 0.008), the actual-G group (25% vs. 4%, p = 0.019), and the no-G group (25% vs. 3%, p = 0.029). Although the difference was not significant, the incidence of FN in all cycles was lower in the pegfilgrastim group than in the neut-adjusted-G group and the actual-G group. However, there was no difference between the pegfilgrastim group and the no-G group in terms of the incidence of FN. There were no significant differences in medical costs among the pegfilgrastim group, the neut-adjusted-G group, and the actual-G group. The no-G group had a tendency toward lower costs than the pegfilgrastim group.
The PFS and OS analysis results of patients who completed all cycles are shown in Figs. 1 and 2, and Supplementary Figs. 1 and 2. Supplementary Figs. 3 and 4 present the results of the PFS and OS analyses including 14 patients who could not complete all cycles. Although there were no significant differences between the groups, the pegfilgrastim group presented a higher PFS and OS than the neut-adjusted-G group (Figs. 1a and 2a), the actual-G group (Figs. 1b and 2b), and the no-G group (Supplementary Figs.1 and 2). Even when patients who could not complete all cycles were included in the analyses, the pegfilgrastim group presented a higher PFS and OS than the other groups (Supplementary Figs. 3 and 4).
Discussion
The results of our study indicated that appropriate FN prophylaxis with pegfilgrastim in the first cycle contributed to an increased proportion of patients who maintained a sustained RDI in all cycles, which is important to achieve the preferred treatment response [3, 4]. Our study also showed that the appropriate prevention of FN during the first cycle using pegfilgrastim increased the PFS and OS, although not significantly. In terms of medical cost, the use of pegfilgrastim in the first cycle did not appear to be significantly more expensive than that of conventional G-CSF considering all cycles. However, when comparing the pegfilgrastim group with the no-G group, the no-G group showed a tendency toward lower cost. To determine the difference between patients who needed conventional G-CSF and those who did not, we compared the baseline characteristics of the actual-G group and the no-G group. As previously reported [23], we found that the actual-G group had a more severe lymphoma stage, kidney dysfunction, and lower albumin and hemoglobin levels. Although future studies to examine the underlying mechanisms were needed, these results suggest that patients with low albumin and hemoglobin levels, severe lymphoma stage, and kidney dysfunction are especially at a risk of severe neutropenia.
The high proportion of sustained RDI in all cycles in the pegfilgrastim group probably occurred because unplanned dose reduction seemed to have been avoided. Although there were no significant differences in the initial dose intensity between the groups, the proportion of dose reductions in the second cycle was lower in the pegfilgrastim group than in the neut-adjusted-G group. Effective FN prophylaxis with pegfilgrastim during the first cycle may contribute to the avoidance of dose reduction in the following cycles. However, when considering the 12 patients who used pegfilgrastim after the second cycle in the neut-adjusted-G group, the average RDI in this subgroup was lower than that in the neut-adjusted-G group. This is possibly because two patients developed FN during the first cycle and had dose reduction from the second cycle, and some patients had severe neutropenia during the first cycle, making it difficult to treat hematologists and for patients to increase the dose. Overall, these results suggest that using pegfilgrastim during the first cycle contributes to sustained RDI in all cycles in day-to-day practice.
We did not conclude that pegfilgrastim is superior to conventional G-CSF. Previous prospective studies have shown that there are no significant differences in the FN prevention effect between pegfilgrastim and conventional G-CSF in the treatment of malignant lymphoma [13, 24,25,26]. However, in day-to-day practice, the usage of conventional G-CSF varies. For example, in many hospitals, including our institute, conventional G-CSF is administered after a neutrophil decrease, whereas in these prospective studies, conventional G-CSF was initiated immediately after chemotherapy [12]. This difference may have resulted in the inferior effect of conventional G-CSF compared with pegfilgrastim in our study. In day-to-day practice, the use of pegfilgrastim may be advantageous owing to the fewer injections required.
There were some limitations to the study. First, conventional G-CSF was used differently in the current study compared with that in prospective studies and the recommendations of some guidelines [11, 27]. Thus, the long-term effects of conventional G-CSF could not be accurately evaluated in the current study. Second, patients in the pegfilgrastim group were diagnosed more recently than those in the other groups, possibly because, in recent years, physicians have become more aware of the importance of maintaining RDI for better survival [15]. This bias may skew the results. Third, the observation periods of the pegfilgrastim group were shorter than that of the other groups as pegfilgrastim has only started being used recently. Therefore, we need longer follow up of the pegfilgrastim group. Fourth, our institute does not routinely evaluate MYC-rearrangement and MUM-1 in patients with DLBCL and we could not accurately determine the number of patients of each subtype with a poor prognosis. Finally, this study was restricted by its retrospective nature; thus, a prospective study is warranted in the future.
In conclusion, an appropriate FN prophylaxis with pegfilgrastim in the first cycle contributed to the avoidance of an unnecessary reduction of RDI in all cycles. Especially, for patients with low albumin and hemoglobin levels, severe lymphoma stage, and kidney dysfunction, pegfilgrastim use for neutropenia prophylaxis may need to be considered.
References
Liu Y, Barta SK. Diffuse large B-cell lymphoma: 2019 update on diagnosis, risk stratification, and treatment. Am J Hematol. 2019;94(5):604–16.
Westin EH, Longo DL. Lymphoma and myeloma in older patients. Semin Oncol. 2004;31(2):198–205.
Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(4):235–42.
Lyman GH, Poniewierski MS, Culakova E. Risk of chemotherapy-induced neutropenic complications when treating patients with non-Hodgkin lymphomas. Expert Opin Drug Saf. 2016;15(4):483–92.
Yamaguchi H, Hirakawa T, Inokuchi K. Importance of relative dose intensity in chemotherapy for diffuse large B-cell lymphoma. J Clin Exp Hematopathol. 2011;51(1):1–5.
Yamamoto M, Suzuki I, Saitou K, Tsumanuma R, Okuyama S, Kumagai H, et al. Impact of comorbidity and relative dose intensity on outcomes in diffuse large B-cell lymphoma patients treated with R-CHOP. J Cancer Res Clin Oncol. 2020;146(11):2995–3002.
Wildiers H, Reiser M. Relative dose intensity of chemotherapy and its impact on outcomes in patients with early breast cancer on aggressive lymphoma. Crit Rev Oncol Hematol. 2011;77(3):221–40.
Holmes FA, Jones SE, O’Shaughnessy J, Vukelja S, George T, Savin M, et al. Comparable efficacy and safety profiles of once-per-cycle pegfilgrastim and daily injection filgrastim in chemotherapy-induced neutropenia: a multicenter dose-finding study in women with breast cancer. Ann Oncol. 2002;13(6):903–9.
Tai E, Guy GP, Dunbar A, Richardson LC. Cost of cancer-related neutropenia or fever hospitalizations, United States, 2012. J Oncol Pract. 2017;13(6):e552-561.
Mehta HM, Malandra M, Corey SJ. G-CSF and GM-CSF in Neutropenia. J Immunol. 2015;195(4):1341–9.
Crawford J, Becker PS, Armitage JO, Blayney DW, Chavez J, Curtin P, et al. Myeloid growth factors, Version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2017;15(12):1520–41.
Yokoyama M, Kusano Y, Nishihara A, Inoue N, Nishimura N, Mishima Y, et al. Incidence and risk factors for febrile neutropenia in Japanese patients with non-Hodgkin B cell lymphoma receiving R-CHOP: 2-year experience in a single center (STOP FN in NHL 2). Support Care Cancer. 2020;28(2):571–9.
Almenar D, Mayans J, Juan O, Bueno JM, Lopez JI, Frau A, et al. Pegfilgrastim and daily granulocyte colony-stimulating factor: patterns of use and neutropenia-related outcomes in cancer patients in Spain-results of the learn study. Eur J cancer Care. 2009;18(3):280–6.
Matsuda K, Taisuke J, Miyauchi M, Toyama K, Nakazaki K, Matsui H, et al. Primary prophylaxis with pegfilgrastim in patients with newly-diagnosed diffuse large B-cell lymphoma: propensity score and instrumental variable analyses. Leuk Lymphoma. 2020;61(10):2435–41.
Hirakawa T, Yamaguchi H, Yokose N, Gomi S, Inokuchi K, Dan K. Importance of maintaining the relative dose intensity of CHOP-like regimens combined with rituximab in patients with diffuse large B-cell lymphoma. Ann Hematol. 2010;89(9):897–904.
Lyman GH, Dale DC, Crawford J. Incidence and predictors of low dose-intensity in adjuvant breast cancer chemotherapy: a nationwide study of community practices. J Clin Oncol. 2003;21(24):4524–31.
Crawford J, Dale DC, Kuderer NM, Culakova E, Poniewierski MS, Wolff D, et al. Risk and timing of neutropenic events in adult cancer patients receiving chemotherapy: the results of a prospective nationwide study of oncology practice. J Natl Compr Canc Netw. 2008;6(2):109–18.
Vogel CL, Wojtukiewicz MZ, Carroll RR, Tjulandin SA, Barajas-Figueroa LJ, Wiens BL, et al. First and subsequent cycle use of pegfilgrastim prevents febrile neutropenia in patients with breast cancer: a multicenter, double-blind, placebo-controlled phase III study. J Clin Oncol. 2005;23(6):1178–84.
Culakova E, Thota R, Poniewierski MS, Kuderer NM, Wogu AF, Dale DC, et al. Patterns of chemotherapy-associated toxicity and supportive care in US oncology practice: a nationwide prospective cohort study. Cancer Med. 2014;3(2):434–44.
Chan A, Lee CP, Chiang J, Ng R. Breakthrough febrile neutropenia and associated complications among elderly cancer patients receiving myelosuppressive chemotherapy for solid tumors and lymphomas. Support Care Cancer. 2013;21(8):2137–43.
Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317(17):1098.
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452–8.
Lyman GH, Morrison VA, Dale DC, Crawford J, Delgado DJ, Fridman M, et al. Risk of febrile neutropenia among patients with intermediate-grade non-Hodgkin’s lymphoma receiving CHOP chemotherapy. Leuk Lymphoma. 2003;44(12):2069–76.
Vose JM, Crump M, Lazarus H, Emmanouilides C, Schenkein D, Moore J, et al. Randomized, multicenter, open-label study of pegfilgrastim compared with daily filgrastim after chemotherapy for lymphoma. J Clin Oncol. 2003;21(3):514–9.
Grigg A, Solal-Celigny P, Hoskin P, Taylor K, McMillan A, Forstpointner R, et al. Open-label, randomized study of pegfilgrastim vs daily filgrastim as an adjunct to chemotherapy in elderly patients with non-Hodgkin’s lymphoma. Leuk Lymphoma. 2003;44(9):1503–8.
Kubo K, Miyazaki Y, Murayama T, Shimazaki R, Usui N, Urabe A, et al. A randomized, double blind trial of pegfilgrastim versus filgrastim for the management of neutropenia during CHASE(R) chemotherapy for malignant lymphoma. Br J Haematol. 2016;174(4):563–70.
Smith TJ, Bohlke K, Lyman GH, Carson KR, Crawford J, Cross SJ, et al. Recommendations for the use of WBC growth factors: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2015;33(28):3199–212.
Acknowledgments
The authors would like to thank medical affairs division of the University of Tokyo hospital for calculating medical costs, Editage (www.editage.com) for English language editing.
Author information
Authors and Affiliations
Contributions
MI and KM designed the research. KM, AS, YM and MK advised the research design and analyses. MI collected the patient’s data and analyzed the data. MI and KM wrote the paper. All authors revised the manuscript and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Kensuke Matsuda received the lecture fee from Kyowa Kirin. Arika Shimura received the lecture fee from Eisai. Yosuke Masamoto received the lecture fee from Janssen Pharmaceutical, Otsuka Pharmaceutical, Nihon Pharmaceutical, Nippon Shinyaku, Eisai, Bristol-Myers Squibb, Ono Pharmaceutical, Celgene, and Kyowa Kirin. Mineo Kurokawa received the lecture fee from MSD, Astellas, AbbVie, Amgen, Sanwa Chemistry, Shire Japan, Daiichi Sankyo, Sumitomo Dainippon Pharma, Takeda Pharmaceutical, Chugai Pharmaceutical, Boehringer Ingelheim, Janssen Pharmaceutical, Otsuka Pharmaceutical, Nippon Shinyaku, Eisai, Bioverativ Japan, Ono Pharmaceutical, Celgene, and Kyowa Kirin. Mineo Kurokawa received the research funding from Pfizer, Otsuka Pharmaceutical, Chugai Pharmaceutical, Astellas, Kyowa Kirin, Takeda Pharmaceutical, MSD, Teijin, Eisai, Sumitomo Dainippon Pharma, Nippon Shinyaku, Daiichi Sankyo, Ono Pharmaceutical, and AbbVie. None of these is related to the current study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Ise, M., Matsuda, K., Shimura, A. et al. Primary prophylaxis with pegfilgrastim during the first cycle of R-CHOP to avoid reduction of dose intensity in elderly patients. Int J Hematol 113, 823–831 (2021). https://doi.org/10.1007/s12185-021-03118-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-021-03118-6