Abstract
Background
Although early detection and improved treatment have increased the number of long-term survivors, little is known about the prevalence and associations of clinically relevant fatigue (CRF) in disease-free stomach cancer survivors. Because no effective CRF management strategy yet exists, understanding CRF risk factors is important for developing treatment approaches.
Methods
Stomach cancer survivors (N = 374) completed a mailed survey that included the Brief Fatigue Inventory, Beck Depression Inventory, and the European Organization for Research and Treatment of Cancer QLQ-C30 questionnaire and its gastric module QLQ-STO22. We assessed sociodemographic, clinical, and symptom characteristics using multivariate logistic regression models to identify CRF-associated factors.
Results
Approximately half of disease-free stomach cancer survivors reported CRF, which was associated with female gender, low economic status, rural residence, current smoker, early tumor progress, current depression, and poor performance. Significant relationships of CRF with current depression and poor performance status remained robust after adjusting for potential confounders. Most functional and symptom scores of fatigued survivors deteriorated more than in non-fatigued survivors. Additionally, congruence between tumor progress and surgery type might influence CRF severity.
Conclusion
In disease-free stomach cancer survivors, CRF is a common problem that is strongly associated with quality of life and other symptoms. Current depression, poor performance, and perceived understanding regarding postoperative condition are important CRF risk factors. Thus, CRF management in this population should focus on identifying these factors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer treatment basically has a curative intent, and to date, most post-treatment studies have focused on disease-based outcomes such as treatment response or disease recurrence. However, as the number of long-term cancer survivors increases [1], increasing attention has been directed toward patient-based outcomes including symptom management and quality of life (QoL) [2]. Especially in modern surgical oncology, the importance of these outcomes following cancer resection cannot be overemphasized. Aside from the biological consequences of multimodality therapies, functional or psychological aspects of postoperative QoL deserve extensive investigation.
Fatigue is the most common symptom affecting cancer patients, defined as the subjective sensation of having reduced energy, loss of strength, or becoming easily tired. Clinically relevant fatigue (CRF) is a major factor causing significant decreases in cancer patients’ ability to perform usual activities and reduced QoL [3]. Nevertheless, CRF is often underreported because cancer patients regard it as inevitable [4] and physicians do not pay sufficient attention to it. The CRF prevalence ranges widely, depending on the clinical disease status and on the type of instrument used to measure fatigue [5].
Stomach cancer survivors may continue to suffer from various nutritional or functional problems, summarized as ‘postgastrectomy syndrome’, which likely greatly impacts fatigue from malnutrition and anemia. However, there is only limited evidence on CRF in long-term stomach cancer survivors, and even fewer data concerning fatigue in the context of QoL outcomes [6]. The existent studies reported only mean scores on QoL fatigue subscales, using tools that have not previously been validated against fatigue-specific instruments [7].
We are not aware of any published studies regarding fatigue prevalence in disease-free stomach cancer survivors, or the association of CRF with relevant variables such as depression and QoL. We therefore aimed to determine the prevalence and association of fatigue in disease-free stomach cancer survivors using a validated fatigue-specific instrument. Ultimately, identification of associated risk factors will facilitate the development of targeted interventions for vulnerable patients.
Methods
Study design and population
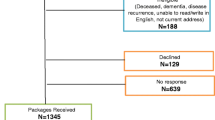
We identified patients from the stomach surgery database at the National Cancer Center and the Seoul Samsung Medical Center in Korea for this cross-sectional study, which was approved by the Institutional Review Boards of both centers. We chose patients treated at two representative hospitals, wherein the study participants resided in 15 different geographic districts across the country. Eligibility required a diagnosis of stages I–III stomach cancer during 2001 or 2002. Patients were excluded if they had a prior history of another cancer, could not speak Korean, or were less than 18 years of age. A total of 855 subjects who had been diagnosed with stages I–III stomach cancer were eligible for the study.
We telephoned eligible subjects to invite them to participate in the study. Each subject who agreed was mailed the questionnaires, a consent form, and a postage-paid return envelope. Any subject who did not return the questionnaire within 1 month received a reminder card and a phone call from a staff member, who further reiterated the purpose of the study and requested that attention be given to the questionnaire. Subjects were asked to sign the informed consent form and to return it with the completed questionnaires. Any subject who did not wish to participate was asked to provide a reason. When efforts were made to contact eligible subjects by telephone to seek their participation in the study, we discovered that 83 patients had died. In addition, 97 refused to participate because of time constraints, due to an inability to communicate either verbally or in writing (i.e., because no one was available to assist them), or because they felt that the study was an inconvenience or invasion of privacy. Each subject who agreed to participate was mailed the questionnaires, but 81 patients who had agreed to participate had changed mailing addresses. Of the 594 patients that received postal material, 165 did not return the questionnaires. Among the remaining subjects, 23 were excluded because they did not complete the questionnaires and 32 were eliminated because they were no longer disease-free. A total of 374 study subjects thus remained, and the recruitment procedures are summarized in Fig. 1. Mean follow-up period was 2.29 ± 0.29 years, and details of the study design have been published previously [8].
Measures
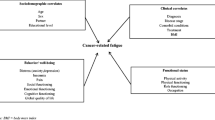
The medical record review provided data on age, gender, tumor characteristics (early vs. advanced, stage, and postoperative period), type of surgery (total gastrectomy vs. subtotal gastrectomy), and type of treatment received (surgery, chemotherapy, or radiation therapy). Patients completed a questionnaire that covered demographic and clinical characteristics as well as a number of standardized instruments designed to assess their fatigue, heath-related QoL, and depression. Demographic information included marital status, employment details, religion, monthly household income, education level, and residential area.
Each variable was categorized as follows: monthly income was categorized into “<2 million Korean Won (KW, KW 1,200 = US$1)” and “≥2 million KW;” education level was categorized into “middle school or lower” and “high school or higher;” residential area was categorized into “rural” and “urban” including metropolitan areas and cities; marital status was classified into “married” and “unmarried,” which included “single” and “divorced/separated/widowed;” religion was classified into “no religion” and “professing a religion” including non-Catholic Christians, Buddhist, Catholics, and others. Patients also provided clinical information on smoking, drinking habits, comorbid conditions, Eastern Cooperative Oncology Group (ECOG) performance status, and family history of cancer. The ECOG performance status is an observer-rated scale of patient physical ability using numbers ranging from 0 (able to carry out all normal activities) to 4 (completely disabled) [9]. As the subjects of this study were disease-free stomach cancer survivors, they were divided into two groups, those with scores of 0 and those with scores of 1 or above.
Instruments
Fatigue was measured using the Brief Fatigue Inventory (BFI). The BFI is a standardized, reliable instrument that rapidly assesses fatigue in cancer patients, and is significantly correlated with other validated fatigue questionnaires [10]. This is a nine-item self-rated scale that includes three items concerning present, usual, and worst fatigue severity and six items concerning how much the fatigue interferes with general activity, mood, walking ability, normal work, relations with other people, and enjoyment of life over the previous week. Each item is scored on an 11-point scale from 0 (‘no fatigue’/‘does not interfere’) to 10 (‘as bad as you can imagine’/‘completely interferes’). The global BFI score is the arithmetic mean of all nine items (range 0–10). The Korean version of the BFI (BFI-K) has been previously validated [11], and the Cronbach’s alpha coefficient for the BFI-K in this study was calculated as 0.96. Here, we defined clinically relevant fatigue as a global BFI score of ≥4 based on National Comprehensive Cancer Network recommendations and previous publications [12, 13].
The validated Korean version of the European Organization for Research and Treatment of Cancer QLQ-C30 [14] and its gastric module (STO22) were used to measure QoL. The EORTC QLQ-C30 is a 30-item cancer-specific questionnaire for assessing the general health-related QoL of cancer patients. The questionnaire incorporates five functioning domains (physical, role, cognitive, emotional, and social), three symptom scales (fatigue, nausea and vomiting, and pain), a global health status and an overall health-related QoL scale, and several single items that assess additional symptoms commonly reported by cancer patients (e.g., dyspnea, appetite loss, sleep disturbance, constipation, and diarrhea) along with the perceived financial impact of disease and treatment. Of the 30 items in QLQ-C30, 28 items are scored on 4-point Likert scales and the two items for the global QoL scale are scored on 7-point linear analog scales. The QLQ-STO22, which was designed to examine specific QoL of gastric patients, contains 22 questions in five symptom scales (dysphagia, pain, reflux symptoms, eating restrictions, and anxiety) and four single items (dry mouth, taste, body image, and hair loss) [15]. The symptom subscales in the EORTC QLQ-C30 were excluded in the analyses because of its overlap with EORTC QLQ-STO22 or BFI. We scored the QLQ-C30 and QLQ-STO22 items according to the EORTC scoring manual. All scales were linearly transformed to a 0 to 100 score, with 100 representing the best global health status or functional status or the worst symptom status, as appropriate. We handled incomplete questionnaires according to the developer’s recommendations. We defined a clinically meaningful difference in health-related QoL as a 10-point difference in the mean score [16].
We measured depression level using the Beck Depression Inventory (BDI). The BDI evaluates 21 symptoms of depression, exploring both cognitive-affective and somatic aspects of the condition. Each symptom is rated on a 4-point scale (0 through 3), and the scores are added to give a total score between 0 and 63. Higher scores represent more severe depressive condition. The Korean version of BDI was standardized [17], but the cutoff scores for the BDI have not been validated in Korean cancer patients. We used a total BDI score of greater than 13 as the cutoff point for depression, which is usual procedure in Korea [18].
Statistical methods
We used a χ 2 test to assess the association of sociodemographic and clinical characteristics with fatigue. Each independent factor that was statistically significant at the P ≤ 0.10 level in the univariate relationship was entered into the final multivariate logistic regression model to calculate adjusted odds ratios (aORs). Using a forward stepwise elimination procedure, we obtained a best-fit multivariate logistic regression model. In multivariate logistic analyses, we considered P values less than 0.05 generated in two-sided tests to indicate statistical significance. We also compared fatigued survivors with non-fatigued survivors on the basis of multivariate-adjusted health-related QoL means in each QoL subscale. Additionally, we classified the subjects into three groups according to the combination of tumor progress and operation type, and compared the adjusted global fatigue score among the groups using the analysis of covariance (ANCOVA) model. All statistical tests were performed using SAS version 9.2 software (SAS Institute, Inc., Cary, NC).
Results
Factors associated with CRF
Table 1 lists the factors significantly associated with CRF. The CRF prevalence was 51.3 %. Stomach cancer survivors who were female, low economic state, current smoker, and had rural residence were more likely to report CRF. Subjects with lower functional status and higher depressive mood were also at increased risk for CRF. Surprisingly, subjects who had been diagnosed with advanced cancer or received radiotherapy were less likely to have CRF. Table 2 shows the results of multivariate analysis of factors associated, by univariate analysis, with a predisposition to CRF. Those who were more depressed (aOR, 7.23) and had lower performance status (aOR, 5.55) were more fatigued than those who were not.
Expectation–outcome consistency and CRF
Based on the assumption that a discrepancy between pre-operative expectation (disease stage) and actual postoperative condition (surgery type) would impact patients’ fatigue, we classified patients into three groups: advanced stage patients with subtotal gastrectomy (n = 94), early stage patients with subtotal gastrectomy or advanced stage patients with total gastrectomy (n = 257), and early stage patients with total gastrectomy (n = 23). Using an ANCOVA model (P < 0.001) after adjusting for ECOG PS status and depression, patients receiving total gastrectomy despite early stage were more likely to be fatigued than those receiving subtotal gastrectomy despite advanced stage (Fig. 2, P = 0.029).
Expectation–outcome consistency and CRF. CRF clinically relevant fatigue, ECOG PS Eastern Cooperative Oncology Group Performance Status, AGC advanced gastric carcinoma, STG subtotal gastrectomy, EGC early gastric carcinoma, TG total gastrectomy. P value was calculated using an ANCOVA model, with adjustment for ECOG PS and depression
CRF and health-related QoL
Figure 3 depicts the relationships between CRF and health-related QoL. In global QoL, physical, emotional, and cognitive scales, non-fatigued subjects presented significantly better function than fatigued subjects (P < 0.05), especially prominent in global QoL, physical, and emotional function (P < 0.01). Additionally, many symptomatic scales such as dysphagia, pain, eating restriction, anxiety, dry mouth, and taste were significantly associated with CRF (P < 0.05). Among these, fatigued subjects had more symptoms, with more than 10-point differences in pain, eating restriction, anxiety, and dry mouth, than non-fatigued subjects.
Health-related quality of life in disease-free stomach cancer survivors according to CRF presence. CRF clinically relevant fatigue, ECOG PS Eastern Cooperative Oncology Group Performance Status, LS linear square, QoL quality of life, PF physical function, RF role function, EF emotional function, SF social function, CF cognitive function, STODYS dysphagia, STORFX reflux, STOEAT eating restriction, STOANX anxiety, STODM dry mouth, STOTA taste, STOBI body image, STOHAIR hair loss. Scores were presented as adjusted mean value for age group, sex, comorbid illness, economic status, residential area, smoking status, tumor progress, ECOG PS, and depression. Higher score means better function or more symptoms. aA ‘clinical meaningful difference’ by 10-point. *P < 0.05 and **P < 0.01
Discussion
Advancements in surgical techniques and increased surveillance are responsible for the current high prevalence of long-term stomach cancer survivors [19]. Gastrointestinal symptoms in conjunction with a gastrointestinal disease were generally associated with fatigue; particularly in stomach cancer survivors, these symptoms contribute to fatigue via malnutrition or anemia. In recent prospective studies with disease-free stomach cancer survivors, fatigue was the representative unrecovered symptom that persisted at 12 months after surgery [20, 21].
To our knowledge, this is the first study to focus on CRF in disease-free stomach cancer survivors. The main findings were that: (1) the prevalence of CRF in the sample was 51.3 % and patients with CRF had worse quality of life, poorer functioning, and more symptoms than those without fatigue; (2) CRF was associated with female gender, low economic status, current smoker, and rural residence, and the independent associations with CRF were current depression and performance state; and (3) congruence between tumor progress and operation type might influence the severity of CRF in disease-free stomach cancer survivors.
Fatigue is also a problem for survivors of other cancers. We are, however, only aware of two studies that specifically addressed fatigue in disease-free survivors using the same fatigue measures as our study. One study examined 1,933 breast cancer patients who were 4.6 years post-surgery [22], and the prevalence of moderate/severe fatigue (BFI single item ‘worst fatigue’ >3) was similar (66 % compared with 65.1 % in our female sample after applying the same criteria). The other study [23] demonstrated that CRF prevalence in recurrence-free prostate cancer survivors was lower than ours (45 % compared with 50.9 % in our male sample with the same criteria).
Performance status was highly relevant to CRF in this study. The relationship between fatigue and physical performance has rarely been investigated in the literature and then only during active treatment for cancer [24, 25] or did not consider disease status [26]; less is known about fatigue in long-term disease-free cancer survivors. Although subjective fatigue is often related to objectively impaired performance, the two phenomena are not synonymous. In some clinical situations, fatigue can exist independently of deterioration in physical performance [27]. Because patients affected by chronic loss of physical performance can gradually become accustomed to this impairment and finally experience it as normal [28], many cancer patients deny feeling fatigued despite severe limitations in physical performance. To assess the actual degree of physical impairment, patients have to be explicitly asked about the objective limitations they experience in daily activities.
Whether impaired performance in these patients is a consequence or the cause of CRF is difficult to determine; performance and fatigue may be reciprocally related. Impaired performance can result in limitations in daily life, increased dependence, decreased self-esteem, and reduced involvement in social activities. Furthermore, impaired performance can be interpreted by the patient as a sign of poor health, which thereby increases their psychological distress and sense of exhaustion [29]. On the other hand, fatigued patients are more likely to reduce their outdoor activity and revert to a passive lifestyle, and this can result in muscular deconditioning and loss of physical performance [30]. Clearly, the association between impaired performance and increased fatigue in cancer patients can have therapeutic consequences. Improving physical performance through a physical training program may reduce fatigue prevalence and severity in cancer survivors.
Similar to other studies [31, 32], one of the strongest risk factors for CRF was current depression; these findings highlight the importance of carefully screening for depression in disease-free stomach cancer survivors who complain of fatigue. Depression and fatigue is a common symptom cluster in cancer patients, and depression is reportedly associated with fatigue [31]. However, fatigue and depression are not equivalent because only 62.5 % of CRF patients were depressed according to our criteria (BDI > 13). These results should be interpreted with caution because our criteria made the definition of depression less stringent; other authors have used case definition thresholds for depression as high as 13 [33].
Essentially all patients who undergo gastric cancer surgery suffer from various gastrointestinal symptoms and malfunction. Recent studies have explored this issue scientifically and quantitatively [34–36]. QoL encompasses the negative aspects of the disease and treatment, including fatigue. Fatigue impacts many aspects of patient QoL [37]. Zieren et al. [38] investigated the QoL in gastric cancer patients after curative resection and found that fatigue had the strongest impact on overall QoL. As expected, non-fatigued survivors were more likely to report better QoL than fatigued survivors in terms of impaired role, emotional, and social function. Non-fatigued survivors also reported a clinically meaningful reduction in deterioration symptoms such as eating restriction, anxiety, dry mouth, and body image versus CRF patients [16]. Health care providers need to assess these symptoms and functional impairment with reliable tools, to assure their identification even long after completion of treatment for stomach cancer. Furthermore, simultaneous supportive care should be provided to minimize these problems.
Literature on fatigue in disease-free stomach cancer survivors is limited [8, 35, 39], and data are largely based on results obtained using the EORTC QLQ-C30 questionnaire. Additionally, many researchers have paid more attention to the operation type, which impacts the QoL [21]. Although there is no clear relationship between fatigue and preceding illness or treatment modalities [40], it is still a logical proposition that fatigue can be predicted by these parameters [41]. Generally, patients who had a total gastrectomy are less likely to have a normal diet, and are more likely to lose weight, lose appetite, and experience severe nausea and vomiting than patients who had a subtotal gastrectomy [35]. In a recent large cohort study, Kim et al. [20] reported that the fatigue score 12 months after surgery was significantly lower in the subtotal gastrectomy group than in the total gastrectomy group, although this difference decreased over time. Additionally, another study with a median follow-up period of 17 months indicated that stomach cancer survivors, once cured, generally enjoy similar QoL levels, including fatigue symptoms, regardless of their original disease stage or surgical procedure type [35]. In the current study, CRF was not related to the surgical technique, and early stage cancer survivors complained of significantly more fatigue than advanced stage cancer survivors.
We found a clue to this unexpected result from the fact that, unlike other cancers, the stomach cancer surgery type, which could impact fatigue, is primarily determined by the lesion locations, regardless of the preoperative tumor staging. Namely, patients undergoing subtotal gastrectomy were not necessarily more likely to be diagnosed with early stage tumors, and therefore were less likely to receive chemotherapy or radiotherapy than those undergoing total gastrectomy. Early-stage patients might have an inappropriately optimistic view of their postoperative condition. In understanding preoperative status, patients would be more susceptible to cancer staging (early vs. advanced) than to operation type (total vs. subtotal). Therefore, we hypothesized that insufficient informed consent increases the possibility of dysfunctional cognition concerning fatigue [29]. Fatigue may be caused by discrepancy between patient pre-operative expectation and their actual postoperative condition, which will influence their coping with fatigue. In this study, patients who had total gastrectomy despite early staging were significantly more likely to be fatigued than those who had subtotal gastrectomy despite advanced staging. Our results suggest that surgeons should fully inform patients about their state and potential postoperative sequelae before surgery, which will help to fill in this gap.
Our study had several limitations. First, as with all cross-sectional studies, we can only offer a ‘snap shot’ view of fatigue and its causative factors. Prospective studies are required to reliably determine whether newly identified relevant variables influence subsequent fatigue [42]. Second, the cutoff value for the BFI global score has not been validated in Korean cancer patients. We acknowledge that other cut-off scores could have been chosen. There currently is no universally agreed upon CRF definition in this population, so we used a severity cut-off level that is justified by clinical guidelines and has previously been used by other researchers. Third, we did not assess psychosocial factors such as anxiety that might strongly impact fatigue [43]. In addition, we did not consider other potential conditions relating to fatigue such as anemia or thyroid dysfunction [5]. Fourth, we could not differentiate CRF from chronic fatigue syndrome [44], which has been described as an initial symptom that can cause a patient to seek medical attention prior to their cancer diagnosis. It might be important to assess fatigue among cancer patients in relation to the background fatigue level within the population, because fatigue is a common complaint that may vary by nationality. Finally, asking patients about their other medical conditions relied heavily on the patients’ interpretation of the questions, accurate recall, and detailed understanding of their medical history.
Despite these limitations, we believe that our study facilitates better understanding of fatigue causes in disease-free stomach cancer survivors. We found that CRF affected approximately half of patients and had a strong association with QoL and symptoms. It also appears that depression, performance status, and a difference between preoperative expectations and postoperative experience may be very important determinants of postoperative CRF. Managing fatigue in disease-free stomach cancer survivors should therefore focus on identifying these associated factors, which can improve therapeutic interventions.
References
Jemal A, Siegel R, Ward E et al (2006) Cancer statistics, 2006. CA Cancer J Clin 56:106–130
Yabroff KR, Lawrence WF, Clauser S, Davis WW, Brown ML (2004) Burden of illness in cancer survivors: findings from a population-based national sample. J Natl Cancer Inst 96:1322–1330
Curt GA, Breitbart W, Cella D et al (2000) Impact of cancer-related fatigue on the lives of patients: new findings from the Fatigue Coalition. Oncologist 5:353–360
Stone P, Richardson A, Ream E, Smith AG, Kerr DJ, Kearney N (2000) Cancer-related fatigue: inevitable, unimportant and untreatable? Results of a multi-centre patient survey. Cancer Fatigue Forum. Ann Oncol 11:971–975
Berger AM, Abernethy AP, Atkinson A et al (2010) Cancer-related fatigue. J Natl Compr Cancer Netw 8:904–931
Kaptein AA, Morita S, Sakamoto J (2005) Quality of life in gastric cancer. World J Gastroenterol 11:3189–3196
Knobel H, Loge JH, Brenne E, Fayers P, Hjermstad MJ, Kaasa S (2003) The validity of EORTC QLQ-C30 fatigue scale in advanced cancer patients and cancer survivors. Palliat Med 17:664–672
Bae JM, Kim S, Kim YW et al (2006) Health-related quality of life among disease-free stomach cancer survivors in Korea. Qual Life Res 15:1587–1596
Oken MM, Creech RH, Tormey DC et al (1982) Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5:649–655
Mendoza TR, Wang XS, Cleeland CS et al (1999) The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer 85:1186–1196
Yun YH, Wang XS, Lee JS et al (2005) Validation study of the korean version of the brief fatigue inventory. J Pain Symptom Manag 29:165–172
NCCN (2013. http://www.nccn.org/professionals/physician_gls/pdf/fatigue.pdf (accessed 28 Dec 2012)) Clinical Practice Guidelines in Oncology: Cancer-Related Fatigue v. 1
Shafqat A, Einhorn LH, Hanna N et al (2005) Screening studies for fatigue and laboratory correlates in cancer patients undergoing treatment. Ann Oncol 16:1545–1550
Yun YH, Park YS, Lee ES et al (2004) Validation of the Korean version of the EORTC QLQ-C30. Qual Life Res 13:863–868
Vickery CW, Blazeby JM, Conroy T et al (2001) Development of an EORTC disease-specific quality of life module for use in patients with gastric cancer. Eur J Cancer 37:966–971
Osoba D, Rodrigues G, Myles J, Zee B, Pater J (1998) Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol 16:139–144
Hahn HM, Yum TH, Shin YW, Kim KH, Yoon DJ, Chung KJ (1986) A standardization study of Beck Depression Inventory in Korea. Korean J Neuropsychiatry 25:487–502
Rhee YS, Yun YH, Park S et al (2008) Depression in family caregivers of cancer patients: the feeling of burden as a predictor of depression. J Clin Oncol 26:5890–5895
Roukos DH (1999) Current advances and changes in treatment strategy may improve survival and quality of life in patients with potentially curable gastric cancer. Ann Surg Oncol 6:46–56
Kim AR, Cho J, Hsu YJ et al (2012) Changes of quality of life in gastric cancer patients after curative resection: a longitudinal cohort study in Korea. Ann Surg 256:1008–1013
Kobayashi D, Kodera Y, Fujiwara M, Koike M, Nakayama G, Nakao A (2011) Assessment of quality of life after gastrectomy using EORTC QLQ-C30 and STO22. World J Surg 35:357–364
Kim SH, Son BH, Hwang SY et al (2008) Fatigue and depression in disease-free breast cancer survivors: prevalence, correlates, and association with quality of life. J Pain Symptom Manag 35:644–655
Storey DJ, McLaren DB, Atkinson MA et al (2012) Clinically relevant fatigue in recurrence-free prostate cancer survivors. Ann Oncol 23:65–72
Cheng KK, Lee DT (2011) Effects of pain, fatigue, insomnia, and mood disturbance on functional status and quality of life of elderly patients with cancer. Crit Rev Oncol Hematol 78:127–137
Dimeo F, Stieglitz RD, Novelli-Fischer U, Fetscher S, Mertelsmann R, Keul J (1997) Correlation between physical performance and fatigue in cancer patients. Ann Oncol 8:1251–1255
Ferreira KA, Kimura M, Teixeira MJ et al (2008) Impact of cancer-related symptom synergisms on health-related quality of life and performance status. J Pain Symptom Manag 35:604–616
Sprangers MA, Van Dam FS, Broersen J et al (1999) Revealing response shift in longitudinal research on fatigue—the use of the thentest approach. Acta Oncol 38:709–718
Breetvelt IS, Van Dam FS (1991) Underreporting by cancer patients: the case of response-shift. Soc Sci Med 32:981–987
Servaes P, Verhagen S, Bleijenberg G (2002) Determinants of chronic fatigue in disease-free breast cancer patients: a cross-sectional study. Ann Oncol 13:589–598
Neil SE, Klika RJ, Garland SJ, McKenzie DC, Campbell KL (2013) Cardiorespiratory and neuromuscular deconditioning in fatigued and non-fatigued breast cancer survivors. Support Care Cancer 21:873–881
Reuter K, Harter M (2004) The concepts of fatigue and depression in cancer. Eur J Cancer Care (Engl) 13:127–134
Jacobsen PB, Donovan KA, Weitzner MA (2003) Distinguishing fatigue and depression in patients with cancer. Semin Clin Neuropsychiatry 8:229–240
Chang MY, Rogers SN, Lowe D et al (2012) The Korean version of the University of Washington Quality of Life Questionnaire for Patients with Head and Neck Cancer, and its use in an initial validation study of 56 patients. Int J Oral Maxillofac Surg 41:1201–1205
Avery K, Hughes R, McNair A, Alderson D, Barham P, Blazeby J (2010) Health-related quality of life and survival in the 2 years after surgery for gastric cancer. Eur J Surg Oncol 36:148–154
Huang CC, Lien HH, Wang PC, Yang JC, Cheng CY, Huang CS (2007) Quality of life in disease-free gastric adenocarcinoma survivors: impacts of clinical stages and reconstructive surgical procedures. Dig Surg 24:59–65
Wu CW, Chiou JM, Ko FS et al (2008) Quality of life after curative gastrectomy for gastric cancer in a randomised controlled trial. Br J Cancer 98:54–59
Pud D, Ben Ami S, Cooper BA et al (2008) The symptom experience of oncology outpatients has a different impact on quality-of-life outcomes. J Pain Symptom Manag 35:162–170
Zieren HU, Zippel K, Zieren J, Muller JM (1998) Quality of life after surgical treatment of gastric carcinoma. Eur J Surg 164:119–125
Tyrvainen T, Sand J, Sintonen H, Nordback I (2008) Quality of life in the long-term survivors after total gastrectomy for gastric carcinoma. J Surg Oncol 97:121–124
Prue G, Rankin J, Allen J, Gracey J, Cramp F (2006) Cancer-related fatigue: a critical appraisal. Eur J Cancer 42:846–863
Lawrence DP, Kupelnick B, Miller K, Devine D, Lau J (2004) Evidence report on the occurrence, assessment, and treatment of fatigue in cancer patients. J Natl Cancer Inst Monogr:40–50
Goedendorp MM, Gielissen MF, Verhagen CA, Bleijenberg G (2012) Development of fatigue in cancer survivors: a prospective follow-up study from diagnosis into the year after treatment. J Pain Symptom Manag
Stone P, Richards M, A’Hern R, Hardy J (2000) A study to investigate the prevalence, severity and correlates of fatigue among patients with cancer in comparison with a control group of volunteers without cancer. Ann Oncol 11:561–567
Giacalone A, Spina M, Berretta M, Tirelli U (2012) Two types of fatigue in cancer patients. Br J Cancer 106:424, author reply 425
Conflict of interest
We have no conflicts of interest concerning this article. We also have full control of all primary data and agree to allow the journal to review our data if requested.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hwang, I.C., Yun, Y.H., Kim, YW. et al. Factors related to clinically relevant fatigue in disease-free stomach cancer survivors and expectation–outcome consistency. Support Care Cancer 22, 1453–1460 (2014). https://doi.org/10.1007/s00520-013-2110-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-013-2110-2