Abstract
Goals of work
It has been observed that certain cancer symptoms frequently occur together. Prior research on symptom patterns has focused mainly on inpatients, early stage cancers, or a single cancer type or metastatic site. Our aim was to explore symptom clusters among outpatients with different advanced cancers.
Materials and methods
Symptom scores by the Edmonton Symptom Assessment Scale (ESAS) were routinely collected for patients attending the Oncology Palliative Care Clinics at Princess Margaret Hospital from January 2005 to October 2007. Principal component analysis (PCA) was performed for the entire patient cohort and within specific disease sites to determine inter-relationships of the nine ESAS symptoms.
Main results
A total of 1,366 patients was included: 682 (50%) were male and 684 (50%) were female. The median age was 64 years (range 18 to 74 years). The most common primary cancer sites were gastrointestinal (27%), lung (14%), and breast (11%). The three most distressful symptoms were fatigue, poor general well-being, and decreased appetite. PCA of symptoms for the entire patient cohort revealed two major symptom clusters: cluster 1 included fatigue, drowsiness, nausea, decreased appetite, and dyspnea, which accounted for 45% of the total variance; cluster 2 included anxiety and depression, which accounted for 10% of the total variance. There was high internal reliability in the clusters (Cronbach’s alpha coefficient ∼0.80). PCA of symptoms within the various primary cancer sites revealed differences in the pattern of symptom clusters.
Conclusions
In patients with advanced cancers, distinct symptom clusters can be identified, which are influenced by primary cancer site. Treatments directed at symptom clusters rather than individual symptoms may provide greater therapeutic benefit. Further prospective studies are warranted in order to develop more effective targeted palliative interventions for the advanced cancer patient population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pain and symptom control are important components of comprehensive cancer care, especially for patients with advanced cancer, whose symptoms are frequently complex and where palliation is typically the primary goal. However, most prior research on pain and symptom management has focused on the study of individual symptoms in isolation rather than exploring potential interactions and inter-relationships among various symptoms. Knowledge of whether and how different symptoms may occur in combination or influence each other can contribute to an improved understanding of the pathophysiology and mechanisms that underlie specific symptoms. Such information can provide a basis for the development of more effective symptom-directed interventions and improve quality of life for cancer patients.
Dodd et al. first highlighted that symptoms may not always exist as distinct entities and that certain symptoms, such as fatigue and sleep disturbances, often occur together for patients who are receiving chemotherapy [14]. The term “symptom clusters” has been defined as two or more inter-related symptoms that are present together, independent of other symptom clusters, and may or may not suggest a common etiology or underlying mechanism [14, 17, 26]. Investigations of symptom clusters have primarily concentrated on inpatients [35, 39], early stage cancers [8, 33], a single cancer type [3, 19, 20], or a specific metastatic site [11, 12] and have usually involved only a small sample of patients. Only a few studies have specifically examined symptom clusters in patients with advanced disease [35, 39].
The study of symptom clusters may be especially relevant for patients with advanced cancer, who commonly manifest with multiple, concurrent symptoms from their disease, their treatments, or both [7, 31]. Moreover, due to a growing emphasis on outpatient cancer care, patients with advanced cancer are increasingly treated in ambulatory settings [27]. For these outpatients, it is important to develop therapies that are convenient, easily administered, and amenable to use by caregivers. The study of symptom clusters may help to streamline treatments to target several symptoms simultaneously and result in greater therapeutic benefit. In the current study, the main objectives were to characterize the pattern of symptom clusters among advanced cancer outpatients and to explore the impact of different cancer sites on these clusters.
Materials and methods
Characteristics of the study hospital and the symptom assessment tool
Princess Margaret Hospital (PMH) is a large tertiary cancer center located in Toronto, Canada. The palliative care program is housed in the Department of Psychosocial Oncology and Palliative Care and includes a 12-bed acute palliative care inpatient unit, a consultation service that assesses urgent cases on a same-day basis, and a daily oncology palliative care clinic. Patients are referred to this outpatient clinic by their oncologists for pain management, control of other symptoms, and palliative care planning [42]. Since 2005, all patients who are assessed in the palliative care clinic are requested to complete the Edmonton Symptom Assessment Scale (ESAS) as a routine component of the initial consultation and at each subsequent clinic visit.
The ESAS is a standardized assessment tool that is commonly used by palliative care teams to evaluate the severity of nine physical and psychological symptoms: pain, dyspnea, appetite, nausea, fatigue, drowsiness, anxiety, depression, and general well-being [4, 6]. Patients grade each item on a scale from 0 to 10 with a score of 0 representing complete absence of the symptom and 10 indicating the worst possible symptom intensity. This scale has been successfully validated for use in cancer patients [6]. Results of ESAS assessments for all patients in the outpatient palliative oncology clinic are prospectively entered into the PMH palliative care clinical database. Random chart audits for a provincial palliative care project initiated in 2006 that includes our cancer center [15] have consistently found completion rates of greater than 90% for the ESAS in our palliative care clinic.
Patient selection and data collection
This study was conducted upon obtaining full research ethics approval from the institutional review board at PMH. The PMH palliative cancer clinical database was used to identify all eligible subjects. Consecutive patients with metastatic cancer who were seen in consultation in the palliative care clinic between January 1, 2005 and October 31, 2007 and who responded to an ESAS questionnaire were considered for inclusion in the study. Patients with early cancers (stage I to III as per the American Joint Committee on Cancer staging system) were excluded. Medical records and database entries for eligible patients were reviewed to extract information on baseline patient demographics, tumor characteristics, and ESAS symptom scores.
Statistical analyses
Descriptive statistics were used to summarize baseline patient demographics, disease characteristics, and distribution of ESAS scores. Spearman correlation analyses were performed on the nine ESAS items to determine the strength of correlation between any two of the nine ESAS symptoms. To detect symptom clusters and to examine whether any inter-relationships existed among symptoms, a principal component analysis (PCA) with varimax rotation was conducted on the intensity scores of the nine ESAS symptoms.
PCA is an exploratory statistical technique that can be applied to a set of variables to determine which variables (or ESAS symptoms, in our study), if any, correlate with each other to form a distinct and stable pattern, which is known as a “component” in the PCA model [2, 29]. A variable that consistently associates with other results in a higher factor loading score in the PCA and predicts its assignment into an independent component or “cluster.” To be considered a true symptom cluster, we defined a symptom within a cluster as having a loading score of at least 0.60 and the cluster itself as accounting for ≥10% of the total variance. The internal consistency and reliability of the derived symptom clusters were assessed with the Cronbach’s alpha coefficient where a higher value indicates better consistency. All statistical analyses were performed with SAS® (V9.1; SAS Institute, Cary, NC, USA).
Results
Our analyses were conducted on the initial ESAS assessments from 1,366 outpatients with metastatic cancer who were assessed in the palliative care clinic between January 1, 2005 and October 31, 2007 (Table 1). Among them, 747 patients had only one assessment and 619 had further assessments, which were not included in our analyses. For those with further assessments, the median number of total assessments per patient was 3 (range 2–18). The median age was 64 years (range 18–74 years); 682 (50%) patients were male and 684 (50%) were female. The most common primary cancer sites were gastrointestinal (27%), lung (14%), and breast (11%). The category “other cancers” consisted mainly of unknown primary tumors, sarcomas, and concurrent cancers. In Table 2, symptom prevalence and severity are described. A symptom was considered to be present if the score for that particular symptom was rated as >0. All nine ESAS symptoms were present in at least 50% of patients, with fatigue being the most prevalent (96%) and nausea the least (53%). Fatigue ranked highest in symptom severity, followed by poor general well-being and decreased appetite.
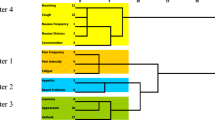
Table 3 lists the findings from the Spearman correlation analyses of the nine ESAS symptoms and reveals that the associations between symptom items were all highly significant (p < 0.0001). The strongest correlations were 0.70 for anxiety and depression, 0.56 for fatigue and drowsiness, and 0.53 for fatigue and well-being. The PCA results are highlighted in Table 4. Two symptom clusters were identified, which accounted for 55% of the total variance. Cluster 1 included fatigue, drowsiness, nausea, decreased appetite, and dyspnea and contributed to 45% of the total variance. Cluster 2 included anxiety and depression, which accounted for 10% of the total variance. Both symptom clusters demonstrated good internal consistency and validity with Cronbach’s alpha coefficients that were high (0.76 and 0.83, respectively).
PCAs were subsequently conducted within the various disease sites to assess the impact of different primary cancers on the presence and pattern of symptom clusters. The two main symptom clusters for each of the disease sites are shown in Table 5. PCA were not performed for the “skin cancer” and “other cancer” categories due to the small sample sizes and heterogeneity in these groups, respectively. Although the constituents of the symptom clusters differed among the various primary cancers, anxiety and depression consistently clustered together in solid tumors, regardless of the disease site. Specifically, pain and drowsiness clustered together for cancers involving the central nervous system and head and neck, while decreased appetite and poor general well-being clustered together for breast, lung, gastrointestinal, genitourinary, and gynecological malignancies. The cluster of anxiety, drowsiness, fatigue, and dyspnea was unique to hematological malignancies.
Discussion
Symptom clusters have not been investigated in detail in the advanced cancer population. In contrast to prior studies that have primarily focused on early stage cancers [8, 33], inpatients [35, 39], or a single cancer type [3, 19, 20] or metastatic site [11, 12], we specifically explored symptom clusters in a large cohort of outpatients with a variety of advanced cancers. Outpatients represent a substantial proportion of the individuals in need of palliative care services near the end of life [27]. Furthermore, advanced cancer outpatients experience a substantial physical and psychological symptom burden [5, 32, 37], which may increase for specific physical symptoms as the time of death approaches [9].
Our study identified two main symptom clusters: one included anxiety and depression and the second consisted of fatigue, drowsiness, nausea, decreased appetite, and dyspnea. Several previous investigations have identified the cluster of anxiety and depression. In one analysis of breast cancer patients by Bender et al., anxiety and depression formed a consistent symptom cluster that was prevalent in all stages of breast cancer [3]. Likewise, two studies conducted by Chow et al., involving patients with bone [11] and brain metastases [12], respectively, found that anxiety and depression occurred together before and after palliative radiotherapy. Similar cluster patterns involving various affective symptoms, such as emotional distress, sadness, anxiety, and depression, were noted in three additional studies [8, 23, 39], including patients with different cancers at various disease stages. Although these studies contained a mixture of inpatients and outpatients, patients admitted to the hospital comprised the majority in all of them (range 54–85%).
The findings noted above, together with those of our current study in outpatients, indicate that psychological symptoms such as anxiety and depression remain a significant source of distress for cancer patients regardless of their primary cancer site, stage, or palliative treatment setting. The tendency of these symptoms to occur together may have implications for the treatment of depression and anxiety in the cancer population, with increased relevance for the use of certain antidepressants, such as selective serotonin uptake inhibitors, that treat both depression and anxiety [22]. However, it should also be noted that, in our study, depression and anxiety were measured using single-item scores and may represent more general psychological distress, rather than clinically significant depression or anxiety. Further prospective studies using both validated measures and structured interviews are required.
In contrast to the widespread prevalence of the anxiety–depression cluster, the second cluster of fatigue, drowsiness, nausea, decreased appetite, and dyspnea has not been reproduced in other studies. Most previous studies utilized the 13-item MD Anderson Symptom Inventory (MDASI), and in these reports, nausea and dyspnea tended to coexist with other symptoms that are not captured by the ESAS [8, 13, 23, 30, 40, 41]. However, the three remaining symptoms in this cluster—fatigue, drowsiness, and poor appetite—did show a general tendency to cluster together in studies that used the ESAS, MDASI, or other symptom scales [8, 12, 23, 39]. This trend suggests that these three symptoms might share a common etiology or that one symptom may produce a downstream effect on another.
There are several plausible explanations for the coexistence of the symptoms in this second symptom cluster. All of the symptoms in this cluster, with the exception of dyspnea, are implicated in the anorexia–cachexia syndrome. Recent reports indicate that proinflammatory cytokines such as tumor necrosis factor-α, interleukin-1, interleukin-6, and interferon-γ may play a fundamental role in causing cancer-related anorexia–cachexia and may, therefore, be involved in the etiology of this symptom cluster [1, 25]. In addition, nausea, poor appetite, fatigue, drowsiness, and dyspnea are all potential side effects of chemotherapy, and this may have further contributed to this cluster in our outpatient population. Finally, there may be a temporal aspect to the clustering of these symptoms. In our previous work with the outpatient cancer population, we found that poor appetite, drowsiness, dyspnea, and fatigue all increased in intensity as the end of life approached, whereas other symptoms evaluated by the ESAS did not [9]. While these are plausible hypotheses, the precise manner in which these symptoms interact and the exact sequence in which they occur needs to be confirmed in longitudinal studies.
One reason that clusters may vary from study to study is due to the lack of common criteria for inclusion of symptoms in a cluster. In our study, we clearly defined a 0.6 cut-off for the factor loading score. In some previous studies [12, 18], this value was neither explicitly set in advance nor specifically listed for individual symptoms, leading to the possibility of post hoc definitions of clusters with various factor loading scores. In addition, several studies chose to use different cut-off values, which ranged from 0.4 to 0.9 [8, 11, 21, 39]. In the future, all studies should disclose their cut-off values for the factor loading score, so that their results can be better interpreted. In addition, it would be useful for researchers of symptom clusters to decide on uniform cut-offs for factor loading scores, so that results can be compared more easily from study to study.
Another unique feature of our study is that we conducted an exploratory analysis to examine the impact of different cancer sites on symptom clusters. While the precise constituents of each identified symptom cluster varied based on disease site, certain symptoms consistently aggregated together. As Table 5 illustrates, anxiety and depression continued to cluster for all solid tumors, regardless of cancer site. In addition, pain and drowsiness tended to occur together for primary tumors of the central nervous system as well as for head and neck cancers. This could indicate that pain is particularly difficult to control for these sites, resulting in higher doses of prescribed opioids and consequently more opioid-induced drowsiness [28]. It may also be that this population is particularly susceptible to drowsiness either as a side effect of pain medication or as sequelae of palliative interventions, such as brain radiotherapy, given that tumors of the central nervous system involve the brain, and cancers of the head and neck frequently metastasize there [10, 16].
For cancers below the diaphragm, including gastrointestinal, genitourinary, and gynecological malignancies, lack of appetite and poor well-being were consistent components in the symptom clusters. All of these cancers involve the abdominal and pelvic cavities and can cause ascites or peritoneal carcinomatosis, which can have a detrimental effect on appetite. The consequent decrease in oral intake can lead to general malaise and poor well-being. Interestingly, patients who were initially diagnosed with primary breast or lung cancers also exhibited a symptom cluster consisting of decreased appetite and poor well-being. One possible reason for this finding is that lung cancers often metastasize to abdominal organs, such as the liver and adrenal glands, and thus may have a negative impact on these same symptoms. Likewise, breast cancers also metastasize to the liver; thus a similar clustering of these symptoms may occur.
We also reported on symptom clusters for hematological malignancies, which have not been previously described. The cluster of anxiety, drowsiness, fatigue, and dyspnea was present only for patients with hematological malignancies and not for those with solid tumors. With the exception of anxiety, all of the symptoms in this cluster can be attributed to anemia, which is common among patients with hematological malignancies. In addition, anxiety was associated with anemia in a study of patients with nonmyeloid malignancies and improved with an increase in hemoglobin [36]. However, another study found that anxiety was associated with cancer-related fatigue in patients with and without anemia [34]. Further research in this area is necessary, particularly in patients with hematological malignancies.
In term of limitations, our results may not be generalizable to other palliative care centers or inpatient settings, as the study consisted of a single institutional review of cancer outpatients who were referred to a palliative care clinic. There were high percentages of gastrointestinal and lung cancers in our study population, which likely reflects the fact that these are the cancers with the highest mortality rate. We also excluded all outpatients who did not complete the ESAS in the palliative care clinic. Although the completion rate for the ESAS in our clinic is high, some of the excluded patients may have been too frail or symptomatic to complete these assessments, resulting in a selection bias and an inaccurate characterization of actual symptom clusters for advanced cancers. ESAS also limits the evaluation to only nine symptoms; thus, the clusters identified in this study may represent an oversimplification, as some important symptoms may have been omitted. Finally, it would have been ideal to evaluate the evolution of symptom clusters prospectively and longitudinally from the time of primary cancer diagnosis to the development of advanced cancer and death from cancer. However, such studies are methodologically challenging [24, 38], and our study provides a necessary first step to generate hypotheses that may subsequently be examined prospectively.
In an era when patients with advanced cancer are frequently managed on an outpatient basis, an understanding of the prevalence and pattern of symptom clusters in this patient cohort has increasing clinical relevance and importance. Our study demonstrated that it is possible in this population to identify distinct symptom clusters, which are influenced by primary cancer site. An awareness of these symptom clusters can aid the development of interventions that manage several symptoms in combination. This approach can potentially reduce polypharmacy, lessen drug side effects, provide pharmacoeconomic benefits, and ultimately optimize quality of life for patients with advanced cancers.
References
Argiles JM, Busquets S, Garcia-Martinez C, Lopez-Soriano FJ (2005) Mediators involved in the cancer anorexia–cachexia syndrome: past, present, and future. Nutrition 21:977–985
Barsevick AM, Whitmer K, Nail LM, Beck SL, Dudley WN (2006) Symptom cluster research: conceptual, design, measurement, and analysis issues. J Pain Symptom Manage 31:85–95 doi:10.1016/j.jpainsymman.2005.05.015
Bender CM, Ergyn FS, Rosenzweig MQ, Cohen SM, Sereika SM (2005) Symptom clusters in breast cancer across 3 phases of the disease. Cancer Nurs 28:219–225 doi:10.1097/00002820-200505000-00011
Bruera E, Kuehn N, Miller MJ, Selmser P, Macmillan K (1991) The Edmonton symptom assessment system (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care 7:6–9
Bruera E, Michaud M, Vigano A et al (2001) Multidisciplinary symptom control clinic in a cancer center: a retrospective study. Support Care Cancer 9:162–168 doi:10.1007/s005200000172
Chang VT, Hwang SS, Feuerman M (2000) Validation of the Edmonton symptom assessment scale. Cancer 88:2164–2171 doi:10.1002/(SICI)1097-0142(20000501)88:9<2164::AID-CNCR24>3.0.CO;2-5
Chang VT, Hwang SS, Feuerman M, Kasimis BS (2000) Symptom and quality of life survey of medical oncology patients at a veteran affairs medical centre. Cancer 88:1175–1183 doi:10.1002/(SICI)1097-0142(20000301)88:5<1175::AID-CNCR30>3.0.CO;2-N
Chen ML, Tseng HC (2006) Symptom clusters in cancer patients. Support Care Cancer 14:825–830 doi:10.1007/s00520-006-0019-8
Cheung WY, Barmala N, Zarinehbaf S, Rodin G, Le LW, Zimmermann C (2008) The association of physical and psychological symptom burden with time to death among palliative cancer outpatients. J Pain Symptom Manage. doi:10.1016/j.jpainsymman.2008.03.008
Chow E, Davis L, Holden L, Tsao M, Danjoux C (2005) Prospective assessment of patient-rated symptoms following whole brain radiotherapy for brain metastases. J Pain Symptom Manage 30:18–23 doi:10.1016/j.jpainsymman.2005.02.009
Chow E, Fan G, Hadi S, Filipczak L (2007) Symptom clusters in cancer patients with bone metastases. Support Care Cancer 15:1035–1043 doi:10.1007/s00520-007-0241-z
Chow E, Fan G, Hadi S, Wong J, Kirou-Mauro A, Filipczak L (2008) Symptom clusters in cancer patients with brain metastases. Clin Oncol (R Coll Radiol) 20:76–82 doi:10.1016/j.clon.2007.09.007
Cleeland CS, Mendoza TR, Wang XS et al (2000) Assessing symptom distress in cancer patients: the MD Anderson symptom inventory. Cancer 89:1634–1646 doi:10.1002/1097-0142(20001001)89:7<1634::AID-CNCR29>3.0.CO;2-V
Dodd MJ, Miaskowski C, Paul SM (2001) Symptom clusters and their effect on the functional status of patients with cancer. Oncol Nurs Forum 28:465–470
Dudgeon D, Vaitonis V, Seow H et al (2007) Ontario, Canada: using networks to integrate palliative care province-wide. J Pain Symptom Manage 33:640–644 doi:10.1016/j.jpainsymman.2007.02.001
Faithfull S, Brada M (1998) Somnolence syndrome in adults following cranial irradiation for primary brain tumours. Clin Oncol (R Coll Radiol) 10:250–254 doi:10.1016/S0936-6555(98)80011-3
Fan G, Filipczak L, Chow E (2007) Symptom clusters in cancer patients: a review of the literature. Curr Oncol 14:173–179 doi:10.3747/co.2007.145
Fan G, Hadi S, Chow E (2007) Symptom clusters in patients with advanced-stage cancer referred for palliative radiation therapy in an outpatient setting. Support Cancer Ther 4:157–162 doi:10.3816/SCT.2007.n.010
Gift AG, Jablonski A, Stommel M, Given CW (2004) Symptom clusters in elderly patients with lung cancer. Oncol Nurs Forum 31:202–212 doi:10.1188/04.ONF.203-212
Glaus A, Boehme CH, Thurlimann B (2006) Fatigue and menopausal symptoms in women with breast cancer undergoing hormonal cancer treatment. Ann Oncol 17:801–806 doi:10.1093/annonc/mdl030
Hadi S, Fan G, Hird AE, Kirou-Mauro A, Filipczak LA, Chow E (2008) Symptom clusters in patients with cancer with metastatic bone pain. J Palliat Med 11:591–600 doi:10.1089/jpm.2007.0145
Hoschl C, Svestka J (2008) Escitalopram for the treatment of major depression and anxiety disorders. Expert Review of Neurotherapeutics 8:537–552 doi:10.1586/14737175.8.4.537
Ivanova MO, Ionova TI, Kalyadina SA et al (2005) Cancer-related symptom assessment in Russia: validation and utility of the Russian MD Anderson symptom inventory. J Pain Symptom Manage 30:443–453 doi:10.1016/j.jpainsymman.2005.04.015
Jordhoy MS, Kaasa S, Fayers P et al (1999) Challenges in palliative care research; recruitment, attrition and compliance: experience from a randomized controlled trial. Palliat Med 13:299–310 doi:10.1191/026921699668963873
Kayacan O, Karnak D, Beder S et al (2006) Impact of TNF-alpha and IL-6 levels on development of cachexia in newly diagnosed NSCLC patients. Am J Clin Oncol 29:328–335 doi:10.1097/01.coc.0000221300.72657.e0
Kim HJ, McGuire DB, Tulman L, Barsevick AM (2005) Symptom clusters: concept analysis and clinical implications for cancer nursing. Cancer Nurs 28:270–284
Lidstone V, Butters E, Seed PT, Sinnott C, Beynon T, Richards M (2003) Symptoms and concerns amongst cancer outpatients: identifying the need for specialist palliative care. Palliat Med 17:588–595 doi:10.1191/0269216303pm814oa
Mercadante S, Villari P, Ferrera P, Casuccio A (2006) Opioid-induced or pain relief-induced symptoms in advanced cancer patients? Eur J Pain 10:153–159 doi:10.1016/j.ejpain.2005.02.006
Miaskowski C, Aouizerat BE, Dodd M, Cooper B (2007) Conceptual issues in symptom clusters research and their implications for quality-of-life assessment in patients with cancer. J Natl Cancer Inst Monogr 37:39–46 doi:10.1093/jncimonographs/lgm003
Okuyama T, Wang XS, Akechi T et al (2003) Japanese version of the MD Anderson symptom inventory: a validation study. J Pain Symptom Manage 26:1093–1104 doi:10.1016/j.jpainsymman.2003.05.003
Portenoy RK, Thaler HT, Kornblith AB (1994) Symptom prevalence, characteristics, and distress in a cancer population. Qual Life Res 3:183–189 doi:10.1007/BF00435383
Potter J, Hami F, Bryan T, Quigley C (2003) Symptoms in 400 patients referred to palliative care services: prevalence and patterns. Palliat Med 17:310–314 doi:10.1191/0269216303pm760oa
Ridner SH (2005) Quality of life and a symptom cluster associated with breast cancer treatment-related lymphedema. Support Care Cancer 13:904–911 doi:10.1007/s00520-005-0810-y
Romito F, Montanaro R, Corvasce C et al (2008) Is cancer-related fatigue more strongly correlated to haematological or to psychological factors in cancer patients? Support Care Cancer 16:943–946 doi:10.1007/s00520-007-0357-1
Sarna L, Brecht ML (1997) Dimensions of symptom distress in women with advanced lung cancer: a factor analysis. Heart Lung 26:23–30 doi:10.1016/S0147-9563(97)90006-6
Smith JR, Glaspy JA, Tchekmedyian NS et al (2003) Hemoglobin increase is associated with improved health-related quality of life in patients with cancer not receiving chemotherapy. Support Cancer Ther 1:49–54 doi:10.3816/SCT.2003.n.004
Stromgren AS, Goldschmidt D, Groenvold M et al (2002) Self-assessment in cancer patients referred to palliative care: a study of feasibility and symptom epidemiology. Cancer 94:512–520 doi:10.1002/cncr.10222
Stromgren AS, Sjogren P, Goldschmidt D et al (2005) A longitudinal study of palliative care: patient-evaluated outcome and impact of attrition. Cancer 103:1747–1755 doi:10.1002/cncr.20958
Walsh D, Rybicki L (2006) Symptom clustering in advanced cancer. Support Care Cancer 14:831–836 doi:10.1007/s00520-005-0899-z
Wang XS, Laudico AV, Guo H et al (2006) Filipino version of the MD Anderson Symptom Inventory: validation and multisymptom measurement in cancer patients. J Pain Symptom Manage 31:542–552 doi:10.1016/j.jpainsymman.2005.11.011
Wang XS, Wang Y, Guo H, Mendoza TR, Hao XS, Cleeland CS (2004) Chinese version of the MD Anderson symptom inventory: validation and application of symptom measurement in cancer patients. Cancer 101:1890–1901 doi:10.1002/cncr.20448
Zimmermann C, Seccareccia D, Clarke A, Warr D, Rodin G (2006) Bringing palliative care to a Canadian cancer center: the palliative care program at Princess Margaret Hospital. Support Care Cancer 14:982–987 doi:10.1007/s00520-006-0093-y
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cheung, W.Y., Le, L.W. & Zimmermann, C. Symptom clusters in patients with advanced cancers. Support Care Cancer 17, 1223–1230 (2009). https://doi.org/10.1007/s00520-009-0577-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-009-0577-7