Abstract
Purpose
It has not previously been shown whether there is any benefit to multi-morbid patients with lung cancer who participate in complex interdisciplinary rehabilitation programmes after primary therapy. The purpose of this prospective study was to assess changes in exercise capacity and quality of life before and after an in-patient training programme.
Patients and methods
Forty-five patients with lung cancer (WHO I-III after surgery and/or radiotherapy and/or chemotherapy) were enrolled in a 28-day in-patient rehabilitation programme that included standardised aerobic training. Functional status and health-related quality of life (QLQ-C30, QLQ-LC13, SF-36, and MFI-20) were examined at the beginning of the study and at day 28.
Results
A substantial increase in work performance (bicycle ergometry from 68 ± 3 to 86 ± 4 W, p < 0.001, and 6-minute walk test from 322 ± 11 to 385 ± 13 m, p < 0.001) was registered. In addition, heart rate at rest was reduced (from 84 ± 2 to 80 ± 1 beats per minute, p < 0.05) and heart rate variability (indicator of the efficacy of endurance training) was significantly increased (from 9.7 ± 1 to 12.9 ± 1 root mean square of successive differences, p < 0.001). Moreover, there was also a significant improvement in quality of life (48 ± 3 to 62 ± 2, p < 0.001) while fatigue was reduced from 66 ± 3 to 41 ± 4, p < 0.001.
Conclusion
A standardised, aerobic endurance training programme as part of the in-patient oncological rehabilitation of patients with lung cancer results in improvements in both physiological and psychological parameters after therapy. A follow-on study in order to determine to what extent this benefit persists over the long-term, particularly, in comparison with patients who have not participated in a rehabilitation programme, is currently being conducted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer is characterised by a high rate of incidence, prevalence, and mortality. The initially asymptomatic course and lack of reliable screening methods lead to late diagnosis and bad prognosis. The elderly and often multi-morbid patients undergo ever more complex treatment in order to restore their health or at least prolong their survival. However, multi-modal therapeutic concepts often cause considerable physical and psychological symptoms and impair the functional and participatory capacity of patients—problems that are defined in the International Classification of Functioning, Disability and Health of the World Health Organization (WHO). Therefore, loss of exercise capacity, dyspnoea at rest, fatigue, states of restlessness, polyneuropathic problems, post-thoracotomy syndrome, etc. reduce the patients’ quality of life and make reintegration into both the working force and social life difficult. German cancer rehabilitation concepts comprise a multi-modal approach of the patient including educational elements and a socio-medical evaluation of the potential for restitution of abilities that have been lost.
The main therapeutic elements of cancer/pneumological rehabilitation are clinical counselling and medical supervision, socio-medical evaluation, drug-based therapies, health education, physical activity (endurance, strength, coordination, and mobility), psychological support, initiation of follow-up measures (including ambulatory lung training sport groups and continuation of programmes to help patients give up smoking), healthcare, and nursing (wound management, etc.).
The efficacy of these types of complex interdisciplinary rehabilitation programmes, which are available in German-speaking countries, have so far not been adequately investigated for lung cancer patients. Apart from a literature review with personal remarks relating to cardiopulmonary rehabilitation after treatment for lung cancer (level 5 evidence according to American College of Chest Physicians guidelines [1]), a pilot study in which a rehabilitation programme was tested on ten patients (level 4 evidence [2]) and individual systematic reviews that report positive effects on quality of life or dyspnoea in lung cancer patients after non-drug-based therapeutic measures and interventions by nursing staff (level 1 evidence [3, 4]), only very few publications on this subject have appeared. In order to determine the efficacy of such in-patient lung cancer rehab programme, we investigated whether physical exercise capacity and quality of life of precisely characterised patients (with clearly defined consequential and functional impairments following surgery and/or radiotherapy and/or chemotherapy) could be improved by means of standardised aerobic endurance training and other measures. So this study was the first to evaluate the efficacy of an in-patient rehabilitation programme in a larger group of patients with lung cancer in which the sample size was determined a priori. During the step of literature evaluation for establishing German S3 guidelines for the diagnosis and treatment of lung cancer, it became evident that no other study among a total of 814 reviewed literature abstracts and 66 full text publications on this subject and was evaluated as level 3 evidence.
Patients and methods
Patients
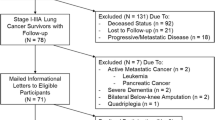
A total of 45 patients with histologically confirmed lung cancer who had undergone surgery and/or radiotherapy and/or chemotherapy participated in the study which was conducted from 2004 to 2005 and involved an in-patient cancer rehabilitation programme lasting for 28 days at the Cecilien-Klinik in Bad Lippspringe, Germany. Generally, the time since last treatment and beginning of rehabilitation is no more than 14 days. The mean age of the patients was 60.2 years (±8 years); minimum and maximum ages were 43 years and 76 years, respectively.
Cancer status and nature of the treatments provided
Thirty-eight of the patients had non-small cell lung cancer (NSCLC) and three patients had small cell lung cancer. In the four remaining study subjects, there was no clear histological differentiation, but it was likely that they had mixed cell lung cancer. Cancer stage at the time of diagnosis was IA in nine study subjects (20.0%), IB in 11 study subjects (24.4%), IIA in one study subject (2.2%), IIB in ten study subjects (22.2%), IIIA in nine study subjects (20.0%), and IIIB in two study subjects (4.4%; see Fig. 1). Of the three patients with small cell lung cancer, two (4.4%) were classified as having “limited disease I”, and one patient (2.2%) was classified as having “extensive disease” (see Fig. 1).
A total of 40 patients underwent surgery (lobectomy in 28 cases (62.2%), pneumectomy was performed in nine cases (20.0%), segment resection in two cases (4.4%), and bilobectomy in one case (2.2%)). Sixteen of the study subjects (35.6%) required radiotherapy, a total of 17 (37.8%) received chemotherapy.
Inclusion and exclusion criteria
Inclusion criteria were histologically confirmed diagnosis, a Karnofsky index of at least 50% and the ability to participate regularly in standardised aerobic interval training. Exclusion criteria were metastatic disease, secondary carcinoma, prior palliative treatment, general physical debility, and major communication problems.
Training programme
The training programme involved heart rate-adapted (maximum heart rate of 180 beats per minute minus individual age of each patient, furthermore, minus ten beats if patients were using beta-blockers or other heart rate-lowering preparations) sub-maximal bicycle ergometer exercise in interval times of 3–5 min for a period of 30 min daily. Interval training was complemented by other oncological rehabilitation measures (see introduction).
Functional status
All parameters described below were registered prospectively at the beginning (t1) and at the end (t2) of the rehabilitation programme. Physical exercise capacity was determined by means of bicycle ergometry according to Löllgen et al. [5] on the basis of the measurement of lactate concentrations. Ergometry (Cardivall 300 ergometer) was started at a work load of 25 W, which was increased by further 25 W intervals every 2 min. Rotational speed was constant within a range of 60–70 rpm. Lactate concentrations were measured at rest, every 2 min after each exercise stage reached and 2 and 10 min after exercise.
The procedure described by Guyatt [6] was used for the 6-minute walk test. Patients were instructed to walk up and down a 20-metre corridor at a speed chosen by themselves (but at a sub-maximal speed, i.e., without running) and to cover the maximum possible distance in a period of 6 min. The same investigator gave exactly the same instructions each time on how the walk test was to be undertaken in order to ensure that baseline conditions were as identical as possible for each test. Every 30 s, the patients were given encouragement by the instructor, who would use phrases such as “you’re doing well” or “carry on” [7].
Patients were then instructed to lay down for 20 min before their heart rate was measured at rest. On completion of this resting phase, heart rate at rest was recorded using S810i heart rate monitor (Polar Electro®) with a Polar T61® chest belt over a period of 12 min, and the mean rate was calculated. Some study subjects had been prescribed a beta-blocker or digitalis for use in the period between the point of time of measurement of heart rate at rest on admission and at the time of discharge. It is well-known that beta-blockers and cardiac glycosides reduce heart-rate at rest and maximum reachable heart-rate under exercise. As a consequence of this, the heart rate measured on admission was reduced by 10 beats per minute in order to get a valid comparison between heart rate on admission and discharge in these cases.
Body plethysmography (Optiplex GL 5100) was used to determine pulmonary function. Forced expiratory volume in 1 s (FEV1) and, as an indicator of static respiratory volume, forced vital capacity (FVC) were evaluated. All the results for body plethysmography were registered as percentages of reference values. Patients were instructed not to use bronchodilatory preparations, such as β2-sympathomimetics within 4 h prior to the pulmonary function test.
Clinical evaluation and quality of life
Quality of life was determined using the EORTC QLQ-C30 core questionnaire (version 3.0), together with the lung cancer-specific supplementary module QLQ-LC13 [8]. Health survey, SF-36, was used to determine health-related quality of life. Quantification of fatigue was performed with the MFI-20 questionnaire. In addition, patient status was clinically evaluated by the physician on the basis of the Karnofsky index and WHO performance score.
Heart rate variability
Heart rate variability (HRV) is the variation in the interval from beat to beat measured over shorter or longer time periods (minutes to up to 24 h) as a measure of the vegetative functioning of the heart. These variations in time intervals between individual beats are measured in milliseconds (time domain), and the results are processed statistically. For the first time in cancer patients, we also measured heart rate variability in addition to the standard functional parameters. To quantify HRV, the parameter root mean square of successive differences (RMSSD) in milliseconds was used (i.e. the root mean square of the total of differences between the squares of successive normal intervals between heart beats). This is considered to be an objective vagal indicator and is proportional to parasympathetic activity. An increase in RMSSD, rather like a reduction in heart rate at rest, represents an improvement in physical status [9, 10]. In order to determine HRV, the S810i heart rate monitor (Polar Electro®) and the Polar T61® chest belt were again used. Löllgen & Mück-Weymann were able to demonstrate that all parameters of heart rate variability measured with the Polar® S810i correlate very closely (p < 0.001 and r = 0.996) to values registered using the standard PowerLab® system. [11].
Statistics
To evaluate significant differences between parameters at the beginning (t1) and at the end (t2) of the rehabilitation programme, the Wilcoxon’s test was used. Significance was reached if p < 0.05. All calculations were made with the Statistical Software Package 13. The standard error of the mean (SEM) is mentioned in all tables and figures.
Results
Functional status
It was demonstrated that the exercise capacity and functional status of patients markedly improved during the rehabilitation programme. There was a significant increase in bicycle ergometer performance from 68 ± 3 to 86 ± 4 W (+27%, p < 0.001), and in the distance covered in the 6-minute walk test increased from 322 ± 11 to 385 ± 13 m (+20%, p < 0.001). Analysis of heart rate variability showed that RMSSD significantly increased from 9.7 ± 1 to 12.9 ± 1 ms (+33%, p < 0.001) during the rehabilitation measures. This development provides evidence of an improvement in physical status. Correspondingly, there was a reduction in heart rate at rest from an initial 84 ± 2 to 80 ± 1 beats per minute (p < 0.05). With regard to pulmonary function, there was a statistically significant but clinically non-relevant increase in FEV1 from 70 ± 3% to 73 ± 3% (p < 0.001) and in FVC from 77 ± 3% to 82 ± 3% (p < 0.001). There was also an improvement over the baseline Karnofsky index of 71 ± 1 to 81 ± 1% (p < 0.001). There was an analogous reduction of 0.5 (p < 0.001) in the WHO performance status score (see Table 1). All figures quoted are mean values ± SEM.
Quality of life
In addition to the improvements in physical functional capacity, there was a considerable and generally, also significant improvement in the quality of life. All functional scales, global quality of life and specific symptom scales of the QLQ-C30 survey showed significantly improved scores at discharge compared with those recorded on admission. A review of the results of the QLQ-LC13 survey shows that all symptom scores were lower at the time of discharge compared with those recorded at the time of admission, indicating an improvement in status. There were statistically significant differences for the parameters “dyspnoea”, “coughing”, and “pain”, which represent the central problems experienced by patients with lung cancer (see Table 2). The results for SF-36 corroborate, in general, the results for the below surveys.
Fatigue
There were also significant reductions in the intensity of fatigue. As the diagram shows, the results for all five scales were significantly lower at time of discharge compared with those recorded at the time of admission. Lower results represent reduced fatigue (see Fig. 2).
Discussion
It has been repeatedly reported in the literature that endurance sports can be performed effectively during oncological therapy, even by patients receiving high-dose of chemotherapy (e.g., in connection with bone marrow transplantation) [12, 13]. An increase in physical performance capacity and emotional stabilisation of cancer patients has been reported as a result of participation in an aerobic training programme comparable to that described in this study [14]. In patients who had undergone lung surgery (N = 27), another research group, has demonstrated that aerobic endurance and relaxation training can have positive effects on fatigue and physical exercise capacity in cancer patients [15]. Heart rate at rest was used as the main indicator for performance capacity. This parameter, which is gradually reduced over the course of training, is one measure for training effect [16].
However, it has not previously been shown whether there is any benefit to multi-morbid patients with lung cancer who participate in complex interdisciplinary rehabilitation programmes after surgery and/or radiotherapy and/or chemotherapy. To date, only Schultz, Bergmann, and Lang et al. [17] have identified and analysed physiological and quality of life-related parameters in 207 patients during a rehabilitation programme (2006). Their investigation included a sub-group of 24 patients with lung cancer. As there was no a priori estimate of the sample size required, it was not possible to demonstrate a statistically significant difference in this specific group in terms of a pre- and post comparison (level 4 evidence).
Other investigations focused on rehabilitation programmes used for groups of specially selected (surgical) patients in a hospital [18], summarised subjectively selected results of pneumological rehabilitation programmes without considering end points or quality criteria [19], or concentrated on particular time points, such as the time during radiotherapy, to verify the effects of measures, such as information and relaxation techniques, on the “activity” of patients [20–22].
With the results of this study, we can now demonstrate that elderly and frequent multi-morbid lung cancer patients after primary therapy, who often also suffer from secondary problems such as chronic obstructive pulmonary disease, coronary heart disease, and/or peripheral vascular disease, can benefit from an in-house rehabilitation programme. This is shown by the increase in physical exercise capacity during the course of the rehabilitation programme, which is objectively substantiated by significant reduction in heart rate at rest. In addition, heart rate variability as an objective quantifier of physical performance in cardiological function and sport medicine (an indicator of neurovegetative activity and the autonomous functioning of the heart [9], see Patients and methods) increased significantly to verify increased endurance capacity. Physical training, thus, improves physical performance capacity and increases consequently parasympathetic activity. This results not only in a reduced heart rate at rest, but also in an increase in heart rate variability [10]. Enhancement in physical status also improves quality of life (QLQ-C30, LC13, and SF-36) and similarly reduces fatigue (MFI-20).
Probably the aerobic training programme was of considerable value as, although, it was only one aspect of the rehabilitation programme as a whole, it was specifically tailored to the needs of lung surgery patients. It is very likely that aspects such as psychological counselling and training relaxation techniques had a positive effect on quality of life and fatigue, but in the context of our study we are unable to determine this effect. In order to investigate this question, we are currently conducting a further study in which the quality of life and physical performance capacity of patients who have not participated in a rehabilitation programme are compared with those of the patients who participated in our study.
References
Nazarian J (2004) Cardiopulmonary rehabiliation after treatment for lung cancer. Curr Treatm Opt Oncol 5:75–82
Spruit MA, Janssen PP, Willemsen SCP et al (2006) Exercise capacity before and after an 8-week multidisciplinary inpatient rehabilitation program in lung cancer patients: a pilot study. Lung Cancer 52:257–260
Thompson E, Sola I, Subirana M (2005) Non-invasive interventions for improving well-being and quality of life in patients with lung cancer—A systematic review of the evidence. Lung Cancer 50:163–176
Sola I, Thompson E, Subirana M et al (2004) Non-invasive interventions for improving well-being and quality of life in patients with lung cancer. Cochrane Database Syst Rev: CD004282
Löllgen H, Goedel-Meinen L, Webering F et al (2000) Vorschlag: Richtlinien für das Belastungs-EKG. Medizin im Dialog 4:19
Guyatt GH, Sullivan MJ, Thompson PJ et al (1985) The 6-minute-walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J 132(8):919–923
Guyatt GH, Pugsley SO, Sullivan MJ et al (1984) Effect of encouragement on walking test performance. Thorax 39:818–822
Aaronsen NK, Ahmedzai S, Bergman B et al (1993) The European organisation for research and treatment of cancer QLQ-C30: a quality-of-life instrument for use in international clinical studies in oncology. Journal of the National Cancer Institute 85:365–376
Löllgen H (1999) Herzfrequenzvariabilität. Neue Methoden in der kardialen Funktionsdiagnostik. Dt Ärztebl 96:2029–2032
Mück-Weymann M (2003) Körperliche und seelische Fitness im Spiegel der Herzfrequenzvariabilität. Hans Jacobs Verlag, Lage
Löllgen D, Jung K, Mück-Weymann M (2004) Herzratenvariabilität (HRV) im Sport – Methodische Überlegungen zur vergleichenden Messung mittels Polar® S810 und Standardmethoden der Medizin. In: Hottenrott K (ed) Herzfrequenzvariabilität im Fitness- und Gesundheitssport. Czwalina Verlag, Hamburg, pp 121–135
Dimeo FC, Tilmann MH, Bertz H et al (1997) Aerobic exercise in the rehabilitation of cancer patients after high dose chemotherapy and autologous peripheral stemm cell transplantation. Cancer 79:1717–1722
Dimeo F, Stieglitz RD, Novelli-Fischer U et al (1999) Effects of physical activity on the fatigue and psychologic status of cancer patients during chemotherapy. Cancer 85:2273–2277
Dimeo F, Rumberger BG, Keul J (1998) Aerobic exercise a therapy for cancer fatigue. Med Sci Sport Exercise 30(4):475–478
Dimeo F, Thomas F, Raabe-Menssen C et al (2004) Effect of aerobic exercise and relaxation training on fatigue and physical performance of cancer patients after surgery. A randomised controlled train. Support Care Cancer 12(11):774–779
Dimeo F, Thiel E, Böning D (1999) Körperliche Aktivität in der Rehabilitation von onkologischen Patienten. Die Rolle des aeroben Trainings. Dt Ärztebl 96:1340–1345
Schultz K, Bergmann KC, Kenn K et al (2006) Effektivität der pneumologischen Anschluss-Rehabilitation (AHB). Dtsch Med Wochenschr 131:1793–1798
Wilson DJ (1997) Pulmonary rehabilitation exercise program for high-risk thoracic surgical patients. Chest Surg Clin N Am 7(4):697–706
Bozzone A, Romanelli A, Magrone G et al (2004) Pulmonary rehabilitation: pre- and postoperative treatment. Rays 29(4):431–433
Christman NJ, Cain LB (2004) The effects of concrete objective information and relaxation on maintaining usual activity during radiation therapy. Oncol Nurs Forum 31(2):39–45
Doorenbos A, Given B, Given C et al (2006) Physical functioning: effect of behavioural intervention for symptoms among individuals with cancer. Nurs Res 55(3):161–171
Wall LM (2000) Changes in hope and power in lung cancer patients who exercise. Nurs 13(3):234–242
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Riesenberg, H., Lübbe, A.S. In-patient rehabilitation of lung cancer patients—a prospective study. Support Care Cancer 18, 877–882 (2010). https://doi.org/10.1007/s00520-009-0727-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-009-0727-y