Abstract
Background
Few studies have explored demographic variations in symptom patterns. Our goals were to examine age and gender differences in symptom intensity and symptom clusters among outpatients with advanced cancer.
Methods
Symptom scores by the Edmonton Symptom Assessment System (ESAS) were collected for patients attending the Oncology Palliative Care Clinics at Princess Margaret Hospital from 2005 to 2007. Symptom intensity was compared between individuals aged ≤60 and >60 years and between males and females. Principal component analysis (PCA) was performed to determine inter-relationships of the nine ESAS symptoms and to compare symptom clusters within age and gender subgroups.
Results
From a total of 1,358 patients, 49.8% were male and 50.2% were female. The median age was 64 (range 19 to 99): 39.6% were ≤60 and 60.4% were >60. The most common primary cancer sites were gastrointestinal (27%), lung (15%), and breast (11%). Younger patients reported worse pain (4.9 vs. 4.5, p = 0.02) and better appetite (4.7 vs. 5.3, p = 0.002) than older patients. Females reported poorer scores than males for nausea (2.6 vs. 2.2, p = 0.02). Analyses of symptom clusters revealed that fatigue and drowsiness were included in the cluster of pain, nausea, and appetite in younger but not older patients. In men, pain clustered together with depression and anxiety; for women, physical and psychological symptoms formed separate clusters.
Conclusions
In patients with advanced cancers, symptom patterns differ according to age and gender. Palliative interventions tailored for symptoms that are more prominent in specific patient subgroups may offer greater therapeutic benefit.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients with metastatic cancer experience substantial psychological and physical distress [1–3], which may intensify near the end of life [4]. Because the goals of palliative care are to minimize symptoms and improve quality of life, studies have been conducted to better characterize the end-of-life symptoms experienced by patients with advanced cancer [2]. Although advances in symptom control have occurred, there is increasing recognition that not all patients experience symptoms similarly and that responses to therapies can vary between different patients. Few studies have specifically examined demographic variations in symptom patterns among advanced cancer patients [5–8], and none have examined the impact of such variations on symptom clusters. However, this knowledge can be valuable for tailoring care to the needs of this vulnerable population. Importantly, this information can also be used to help design more effective clinical trials of cancer symptom palliation.
We previously reported on symptom clusters in a large cohort of advanced cancer outpatients who were attending oncology palliative care clinics at a tertiary cancer center [9]. Symptoms were measured with the Edmonton Symptom Assessment System (ESAS) [10], and symptom clusters were defined as two or more inter-related symptoms that frequently occur together, which may or may not suggest a common etiology [11–13]. The study of symptom clusters can be especially pertinent for patients with advanced cancer because they frequently manifest with multiple, concurrent symptoms that are difficult to control. An understanding of symptom clusters may help to streamline treatments to target several symptoms concurrently and thus result in greater therapeutic benefit. In our prior work, we identified two distinct symptom clusters in advanced cancer outpatients, namely a psychological cluster (anxiety and depression) and a physical cluster (fatigue, drowsiness, nausea, decreased appetite, and shortness of breath), and found that symptom clusters were influenced by the site of the primary cancer [9].
The main objective of the current analysis was to explore the impact of demographic characteristics, specifically age and gender, on symptom clusters and symptom intensity in advanced cancer outpatients. Awareness of age- and gender-related symptom variations may help to develop and provide better symptom-directed interventions for specific subgroups of palliative cancer outpatients.
Methods
Study procedures
This study was conducted at Princess Margaret Hospital (PMH), a large tertiary cancer center in Toronto, Canada. The center has a multi-disciplinary psychosocial oncology team and a palliative care program, which consists of a 12-bed acute inpatient unit, a consultation service that assesses patients with urgent palliative care needs, and daily outpatient oncology clinics that provide pain management, symptom control, and end-of-life care planning [14]. All patients referred to and evaluated in one of the outpatient clinics are asked to complete the ESAS questionnaire as part of each clinic assessment. Full approval for this study was obtained from the University Health Network Research Ethics Board.
The ESAS is a standardized instrument for evaluating the severity of nine physical and psychological symptoms: pain, fatigue, nausea, anxiety, depression, drowsiness, appetite, general wellbeing, and shortness of breath [10]. Each of the nine items is rated by respondents on a scale of 0–10, where a 0 means that the symptom is completely absent and a 10 indicates that the symptom is at its worst possible intensity. This scale has been validated for patients with cancer [15]. Results of ESAS assessments for patients who were seen in the outpatient oncology palliative care clinics were prospectively entered into the PMH palliative care clinical database. Chart audits for a regional palliative care improvement project have recently shown an ESAS completion rate of above 90% in our palliative care clinics [16].
Analytical and statistical considerations
The study sample consisted of patients aged 18 years or older with metastatic cancer who were seen in the oncology palliative care clinics at PMH between January 1, 2005 and December 31, 2007 and completed at least one ESAS questionnaire. Analyses were limited to the initial ESAS assessment for all patients. Information on patient demographics, disease characteristics, and ESAS scores was extracted from the PMH palliative care clinical database for eligible patients, and then summarized with descriptive statistics. Overall severity for each of the nine symptoms was evaluated with mean scores.
We calculated the ESAS total symptom distress score (TSDS) by adding the individual scores from each of the nine symptoms [10]. The ESAS was also categorized into a physical sub-score (PHS) and a psychological sub-score (PSS). Scores from the physical domain (pain, fatigue, nausea, drowsiness, appetite, shortness of breath) and the psychological domain (anxiety, depression) were summated to generate PHS and PSS, respectively. Because ‘general wellbeing’ is a measure of both physical and psychological function, this item was excluded from the sub-scores. For patients who did not respond to all of the ESAS items, scores were prorated if at least 50% of the items were completed (five items for TSDS, three items for PHS, and one item for PSS), as suggested for quality-of-life scales [17, 18]. Prorated scores were determined by summing the individual scores of each available item, multiplying by the number of possible items (nine items for TSDS, six items for PHS, and two items for PSS), and dividing by the total number of completed items [17, 18].
To detect symptom clusters, a principal component analysis (PCA) with varimax rotation was conducted on the nine ESAS symptoms. PCA is an exploratory statistical technique that can be applied to a set of variables to determine which variables, if any, correlate with each other to form a distinct and stable pattern, which is called a “component” in the PCA model [19, 20]. A variable that consistently associates with another results in a higher factor loading score in the PCA and predicts its assignment into an independent component or “cluster”. To be considered a true symptom cluster, a symptom within a cluster must have a factor loading score >0.60 and the cluster must account for ≥10% of the total variance, as proposed in prior literature [19, 20]. The internal consistency and reliability of the derived symptom clusters were assessed with the Cronbach's alpha coefficient, where a higher value indicates better consistency.
To study the effect of age, analyses were performed whereby patients were dichotomized into ≤60 (“younger” cohort) and >60 years old (“older” cohort) based on cut-offs used previously in studies of cancer and chronic pain [21–23]. Prior to these analyses, the relationship between age (as a continuous variable) and ESAS score was investigated for each symptom using linear regression. There was no indication of any biphasic or other nonlinear relationship, thus supporting our decision to dichotomize our age analysis. ESAS intensity scores were compared between age and gender subgroups using t tests with and without Bonferroni correction. Symptom clusters were compared by performing PCA on the entire study cohort and then separately within age and gender subgroups. We also evaluated the clinical significance of the findings, defined based on prior symptom and quality-of-life analyses as a minimum difference of 5% between patient-reported outcome measures [24–26]. All statistical analyses were performed with SAS® (V9.1; SAS Institute, Cary, NC).
Results
A total of 1,358 outpatients with metastatic cancer were identified. Our analyses were conducted on the initial ESAS assessments from 1,358 outpatients with metastatic cancer who were assessed in the palliative care clinic. Among them, 739 patients had only the baseline assessment and 619 had further assessments (the median number of total assessments per patient was three; range two to 18). To minimize the impact of palliative interventions on symptom intensity and symptom clusters, analyses for this study were conducted using only the baseline assessment of each patient. The median age was 64 years (range 19–99); 538 (39.6%) patients were ≤60 years of age and 820 (60.4%) patients were >60 years old. For gender, 676 (49.8%) patients were male and 682 (50.2%) were female. Cancers originated most frequently from the gastrointestinal (GI) tract (27%), lung (15%), and breast (11%). The category “other cancers” consisted mainly of unknown primary tumors, sarcomas, and concurrent cancers. Table 1 outlines the distribution of primary cancers by age and gender subgroups. The two most prevalent cancers were breast and GI in the younger cohort and among women, whereas lung and GI cancers were more common in the older cohort and among men.
Table 2 describes the differences in mean symptom intensity scores by age and gender. Across all ages and gender subgroups, fatigue was the only symptom for which mean severity was greater than six out of ten. Younger patients reported more pain, whereas older patients reported worse appetite; the results for appetite remained statistically significant after Bonferroni correction and were also clinically significant. In our analysis on the effect of gender, women reported poorer scores than men only for nausea. Distress from physical and psychological symptoms, as measured by the PHS and PSS, was not significantly different between age groups or between genders. TSDS, which reflected overall symptom burden, was also similar among all of the demographic subgroups.
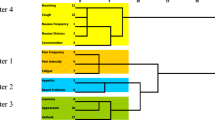
Analyses of symptom clusters stratified by age and gender are illustrated in Tables 3 and 4, respectively. For patients ≤60 and >60 years old, pain, nausea, and decreased appetite were components of the primary symptom cluster, although fatigue and drowsiness were also included in this cluster for those who were ≤60. Depression and anxiety were reflected in the secondary cluster for both age groups, but poor general wellbeing was also present in this cluster for the younger cohort. In men, the main symptom cluster consisted of pain, depression and anxiety; a secondary cluster consisted of fatigue, decreased appetite, and dyspnea. For women, pain, fatigue, nausea, drowsiness and decreased appetite grouped together in the main symptom cluster; depression, anxiety and poor general wellbeing formed a second cluster.
Discussion
The symptom experiences of advanced cancer patients are diverse, and the severity of symptoms can vary widely. Furthermore, symptom burden in this population is high [1–3], often requiring various palliative interventions to control symptoms adequately [27]. Thus, these patients are at high risk for potential drug interactions [28] and a poor quality of life [29, 30]. In order to address advanced cancer symptoms effectively, there is growing interest in understanding the extent to which symptoms differ between patients and in identifying factors that influence symptoms at the end of life. To date, few studies have explored the relationship of demographic characteristics to symptom intensity for advanced cancers specifically [5–8], and none have examined their impact on symptom clusters. In this analysis of outpatients with metastatic cancer, we examined the effects of age and gender on advanced cancer symptoms. Our findings suggest that symptom intensity is modified by both age and gender, whereas symptom clusters are influenced primarily by gender alone.
Although we found few clinically or statistically significant differences in symptom severity according to age or gender, those that we did observe were consistent with previous studies. The relationship between age and cancer symptom intensity has been previously documented [6, 31, 32]. Prior studies have demonstrated that older cancer patients experience worse overall physical function, including increased fatigue [31, 32]. This past research, however, involved either early stage cancers [31] or individuals who recently had surgery [32]. Therefore, fatigue described in these studies may be attributed to side effects of curative therapies or longer post-treatment recovery among older patients. Jordhoy et al. reported a lesser impact of sociodemographic characteristics on symptoms in patients with advanced cancer than had been previously described in general populations [6]. However, they did find that increased age was associated with better overall emotional function, less pain, and fewer sleep disturbances. Our analysis is consistent with their findings in that patients who were ≤60 years old reported worse symptom scores for anxiety and pain than those >60 years old; however, only pain was statistically significant. The only symptom in our study for which there was a clinically significant difference was appetite, which was worse in older patients. The relationship between advanced age and worse appetite has not been previously reported for advanced cancers. Reasons for this association are unclear, and may be due to normal, age-related physiologic changes, increased burden of comorbidities, or side effects of polypharmacy in the older age group [33]. This important area warrants further evaluation, especially in the context of its relationship with fatigue, which was also worse for patients >60 years of age.
Women in our study tended to report worse nausea and anxiety than men, supporting previous studies in the general [34, 35] and advanced cancer populations [6, 8]. The majority of this prior research reported worse quality of life for women than for men in both physical and psychological symptom domains. Specifically, studies of cancer patients have described either no gender differences for anxiety [36] or a higher prevalence in women [8, 37–39]. Similarly, an increased prevalence of nausea and vomiting has been reported in women [6, 7]. Reasons for this are uncertain, but palliative chemotherapy regimens used for breast and gynecological cancers, which frequently consist of cyclophosphamide and platinum agents, are highly emetogenic [40, 41]. Furthermore, past studies have suggested that women may have an increased predisposition for chemotherapy-related nausea and vomiting compared to men [42–44]. Similarly, the gender difference in anxiety is likely due to a combination of factors, including varied responses to palliative interventions [8, 45] as well as different coping mechanisms and social support networks between genders [46].
The most distinguishing feature of our study is its focus on symptom clusters. Recent studies have described the prevalence of symptom clusters in various cancer settings [47–53], but none have explored the effect of age and gender on these clusters. Our finding that age and gender had a different impact on symptom intensity than they had on symptom clusters reinforces the fact that symptom clusters do not necessarily depend on symptom intensity. Moreover, the traditional focus of symptom-based research on symptom severity may omit the identification of relevant clusters that are equally important in the treatment decision making process. Our findings suggest that cluster-based endpoints should also be considered in future trials of symptom control.
In patients ≤60 years old, the clusters included additional physical symptoms (namely fatigue, drowsiness, and poor wellbeing) that were absent from those >60 years old. It is possible that younger patients are accustomed to maintaining a higher level of function and activity, and thus the impact of advanced cancer may affect more domains of their physical function. The cluster of depression and anxiety that was noted in both age groups was also seen across many tumor sites in our previous analysis [9], and has been noted in other prior studies. Because the ESAS contains only two psychological symptoms, however, a more thorough understanding of this cluster would require studies that incorporate the use of instruments that measure anxiety, depression, and other psychological symptoms in greater detail.
The observation that pain clustered with anxiety and depression in men only whereas for women, physical and psychological symptoms formed separate clusters is noteworthy. Taken together, these findings suggest that pain may contribute more to anxiety and depression or that pain may be exacerbated to a greater extent by anxiety and depression among men. Conversely for women, pain primarily clustered with other physical symptoms such as fatigue, nausea, drowsiness, and loss of appetite. This observation may be due to an association of pain with these symptoms among women or a result of side effects from commonly used pain medications such as opioids. The latter is consistent with prior research showing a greater susceptibility of women than men to opioid-related toxicities [54, 55]. In contrast, fatigue, loss of appetite, and shortness of breath clustered together in men. The reason for this predisposition among men is less clear, but this particular group of symptoms has been correlated with advancing illness [4], postulating that symptom differences between genders may be more prominent at the end of life.
There are several limitations. While our sample size was large, the study was conducted in a single institution, and therefore results may not be generalizable. Also, we were unable to include outpatients who did not complete the ESAS. Although the completion rate for the ESAS in our clinic was high, excluding patients who were too ill to complete the symptom assessments may have resulted in selection bias. Furthermore, the inclusion of gender-specific cancers in our analyses and the higher proportions of lung, gastrointestinal and genitourinary cancers in the older cohort raises the possibility that the observed differences, which we have primarily attributed to gender and age, respectively, may also reflect differences in cancer sites. The symptom patterns observed in the current analysis by age and gender, however, differed from those seen in our previous analysis based on cancer site [9], thus providing some support for the presence of age- and gender-related symptom variations. Nonetheless, it is important to interpret these differences cautiously because symptom severity in this study was evaluated based on ESAS intensity scores alone. Differences in symptom reporting may not necessarily reflect true differences in symptom experiences [56], and the ESAS emphasizes only nine symptoms. Therefore, future research should focus on different approaches to evaluating symptoms across age and gender, and use of other validated symptom assessment measures.
In conclusion, our study showed an influence of age and gender on symptom severity, though this was not as marked as in other studies. There were distinct differences in symptom clusters according to gender and lesser differences according to age. Symptom severity and symptom clusters represent distinct constructs that can be analyzed separately. To our knowledge, this is the first report to explore demographic characteristics on symptom clusters, and our findings support a need for further research in this emerging area. Future trials that consider specific patient subgroups in their designs might be more effective when evaluating novel palliative care interventions in cancer.
References
Doorenbos AZ, Given CW, Given B, Verbitsky N (2006) Symptom experience in the last year of life among individuals with cancer. J Pain Symptom Manage 32:403–412
Teunissen SC, Wesker W, Kruitwagen C, de Haes HC, Voest EE, de Graeff A (2007) Symptom prevalence in patients with incurable cancer: a systematic review. J Pain Symptom Manage 34:94–104
McCarthy EP, Phillips RS, Zhong Z, Drews RE, Lynn J (2000) Dying with cancer: patients’ function, symptoms, and care preferences as death approaches. J Am Geriatr Soc 48(5 Suppl):S110–S121
Cheung WY, Barmala N, Zarinehbaf S, Rodin G, Le LW, Zimmermann C (2009) The association of physical and psychological symptom burden with time to death among palliative cancer outpatients. J Pain Symptom Manage 37:297–304
Husain AF, Stewart K, Arsenault R, Moineddin R, Cellarius V, Librach SL et al (2007) Women experience higher levels of fatigue than men at the end of life: a longitudinal home palliative care study. J Pain Symptom Manage 33:389–397
Jordhoy MS, Fayers P, Loge JH, Saltnes T, Ahlner-Elmqvist M, Kaasa S (2001) Quality of life in advanced cancer patients: the impact of sociodemographic and medical characteristics. Br J Cancer 85:1478–1485
Walsh D, Donnelly S, Rybicki L (2000) The symptoms of advanced cancer: relationship to age, gender, and performance status in 1,000 patients. Support Care Cancer 8:175–179
Zimmermann C, Burman D, Follwell M, Wakimoto K, Seccareccia D, Bryson J, Le L, Rodin G. Predictors of symptom severity and response in patients with metastatic cancer. Am J Hosp Palliat Med. 2010 Oct 16. [Epub ahead of print] in press
Cheung WY, Le LW, Zimmermann C (2009) Symptom clusters in patients with advanced cancers. Support Care Cancer 17(9):1223–1230
Bruera E, Kuehn N, Miller MJ, Selmser P, Macmillan K (1991) The Edmonton symptom assessment system (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care 7:6–9
Fan G, Filipczak L, Chow E (2007) Symptom clusters in cancer patients: a review of the literature. Curr Oncol 14:173–179
Kim HJ, McGuire DB, Tulman L, Barsevick AM (2005) Symptom clusters: concept analysis and clinical implications for cancer nursing. Cancer Nurs 28:270–284
Dodd MJ, Miaskowski C, Paul SM (2001) Symptom clusters and their effect on the functional status of patients with cancer. Oncol Nurs Forum 28:465–470
Zimmermann C, Seccareccia D, Clarke A, Warr D, Rodin G (2006) Bringing palliative care to a Canadian cancer center: the palliative care program at Princess Margaret Hospital. Support Care Cancer 14:982–987
Chang VT, Hwang SS, Feuerman M (2000) Validation of the Edmonton symptom assessment scale. Cancer 88:2164–2171
Dudgeon D, Vaitonis V, Seow H et al (2007) Ontario, Canada: using networks to integrate palliative care province-wide. J Pain Symptom Manage 33:640–644
Webster K, Cella D, Yost K (2003) The Functional Assessment of Chronic Illness Therapy (FACIT) measurement system: properties, applications, and interpretation. Health Qual Life Outcomes 1:79
Fayers PM, Aaronson NK, Bjordal K et al (2001) European Organisation for Research and Treatment of Cancer QLQ-C30 Scoring Manual, ed 3rd edn. EORTC Quality of Life Group, Brussels
Miaskowski C, Aouizerat BE, Dodd M, Cooper B (2007) Conceptual issues in symptom clusters research and their implications for quality-of-life assessment in patients with cancer. J Natl Cancer Inst Monogr 37:39–46
Barsevick AM, Whitmer K, Nail LM, Beck SL, Dudley WN (2006) Symptom cluster research: conceptual design, measurement, and analysis issues. J Pain Symptom Manage 31:85–95
Gagliese L, Melzack R (2003) Age-related differences in the qualities but not the intensity of chronic pain. Pain 104:597–608
Gagliese L, Katz J (2003) Age differences in post-operative pain are scale dependent: a comparison of measures of pain intensity and quality in younger and older surgical patients. Pain 103:11–20
Gagliese L, Jovellanos M, Zimmermann C, Shobbrook C, Warr D, Rodin G (2009) Age-related patterns in adaptation to cancer pain. Pain Med 10:1050–1061
Ringash J, O’Sullivan B, Bezjak A et al (2007) Interpreting clinically significant changes in patient reported outcomes. Cancer 110:196–202
Osoba D, Rodrigues G, Myles J et al (1998) Interpreting the significance of changes in health-related quality-of-life socres. J Clin Oncol 16:139–144
Barrett B, Brown D, Mundt M et al (2005) Sufficiently important difference: expanding the framework of clinical significance. Med Decis Making 25:250–261
Riechelmann RP, Zimmermann C, Chin SN, Wang L, O’Carroll A, Zarinehbaf S et al (2008) Potential drug interactions in cancer patients receiving supportive care exclusively. J Pain Symptom Manage 35:535–543
Riechelmann RP, Krzyzanowska MK, O’Carroll A, Zimmermann C (2007) Symptom and medication profiles among cancer patients attending a palliative care clinic. Support Care Cancer 15:1407–1412
Bekelman DB, Rumsfeld JS, Havranek EP, Yamashita TE, Hutt E, Gottlieb SH et al (2009) Symptom burden, depression, and spiritual well-being: a comparison of heart failure and advanced cancer patients. J Gen Intern Med 24:592–598
Habraken JM, ter Riet G, Gore JM, Greenstone MA, Weersink EJ, Bindels PJ et al (2009) Health-related quality of life in end-stage COPD and lung cancer patients. J Pain Symptom Manage 37:973–981
Baumann R, Putz C, Rohrig B, Hoffken K, Wedding U (2009) Health-related quality of life in elderly cancer patients, elderly non-cancer patients and an elderly general population. Eur J Cancer Care (Engl) 18:457–465
Schmidt CE, Bestmann B, Kuchler T, Longo WE, Kremer B (2005) Impact of age on quality of life in patients with rectal cancer. World J Surg 29:190–197
Cleary JF, Carbone PP (1997) Palliative medicine in the elderly. Cancer 80:1335–1347
Holzner B, Kemmler G, Cella D, De Paoli C, Meraner V, Kopp M et al (2004) Normative data for functional assessment of cancer therapy—general scale and its use for the interpretation of quality of life scores in cancer survivors. Acta Oncol 43:153–160
Hjermstad MJ, Fayers PM, Bjordal K, Kassa S (1998) Health-related quality of life in the general Norwegian population assessed by the European Organization for Research and Treatment of Cancer Core Quality-of-Life Questionniare: the QLQ = C30(+3). J Clin Oncol 16:1188–1196
Carlson LE, Angen M, Cullum J, Goodey E, Koopmans J, Lamont L et al (2004) High levels of untreated distress and fatigue in cancer patients. Br J Cancer 90:2297–2304
Strong V, Waters R, Hibberd C, Rush R, Cargill A, Storey D et al (2007) Emotional distress in cancer patients: the Edinburgh Cancer Centre symptom study. Br J Cancer 96:868–874
Pascoe S, Edelman S, Kidman A (2000) Prevalence of psychological distress and use of support services by cancer patients at Sydney hospitals. Aust N Z J Psychiatry 34:785–791
Aass N, Fossa SD, Dahl AA, Moe TJ (1997) Prevalence of anxiety and depression in cancer patients seen at the Norwegian Radium Hospital. Eur J Cancer 33:1597–1604
Hamadani M, Chaudhary L, Awan FT et al (2007) Management of platinum-based chemotherapy-induced acute nausea and vomiting: is there a superior serotonin receptor antagonist? J Oncol Pharm Pract 13:69–75
Shih V, Wan HS, Chan A (2009) Clinical predictors of chemotherapy-induced nausea and vomiting in breast cancer patients receiving adjuvant doxorubicin and cyclophosphamide. Ann Pharmacother 43:444–452
Pollera CF, Giannarelli D (1989) Prognostic factors influencing cisplatin-induced emesis. Definition and validation of a predictive logistic model. Cancer 64:1117–1122
Liaw CC, Wang CH, Chang HK, Liau CT, Yeh KY, Huang JS et al (2001) Gender discrepancy observed between chemotherapy-induced emesis and hiccups. Support Care Cancer 9:435–441
Liaw CC, Chang HK, Liau CT, Huang JS, Lin YC, Chen JS (2003) Reduced maintenance of complete protection from emesis for women during chemotherapy cycles. Am J Clin Oncol 26:12–15
Follwell M, Burman D, Le LW, Wakimoto K, Seccareccia D, Bryson J et al (2009) Phase II study of an outpatient palliative care intervention in patients with metastatic cancer. J Clin Oncol 27:206–213
McCaughan E, Prue G, Parahoo K (2009) A systematic review of quantitative studies reporting selected patient experienced outcomes with a specific focus on gender differences in people with colorectal cancer. Eur J Oncol Nurs 13:376–385
Chow E, Fan G, Hadi S, Wong J, Kirou-Mauro A, Filipczak L (2008) Symptom clusters in elderly patients with lung cancer. Clin Oncol (R Coll Radiol) 20:76–82
Chow E, Fan G, Hadi S, Filipczak L (2007) Symptom clusters in cancer patients with bone metastases. Support Care Cancer 15:1035–1043
Chen ML, Tseng HC (2006) Symptom clusters in cancer patients. Support Care Cancer 14:825–830
Walsh D, Rybicki L (2006) Symptom clustering in advanced cancer. Support Care Cancer 14:831–836
Ridner SH (2005) Quality of life and a symptom cluster associated with breast cancer treatment-related lymphedema. Support Care Cancer 13:904–911
Bender CM, Ergyn FS, Rosenzweig MQ, Cohen SM, Sereika SM (2005) Symptom clusters in breast cancer across 3 phases of disease. Cancer Nurs 28:219–225
Gifts AG, Jablonski A, Stommel M, Given CW (2004) Symptom clusters in elderly patients with lung cancer. Oncol Nurs Forum 31:202–212
Fillingim RB, Ness TJ, Glover TL, Campbell CM, Hastie BA, Price DD et al (2005) Morphine responses and experimental pain: sex differences in side effects and cardiovascular responses but not analgesia. J Pain 6:116–124
Bijur PE, Esses D, Birnbaum A, Chang AK, Schechter C, Gallagher EJ (2008) Response to morphine in male and female patients: analgesia and adverse events. Clin J Pain 24:192–198
Barsky AJ, Peekna HM, Borus JF (2001) Somatic symptom reporting in women and men. J Gen Intern Med 16:266–275
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cheung, W.Y., Le, L.W., Gagliese, L. et al. Age and gender differences in symptom intensity and symptom clusters among patients with metastatic cancer. Support Care Cancer 19, 417–423 (2011). https://doi.org/10.1007/s00520-010-0865-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-010-0865-2