Abstract
Introduction
Previous studies by our group and others have demonstrated the importance of sociodemographic factors in cancer-related outcomes. The identification of these factors has led to novel approaches to the care of the high-risk cancer patient, specifically in the adoption of clinical interventions that convey similar benefits as favorable sociodemographic characteristics. This study examined the importance of marital status and race as prognostic indicators in men with prostate cancer.
Methods
This report is a meta-analysis of 3,570 patients with prostate cancer treated in three prospective RTOG clinical trials. The Kaplan–Meier method was used to estimate the survival rate and the cumulative incidence method was used to analyze biochemical failure rate. Hazard ratios were calculated for all covariates using either the Cox or Fine and Gray’s proportional hazards model or logistic regression model with associated 95% confidence intervals and p values.
Results
Hazard ratio (HR) for overall survival (OS) for single status compared to married status was 1.36 (95% CI, 1.2 to 1.53). OS HR for non-White compared to White patients was 1.05 (CI 0.92 to 1.21). In contrast, the disease-free survival (DFS) HR and biochemical failure (BF) HR were both not significantly different neither between single and married patients nor between White patients and non-White patients. Median time to death for married men was 5.68 years and for single men was 4.73 years. Median time for DFS for married men was 7.25 years and for single men was 6.56 years. Median time for BF for married men was 7.81 years and for single men was 7.05 years.
Conclusions
Race was not associated with statistically significant differences in this analysis. Congruent with our previous work in other cancer sites, marital status predicted improved prostate cancer outcomes including overall survival.
Implications for cancer survivors
Prostate cancer is the most common visceral cancer in men in the USA. The stratification of prostate cancer risk is currently modeled solely on pathologic prognostic factors including PSA and Gleason Score. Independent of these pathologic prognostic factors, our paper describes the central sociodemographic factor of being single as a negative prognostic indicator. Single men are at high risk of poorer outcomes after prostate cancer treatment. Intriguingly, in our group of patients, race was not a significant prognostic factor. The findings in this paper add to the body of work that describes important sociodemographic prognostic factors that are currently underappreciated in patients with cancer. Future steps will include the validation of these findings in prospective studies, and the incorporation of clinical strategies that identify and compensate for sociodemographic factors that predict for poorer cancer outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer is the most common visceral cancer in men in the USA, with an estimated 217,730 new cases and 32,050 deaths in 2010 [1]. Previous publications have described the importance of race and socioeconomic status in outcomes of patients with prostate [2, 3] and colorectal [4–6] cancers. Marital status has also been described as a prognostic factor in overall survival in cancer, as well as in other diseases [7–10]. Our group has previously demonstrated, in an analysis of RTOG clinical trials, that single men with head and neck cancer are at a disadvantage for survival [11]. The clinical and public health implications of such prognostic socioeconomic variables are distinct from those of pathologic factors that have been traditionally associated with cancer-related outcomes [12, 13]. The recognition of these factors is increasingly acknowledged as important in cancer care, and they potentially allow for tailored interventions that improve patient outcomes for specific populations at risk.
In this report, we examine the effect of race and marital status on outcomes in men treated with radiation therapy for prostate cancer. We hypothesize that the interaction among the key sociodemographic factors of marital status and race will assist in the identification of critical predictors for poor outcomes for prostate cancer patients.
Methods
Study and patient inclusion criteria
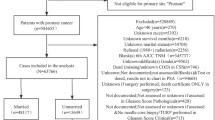
This report is a meta-analysis of patients with prostate cancer treated in three prospective RTOG clinical trials, RTOG 9202, 9406, and 9413 (described in detail below). Sociodemographic data was obtained at the time of enrollment into the trail. The eligibility criteria for entry into each trial differed. None of these trials used race or marital status as a stratification variable before patient randomization. Marital status was defined as being married or living with a partner. These studies accrued a total of 3,734 patients from 1992 to 2000. There were 164 (4%) patients with missing or unknown pretreatment information who were excluded from the analyses; one patient was of unknown race, 71 patients had unknown marital status, and 92 patients had unknown Gleason Score. The resulting analysis therefore included a total of 3,570 patients. Four percent of all eligible patients have missing data and therefore can be excluded from this analysis without missing data bias since this is less than or equal to 5% of the data (complete case analysis).
RTOG 9202
RTOG 9202 was a phase III study examining long-term total androgen suppression following neoadjuvant hormonal cytoreduction and radiation therapy in locally advanced prostate carcinoma. Between June 1992 and April 1995, this study accrued 1,468 patients who were randomized to two arms. Arm 1 was neoadjuvant total androgen suppression starting 2 months prior and then concomitant with radiation therapy. Arm 2 was neoadjuvant total androgen suppression starting 2 months prior to and during radiation therapy, followed by Zoladex for 2 years following radiation therapy.
RTOG 9406
RTOG 9406 was a phase I/II study examining the toxicity of escalating doses of three-dimensional (3D) conformal radiation therapy for treatment of localized prostate carcinoma. Between May 1994 and October 2000, 1,030 patients were enrolled in this study. These patients were stratified based on clinical T-stage, PSA, and Gleason Score, then were treated to one of five dose levels: 68.4 Gy in 1.8 Gy fractions, 73.8 Gy in 1.8 Gy fractions, 79.2 Gy in 1.8 Gy fractions, 74 Gy in 2 Gy fractions, and 78 Gy in 2 Gy fractions.
RTOG 9413
RTOG 9413 was a phase III study comparing whole pelvic irradiation followed by a conedown to irradiation of the prostate only, and comparing neoadjuvant to adjuvant total androgen suppression in patients with localized disease with an elevated prostate-specific antigen (PSA) less than or equal to 100 ng/ml. Between April 1995 and June 1999, this study enrolled 1,236 patients who were randomized to four arms. Arm 1 was neoadjuvant total androgen suppression 2 months prior to and concomitant with radiation therapy to the whole pelvis followed by conedown to the prostate. Arm 2 was neoadjuvant total androgen suppression 2 months prior to and concomitant with radiation therapy to the prostate only. Arm 3 was radiation therapy to the whole pelvis followed by a conedown to the prostate, and then 4 months of adjuvant total androgen suppression. Arm 4 was radiation therapy to the prostate only, followed by 4 months of total androgen suppression.
Statistical methods
An event affecting overall survival (OS) was defined as death due to any cause, and time to death was measured from date of randomization to date of death or the last clinical follow-up. Biochemical failure (BF) was defined as a >2 ng/ml rise in PSA above the PSA nadir, after the end of radiation therapy, or after the end of salvage therapy. An event affecting disease-free survival (DFS) was defined as one of the following: death due to any cause, local failure, biochemical failure (as defined above), distant metastases, or second primary.
The Kaplan–Meier method [14] was used to estimate the survival rate for OS and DFS, and the cumulative incidence method was used for failure rate for BF. To analyze whether each covariate was independently associated with outcomes while adjusting for other covariates, the Cox proportional hazards regression models [9] were used for OS and DFS, and Fine and Gray’s regression models were used for BF. Unadjusted and adjusted hazard ratios (HR) were calculated for all covariates using either the Cox or Fine and Gray’s proportional hazards model or a logistic regression model with associated 95% confidence intervals (CI) and p values. All statistical tests were two-sided and a p value <0.05 was considered to be statistically significant. Statistical Analysis System (SAS Institute, Cary, NC, USA) and R software was used for all statistical analyses.
Results
A total of 3,570 patients with complete covariate information from three RTOG prostate cancer trials were examined in this meta-analysis. Of these, 1,358 patients were enrolled on RTOG 9202, 999 patients were enrolled on RTOG 9406, and 1,213 patients were enrolled on RTOG 9413. At the time of analysis, 1,381 (39%) of these patients had died (OS), 2,547 (71%) had experienced disease recurrence or died (DFS), and 1,537 (43%) had experienced biochemical failure (BF). The median follow-up was 7.22 years for all patients and 7.95 years for the surviving patients. To account for changes in accepted treatment for prostate cancer over time, all analyzable patients were stratified for accrual years: 1992–1994, 1995–1997, and 1998–2000 (Table 1). The major covariates of race and marital status were considered in all outcomes. Other covariates were considered for each outcome in addition to the race and marital status. As indicated in Table 1, these covariates were age, clinical stage, Karnofsky Performance Score (KPS), Gleason Score, Prostate Specific Antigen (PSA), Biologic Effective Dose (BED), and type of treatment received. The median age was 70 years old (range 41–88) with 53% of the patients ≤70 years old and 47% of the patients >70 years old. Two thousand seven hundred and forty-one (77%) of the patients were White and 829 (23%) of the patients were non-White. Of the non-White patients, 78% were Black, 15% were Hispanic, 3% were Asian, 2% were Native American, and 2% were Other. Of the patients, 76% were married and 24% were single. The performance status in this group was generally excellent, with 94% of the patients with KPS 90–100. There was a notable difference between patients stratified across accrual years in that, in later accrual years, more people were in the PSA <10 category and had a lower T and N staging, as well as being treated to a higher BED of radiation.
Heterogeneity testing was next performed in order to determine if a pooled estimate could be used to represent the combined data from the trials in this meta-analysis. This demonstrated that the stratification groups were homogeneous in respect to their adjusted hazard ratios, and the pooled adjusted hazard ratio was noted for each outcome. We therefore proceeded to use pooled hazard ratios for our analysis.
In our patient population, there was a statistically significant overall survival benefit in married men with prostate cancer, with the HR for single status compared to married status being 1.36 (95% CI, 1.2 to 1.53). There was no significant overall survival difference between White patients and non-White patients, with a HR for non-White compared to White patients of 1.05 (CI 0.92 to 1.21). The DFS HR and BF HR were both not significantly different between single and married patients, HR 1.05 (95% CI 0.96 to 1.16) and 0.92 (95% CI 0.82 to 1.05), respectively. Similarly, the DFS HR and BF HR were also not significantly different between White patients and non-White patients, 0.92 (95% CI 0.83 to 1.01) and 0.87 (95% CI 0.77 to 0.99), respectively.
Subgroup analysis of marital status and race was performed (Table 2). Five-year survival and event estimates were calculated for OS, DFS, and BF, in single White, single non-White, married White, and married non-White patients (Table 3). For OS, single White and single non-White patients are at significantly higher risk of death from any cause than married White patients, HR 1.35 (95% CI 1.17 to 1.56) and HR 1.44 (95% CI 1.18 to 1.75). For DFS, single White patients are at significantly higher risk of recurrence than married White patients, with HR 1.17 (95% CI 1.05 to 1.30). All groups are at equal risk for biochemical failure at 5 years.
Further pairwise comparison of subgroups of marital status and race was performed. When compared to single White patients, single non-White patients had a decreased risk of DFS and BF, with DFS HR 0.77 (95% CI 0.64 to 0.92) and BF HR 0.75 (95% CI 0.59 to 0.96). However, despite these trends, there was no statistically significant difference in OS between the two groups. Married non-White and White patients, when compared to single White patients, had improved OS with HR 0.71 (95% CI 0.58 to 0.88) and 0.74 (95% CI 0.64 to 0.86), respectively, as well as improved DFS with HR 0.85 (95% CI 0.73 to 0.99) and 0.87 (95% CI 0.78 to 097), respectively; however, no statistically significant difference was observed. Married non-White patients had no statistically significant difference in OS, DFS, and BF when compared to married White patients. Single non-White patients had worse OS, with OS HR 1.44 (95% CI 1.18 to 1.78), when compared to married White patients. This is despite no significant difference in DFS HR and an apparently decreased risk of BF with a HR of 0.76 (95% CI 0.62 to 0.94). Finally, married non-White patients had improved OS when compared to single non-White patients, with an OS HR of 0.69 (95% CI 0.54 to 0.88), again with no statistical difference.
Because there have been previous reports of poorer disease-free survival outcomes in Black patients compared to White, Hispanic, and Asian patients [15], we performed additional multivariate analysis focusing on race. We performed multivariate analysis on all other variables in the previously performed analysis, and in addition, examined subsets of race as White, Black, Hispanic, and Other. Adjusted by these variables, statistical significance of OS still favors married patients compared to single patients. The pooled OS HR (95%CI) was 1.35 (1.12, 1.53) for all original variables and race subsets as White, Black, Hispanic, and Other. Both DFS and BF HRs did not show significant difference, with DFS pooled HR (95%) of 1.07 (0.98, 1.18) and BF pooled HR (95%) of 0.91 (0.81, 1.04) for all original variables and race subsets as White, Black, Hispanic, and Other.
Figures 1, 2, and 3 demonstrate Kaplan–Meier analysis of OS, DFS, and BF for married compared to single patients. Median time to death (OS) for married men was 5.68 years and for single men was 4.73 years. Median time for DFS for married men was 7.25 years and for single men was 6.56 years. Median time for BF for married men was 7.81 years and for single men was 7.05 years. The median age of death for married men was 72 years old, and for single men was 70 years old.
In order to more carefully examine if there was an association between established prognostic factors and marital status, the variables of PSA, Gleason Score, KPS, and T-stage were compared between married and single patients (Table 3). While Gleason Score, KPS, and T-stage were not significantly different between married and single patients, there was a significant difference in PSA levels between married and single patients. Of note, 45% of single patients and 40% of married patients presented with PSA >20 and 28% of single patients and 33% of married patients presented with PSA <10. This may be related to single men lacking the social support to seek early detection and treatment of prostate cancer. Further, single men were still at higher risk for worse survival when this initial PSA difference was taken into account.
Discussion
While biologic and pathologic factors have traditionally been associated with cancer-related outcomes, sociodemographic factors are increasingly recognized as important in the clinical outcomes of cancer patients. The value in identification of specific sociodemographic factors is that they are potentially amenable to novel approaches of clinical intervention. It is hoped that, by identifying specific characteristics of populations at risk, we will be able to develop strategies that ameliorate these variables.
While there are substantial, although inconsistent, published data on the association between the sociodemographic status of race and prostate cancer outcomes, there are less clear associations among marital status and prostate cancer outcomes. However, there has been extensive analysis of the importance of marital status as a predictive factor of mortality in the general health literature. This effect has been particularly demonstrated for men, in whom marital status is associated with a mortality benefit. Interestingly, this benefit seems not to be seen in women who are married [16]. The duration of marital status is strongly associated with survival, and the interaction between marital status and mortality is complex and appears to be associated with changes in socioeconomic resources, risk behaviors, and social support, all of which contribute to overall better physical and psychological health [17–19]. In life-threatening events such as an acute cardiac event, marital status was associated with transport to a medical facility [20], and this suggests that married patients are more likely to seek help and utilize social and medical support in life-threatening situations. Finally, there is limited data that in cancers such as cervical cancers [21] and testicular cancers [22], marital status has been independently associated with improved cancer outcomes. Because of the importance of general health and of support systems in cancer care, we hypothesized that marital status may also be an important prognostic factor for prostate cancer outcomes. In combining published data, we further hypothesized that there would be an interaction in prostate cancer outcomes between marital status and race.
The analysis of prostate cancer patients presented here demonstrates a significantly increased risk of mortality in single men compared to married men; however, race was not associated with statistically significant differences in this analysis. Despite an extensive literature on the topic, it remains unclear whether race is associated with poorer prostate cancer outcomes. Black race has been reported to be an adverse prognostic factor for prostate cancer outcomes following radical prostatectomy [23] and following either prostatectomy or radiotherapy [24]. However, Peters et al. [25] had surveyed prostate cancer literature from 1992 to 2002 and reported inconsistent findings, with 79% of the studies demonstrating no differences in outcomes between Black and White patients, with the remainder reporting worse prostate cancer related outcomes. Another meta-analysis of prostate cancer articles [26], published from 1968 to 2007, failed to demonstrate prostate-cancer-specific survival differences between Black and White patients. It has been previously demonstrated in a Surveillance Epidemiology and End Results (SEER) data analysis that there are racial differences in disease-free survival for prostate cancer patients, with Black patients experiencing shorter disease-free survival compared to White, Hispanic, or Asian patients [15]. One possibility that we failed to demonstrate a racial disparity in our analysis may be that by grouping multiple races into a broad non-White category, that differences between Black, White, Hispanic, and Asian races may obscure potential effects. We therefore performed additional multivariate analysis examining subsets of race as White, Black, Hispanic, and Other. Adjusted by all these variables, OS HR still favors married patients compared to single patients, regardless of race, and did not change our findings that race was not a statistically significant factor in OS, DFS, or BF.
It is critical to note that previous analysis of race and prostate cancer outcomes in RTOG trials from 1975 to 1992 [27] has demonstrated that Black men with prostate cancer had a lower OS and DFS; however, that when adjusted for prostate cancer risk group and treatment type, that race was no longer associated with poorer outcomes—leading to the conclusion that differences in outcomes was due to higher tumor burden at diagnosis in Black patients. Indeed, in an equal access health care setting, such as a Veterans Affairs [28] or similar military medical system [29], outcomes are similar between Blacks and Whites. Our analysis includes trials from more recent RTOG trials, primarily during the PSA screening era, where differences in tumor burden at diagnosis may be less pronounced than previously noted due to earlier detection by routine PSA screening. This may ameliorate disparities at time of diagnosis that had been present historically. However, it is important to note that in a SEER/Medicare data analysis from 1994 to 2002, there are racial disparities in PSA screening intervals [30] where Blacks, compared to Whites, are more likely to have a longer PSA screening interval prior to prostate cancer diagnosis and a greater likelihood of no pre-cancer diagnosis PSA.
Another possibility why our study did not demonstrate a racial disparity may be that cancer patients in our RTOG clinical trials do not represent the general cancer patient population. However, a sociodemographic analysis of patients in RTOG trials was performed, comparing RTOG patient sociodemographic characteristics to those of U.S. Department of Census data as well as SEER data [31]. This analysis demonstrated that Black patients are proportionally well represented in RTOG trials. In addition, the RTOG enrolled proportionally more Black men to all cancer trials combined, including prostate cancer trials. This previous analysis in fact indicates that our patient population is certainly representative of the Black patient population when compared to U.S. Census and SEER data.
A third possibility why we did not demonstrate a racial disparity is that, in comparison to SEER or Census data, RTOG patients derive a benefit from enrolling in a clinical trial that outweighs the inherent sociodemographic factors of race. Certainly, cancer clinical trial participation provides secondary benefits to patients including cancer treatment per a standardized clinical protocol, medical care that is provided by medical centers that actively enroll in cancer clinical trials, and regular and mandated follow-up care.
In contrast to our findings with race and prostate cancer, we did demonstrate that marital status, being married or living with a partner, predicted for improved prostate cancer outcomes. This survival benefit does not correlate with a biochemical failure benefit or disease-free survival benefit, which suggests that the mortality benefit associated with marital status is independent of cancer disease progression in these prostate cancer patients. This benefit predominates despite the limitation of examining marital status as a simple binomial of married or single, instead of a more complex variable. Sprehn et al. performed a SEER data analysis that examined subsets of unmarried cancer patients at the time of cancer diagnosis [32], including never married, divorced, widowed, or separated. Patients who were separated at the time of diagnosis had worse cancer survival than other unmarried patients. The authors suggest that the acute stress of separation could contribute to these poorer outcomes. It should therefore follow that a more sophisticated prospective analysis, with appropriate psychosocial assessments of stress and quality of life, may lead to a clearer understanding of the factors that convey this survival benefit.
Indeed, there has been increasing interest in exploring the biologic mechanisms of psychosocial prognostic factors and stress in cancer-related outcomes. Stress conditions including social isolation, depression, and decreased coping have been associated with biologic changes, and recent reports have preliminarily examined this through various approaches. Chou et al. [33] have reported that a specific APOE epsilon 2 genotype actually decreased vulnerability to symptoms of depression in response to increased stressors. McClintok’s group [34] has demonstrated, in animal models, the intriguing finding of poorer cancer outcomes induced by social stress. This group examined a rat breast cancer cell model and demonstrated that rats that were socially isolated had a dramatic increase in the number and size of their tumors. This isolation was associated with increases in the production of the stress hormone corticosterone as well as increased expression of metabolic and inflammatory genes. While preclinical and preliminary, these findings certainly suggest a course for future studies that will examine biologic responses to social stress and their correlation to cancer outcomes. Attention should subsequently be directed at clinical interventions that target the complex interactions between biology and socioeconomic factors that result in poorer cancer-related outcomes. Indeed, small proof of principle studies have reported on the efficacy of simple psychosocial interventions on cancer outcomes. One such study from Shrock et al. [35] examined the effect of a course of formal psychology classes on early stage breast and prostate cancer patient outcomes. Their small cohort of 50 patients demonstrated a survival benefit, at a median follow-up time of 4.2 years, when compared to a matched cohort of patients who had been treated at the same hospitals but did not undergo the psychological intervention.
In summary, the analysis presented here identifies a subgroup of prostate cancer patients, defined by the sociodemographic factor of being single, that are at higher risk of mortality. This finding adds to the understanding of how sociodemographic factors, and their complex associations with economic, psychological, social, and biologic determinants, affect the outcome of cancer care. In addition, this presents an opportunity to positively impact cancer patients’ outcomes utilizing strategies that are separate, complementary, and synergistic to conventional cancer treatment strategies that rely solely on modifying traditional markers of cancer outcomes. It may be that the quality of life, performance status, and overall health of married men, prior to diagnosis of cancer, provide a more favorable foundation for tolerating and compensating for the detrimental physical and psychosocial effects of cancer and cancer treatment. Whether strategies could be developed that would allow single patients to obtain the benefits of being married remains to be determined; however, simple targeted clinical interventions are certainly feasible and should be implemented in prospective analyses that examine the clinical and biologic implications of such therapies.
References
Jemal A, Siegel R, Xu J, Ward E (2010) Cancer statistics, 2010. CA Cancer J Clin 60(5):277–300
Godley PA, Schenck AP, Amamoo MA, Schoenbach VJ, Peacock S, Manning M et al (2003) Racial differences in mortality among Medicare recipients after treatment for localized prostate cancer. J Natl Cancer Inst 95(22):1702–1710
Harvei S, Kravdal O (1997) The importance of marital and socioeconomic status in incidence and survival of prostate cancer. An analysis of complete Norwegian birth cohorts. Prev Med 26(5 Pt 1):623–632
Wrigley H, Roderick P, George S, Smith J, Mullee M, Goddard J (2003) Inequalities in survival from colorectal cancer: a comparison of the impact of deprivation, treatment, and host factors on observed and cause specific survival. J Epidemiol Commun Health 57(4):301–309
Wudel LJ Jr, Chapman WC, Shyr Y, Davidson M, Jeyakumar A, Rogers SO Jr et al (2002) Disparate outcomes in patients with colorectal cancer: effect of race on long-term survival. Arch Surg 137(5):550–554, discussion 4–6
Johansen C, Schou G, Soll-Johanning H, Mellemgaard A, Lynge E (1996) Influence of marital status on survival from colon and rectal cancer in Denmark. Br J Cancer 74(6):985–988
Lund R, Due P, Modvig J, Holstein BE, Damsgaard MT, Andersen PK (2002) Cohabitation and marital status as predictors of mortality—an eight year follow-up study. Soc Sci Med 55(4):673–679
Vercelli M, Lillini R, Capocaccia R, Micheli A, Coebergh JW, Quinn M et al (2006) Cancer survival in the elderly: effects of socio-economic factors and health care system features (ELDCARE project). Eur J Cancer 42(2):234–242
Cox D (1972) Regression models and life tables. J Roy Stat Soc 34:187–220
Coyne JC, Rohrbaugh MJ, Shoham V, Sonnega JS, Nicklas JM, Cranford JA (2001) Prognostic importance of marital quality for survival of congestive heart failure. Am J Cardiol 88(5):526–529
Konski AA, Pajak TF, Movsas B, Coyne J, Harris J, Gwede C et al (2006) Disadvantage of men living alone participating in Radiation Therapy Oncology Group head and neck trials. J Clin Oncol 24(25):4177–4183
Yates BC (1995) The relationships among social support and short- and long-term recovery outcomes in men with coronary heart disease. Res Nurs Health 18(3):193–203
Trief PM, Wade MJ, Britton KD, Weinstock RS (2002) A prospective analysis of marital relationship factors and quality of life in diabetes. Diabetes Care 25(7):1154–1158
Kaplan E, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457–481
Cohen JH, Schoenbach VJ, Kaufman JS, Talcott JA, Schenck AP, Peacock S et al (2006) Racial differences in clinical progression among Medicare recipients after treatment for localized prostate cancer (United States). Cancer Causes Control 17(6):803–811, Research Support, N.I.H., Extramural Research Support, U.S. Gov't, Non-P.H.S
Scafato E, Galluzzo L, Gandin C, Ghirini S, Baldereschi M, Capurso A et al (2008) Marital and cohabitation status as predictors of mortality: a 10-year follow-up of an Italian elderly cohort. Soc Sci Med 67(9):1456–1464
Dupre ME, Beck AN, Meadows SO (2009) Marital trajectories and mortality among US adults. Am J Epidemiol 170:546–555
Ebrahim S, Wannamethee G, McCallum A, Walker M, Shaper AG (1995) Marital status, change in marital status, and mortality in middle-aged British men. Am J Epidemiol 142(8):834–842
Ikeda A, Iso H, Toyoshima H, Fujino Y, Mizoue T, Yoshimura T et al (2007) Marital status and mortality among Japanese men and women: the Japan Collaborative Cohort Study. BMC Public Health 7:73
Barnett E, Reader S, Ward BG, Casper ML (2006) Social and demographic predictors of no transport prior to premature cardiac death: United States 1999–2000. BMC Cardiovasc Disord 6:45
Patel MK, Patel DA, Lu M, Elshaikh MA, Munkarah A, Movsas B (2010) Impact of marital status on survival among women with invasive cervical cancer: analysis of population-based surveillance, epidemiology, and end results data. J Low Genit Tract Dis 14(4):329–338, Research Support, N.I.H., Extramural
Abern MR, Dude AM, Coogan CL (2010) Marital status independently predicts testis cancer survival—an analysis of the SEER database. Urol Oncol [Epub ahead of print]
Moul JW, Douglas TH, McCarthy WF, McLeod DG (1996) Black race is an adverse prognostic factor for prostate cancer recurrence following radical prostatectomy in an equal access health care setting. J Urol 155(5):1667–1673, Clinical Trial Research Support, U.S. Gov't, Non-P.H.S
Sohayda CJ, Kupelian PA, Altsman KA, Klein EA (1999) Race as an independent predictor of outcome after treatment for localized prostate cancer. J Urol 162(4):1331–1336
Peters N, Armstrong K (2005) Racial differences in prostate cancer treatment outcomes: a systematic review. Cancer Nurs 28(2):108–118, Review
Sridhar G, Masho SW, Adera T, Ramakrishnan V, Roberts JD (2010) Do African American men have lower survival from prostate cancer compared with White men? A meta-analysis. Am J Mens Health 4(3):189–206 [Meta-Analysis Review]
Roach M 3rd, Lu J, Pilepich MV, Asbell SO, Mohiuddin M, Grignon D (2003) Race and survival of men treated for prostate cancer on radiation therapy oncology group phase III randomized trials. J Urol 169(1):245–250 [Research Support, U.S. Gov't, P.H.S.]
Fowler JE Jr, Terrell F (1996) Survival in Blacks and Whites after treatment for localized prostate cancer. J Urol 156(1):133–136 [Comparative Study]
Optenberg SA, Thompson IM, Friedrichs P, Wojcik B, Stein CR, Kramer B (1995) Race, treatment, and long-term survival from prostate cancer in an equal-access medical care delivery system. JAMA 274(20):1599–1605
Carpenter WR, Howard DL, Taylor YJ, Ross LE, Wobker SE, Godley PA (2010) Racial differences in PSA screening interval and stage at diagnosis. Cancer Causes Control 21(7):1071–1080 [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S.]
Chamberlain RM, Winter KA, Vijayakumar S, Porter AT, Roach M 3rd, Streeter O et al (1998) Sociodemographic analysis of patients in radiation therapy oncology group clinical trials. Int J Radiat Oncol Biol Phys 40(1):9–15 [Comparative Study Research Support, U.S. Gov't, P.H.S.]
Sprehn GC, Chambers JE, Saykin AJ, Konski A, Johnstone PA (2009) Decreased cancer survival in individuals separated at time of diagnosis: critical period for cancer pathophysiology? Cancer 115(21):5108–5116 [Research Support, N.I.H., Extramural]
Chou KL (2010) Moderating effect of apolipoprotein genotype on loneliness leading to depressive symptoms in Chinese older adults. Am J Geriatr Psychiatry 18(4):313–322
Hermes GL, Delgado B, Tretiakova M, Cavigelli SA, Krausz T, Conzen SD et al (2009) Social isolation dysregulates endocrine and behavioral stress while increasing malignant burden of spontaneous mammary tumors. Proc Natl Acad Sci USA 106(52):22393–22398
Shrock D, Palmer RF, Taylor B (1999) Effects of a psychosocial intervention on survival among patients with stage I breast and prostate cancer: a matched case–control study. Altern Ther Health Med 5(3):49–55
Conflicts of interest statement
The authors indicated no potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Charlene Bryan is deceased.
Supported by RTOG U10 CA21661 and CCOP U10 CA37422 grants from the NCI, as well as Pennsylvania Commonwealth Universal Research Enhancement (CURE) Program ME-02-149. This paper's contents are the sole responsibility of the authors and do not necessarily represent the official views of the NCI.
Rights and permissions
About this article
Cite this article
Du, K.L., Bae, K., Movsas, B. et al. Impact of marital status and race on outcomes of patients enrolled in Radiation Therapy Oncology Group prostate cancer trials. Support Care Cancer 20, 1317–1325 (2012). https://doi.org/10.1007/s00520-011-1219-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-011-1219-4