Abstract
Purpose
Lung cancer survivors are at risk for health impairments resulting from the effects and/or treatment of lung cancer and comorbidities. Practical exercise capacity (EC) assessments can help identify impairments that would otherwise remain undetected. In this study, we characterized and analyzed the association between functional EC and cancer-specific quality of life (QoL) in lung cancer survivors who previously completed curative intent treatment.
Methods
In a cross-sectional study of 62 lung cancer survivors who completed treatment ≥ 1 month previously, we assessed functional EC with the 6-min walk distance (6MWD) and cancer-specific QoL with the European Organization for Research and Treatment of Cancer QoL Questionnaire Core 30 (EORTC-QLQ-C30). Cancer-specific QoL was defined using a validated composite EORTC-QLQ-C30 summary score. Univariable (UVA) and multivariable linear regression analyses (MVA) were performed to assess the relationship between functional EC and cancer-specific QoL.
Results
Lung cancer survivors had reduced functional EC (mean 6MWD = 335 m, 65% predicted) and QoL (mean EORTC-QLQ-C30 summary score = 77, scale range 0–100). In UVA, 6MWD was significantly associated with cancer-specific QoL (R2 = 0.16, p = 0.001). In MVA, in a final model that also included heart failure, obstructive sleep apnea, and psychiatric illness, 6MWD was independently associated with cancer-specific QoL (partial R2 = 0.20, p = 0.001).
Conclusions
Functional EC was independently associated with cancer-specific QoL in lung cancer patients postcurative intent treatment. Exercise-based interventions aimed at improving EC may improve cancer-specific QoL in these patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Up to 50% of nonsmall cell lung cancer (NSCLC) cases present at stage I–IIIA [1], the treatment of which involves a combination of modalities including surgical resection, ablative therapy, and chemoradiotherapy aimed at achieving a cure. As of 2016, there were more than 526,000 lung cancer survivors in the US [2].
Lung cancer survivors are at risk for cardiopulmonary impairments resulting from the effects and/or treatment of lung cancer and comorbidities. Perioperative pulmonary [3] and cardiopulmonary [4] complications have been reported in 15 and 35%, respectively, of patients undergoing lung cancer resection surgery and can result in negative health consequences well beyond the perioperative period (e.g., atrial arrhythmias, prolonged respiratory failure/intensive care unit stay). At 6 months following surgery, a loss of forced expiratory volume in 1 s (FEV1) of 10–15% for lobectomy and 30–35% for pneumonectomy [5] is expected. Chemotherapy and radiotherapy, often part of the treatment for stage IB–IIIA lung cancer, can also lead to long-term cardiopulmonary impairments (e.g., cardiomyopathy, cardiac conduction disturbances, coronary artery disease, valvular disease, pneumonitis, pulmonary fibrosis) [6]. In those undergoing definitive external-beam radiation, it is common to develop some degree of focal pulmonary fibrosis, and a minority will subsequently develop progressive pulmonary fibrosis, cor pulmonale, and respiratory failure [6]. In the peripheral vascular and musculoskeletal systems, altered blood flow response to exercise [7], and decreased skeletal microvascular function [8] have been recently described in cancer survivors treated with adjuvant therapy.
Lung cancer patients can also have major comorbidities that limit health. In a large cohort study of 5683 lung cancer patients, the most common comorbidities included chronic obstructive pulmonary disease (COPD, in 53% of patients), diabetes (16%), and congestive heart failure (13%) [9]. In time, partly due to the long-term effects of lung cancer treatment and comorbidities, patients experience disabling symptoms, which in turn can lead to a downward spiral of health. Dyspnea and fatigue were reported to be worse compared to baseline in 40–50% of lung cancer survivors at 2 years postresection surgery [10]. Long-term respiratory symptoms are highly prevalent and can be present in up to 60–70% of patients at ≥ 5 years [11]. These symptoms have been shown to limit generic quality of life (QoL) [11], which can be more important than the duration of survival for some patients. According to a survey of 660 lung cancer patients, health issues that are deemed important or very important include QoL, maintaining independence, ability to perform normal activities, ability to sleep, and not being fatigued [12].
It is important to characterize health limitations and to identify potential therapeutic options in lung cancer survivors. Practical clinical tools to assess and identify these health limitations are currently lacking. Functional exercise testing offers an opportunity to measure objectively patients’ exercise capacities and identify exercise limitations that would otherwise remain undetected. In lung cancer, exercise testing is used most often to risk-stratify patients undergoing evaluation for lung cancer resection [13,14,15]. In recent years, its use outside this context has been described, including in nonsurgical candidates [16, 17] and lung cancer survivors [18]. In this study, we characterized functional exercise capacity (EC) in lung cancer survivors who have received curative-intent treatment and analyzed the relationship between functional EC and cancer-specific QoL. We hypothesize that functional EC is an important, independent predictor of cancer-specific QoL.
Methods
Study overview
We performed a cross-sectional study of patients who completed curative-intent treatment of stage I–IIIA lung cancer (i.e., anatomic lung cancer resection surgery, ablative therapy, or concurrent chemoradiation) ≥ 1 month previously. Eligible participants were identified from a database of consecutive lung cancer patients diagnosed and managed at the VA San Diego Healthcare System (VASDHS), maintained since 2010 to shorten time to diagnosis and improve the quality of care. We allowed at least a 1-month period for recovery following any acute health decrements associated with treatment [19]. Between July 2016 and July 2017, we mailed informational letters to potential candidates identified from October 2010 to July 2017 and followed-up with a telephone call approximately 1 week later to gauge their interest. All exercise testing and patient-reported outcome (PRO) assessments were conducted in person by one observer (DH). We obtained written informed consent from each participant. The protocol was approved by the VASDHS Institutional Review Board (no. H150158). We followed standard guidelines [20] to report the findings of our study.
Participants
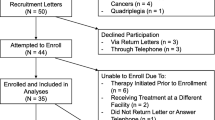
We included participants (Fig. 1) over 18 years of age and collected available baseline clinical characteristics and potential confounders related to cardiopulmonary/physical health and QoL through electronic chart review including age, gender, body mass index, tobacco exposure, comorbidities [e.g., COPD, heart failure (HF), psychiatric illnesses (anxiety, depression, posttraumatic stress disorder)], lung function [FEV1, total lung capacity (TLC), diffusion capacity of the lung for carbon monoxide (DLCO)], and echocardiography (ejection fraction, diastolic dysfunction, valvular disease). We confirmed the accuracy of data collected using available documentation from clinical specialists where applicable. Lung cancer-related information included clinical stage as defined by the American Joint Committee on Cancer TNM staging system (7th and 8th editions) and the primary curative-intent mode of treatment (surgical resection, ablative therapy, chemoradiotherapy).
Exercise testing
Based on our previous review of the utility of exercise testing in patients with lung cancer [18], we chose the 6MWT based on practical considerations (availability and ease of performance), the likelihood that daily activities are performed at submaximal exercise levels, and previous validation in cancer [21], including lung cancer [22] clinical populations. We performed the 6MWT according to the standard protocol at the VASDHS following the American Thoracic Society (ATS) Pulmonary Function Standards Committee recommendations [23] using a ~ 130-ft (~ 40-m) hallway with a flat and hard surface marked with alternating-colored tiles; a finger-probe pulse oximeter was used to obtain oxygen saturation and heart rate before and at the end of the 6MWT. Patients requiring oxygen supplementation used their own equipment at the same flow rate as their regular prescription. No practice test was conducted, as per ATS recommendations in most clinical settings [23].
Patient-reported outcome assessments
We chose the European Organization for Research and Treatment of Cancer QoL Questionnaire Core 30 (EORTC-QLQ-C30) [24] instrument based on its availability and inclusion of core domains of QoL and other subdomains of health relevant to lung cancer survivors (e.g., dyspnea, fatigue, insomnia). Our primary endpoint was a validated composite score of cancer-specific QoL as defined by the EORTC-QLQ-C30 summary score [25]. We also performed exploratory PRO assessments for lung cancer-specific symptoms, generic health, sleep quality, dyspnea, fatigue, and anxiety/depression using the EORTC-QLQ-Lung Cancer Module 13 (LC13) [26], EuroQoL-5 Dimensions/visual analogue scale (EQ-5D/VAS) [27], Pittsburgh Sleep Quality Index (PSQI) [28], University California San Diego Shortness of Breath Questionnaire (UCSD SOBQ) [29], Brief Fatigue Inventory (BFI) [30], and Hospital Anxiety and Depression Scale (HADS) [31] questionnaires, respectively. All questionnaires were self-administered on printed forms without modifications, scored per their respective instruction manuals, and analyzed as continuous variables.
Statistical analyses
Descriptive statistics were summarized as means and standard deviations or medians and ranges for all continuous variables and as counts and percentages for all categorical variables. The 6MWD was recorded and analyzed as a continuous variable, and interpreted using the reference equations for the 6MWT in healthy adults [32]. Correlation coefficients were obtained using Pearson’s r and Spearman’s ρ for variables with parametric and nonparametric distributions, respectively. Univariable linear regression analyses (UVAs) were performed to assess the relationship between baseline characteristics including functional EC as reflected by the 6MWD and cancer-specific QoL as reflected by the EORTC-QLQ-C30 summary score. Multivariable linear regression analyses (MVAs) were performed using stepwise backward selection modeling of all baseline characteristics with p < 0.15. Regression coefficients, 95% confidence intervals (CIs), and coefficients of determination (R2 and partial R2) were used to interpret the association between dependent and independent variables. Additional analyses were performed to assess the relationship between the 6MWD and the functional subscales of the EORTC-QLQ-C30 questionnaire (using p value cutoff < 0.01 to account for multiple comparison), as well as baseline clinical characteristics associated with the 6MWD. One-way analyses of variance (ANOVA) with Bonferroni post hoc analyses were performed to assess the differences in 6MWD and cancer-specific QoL between the three most common curative-intent treatment modalities. Exploratory UVAs were performed to assess the relationship between functional EC and other PROs, corrected for multiple comparisons by multiplying the p values for each comparison by the total number of comparisons. All tests were two-tailed. Statistical significance was defined as p < 0.05. All data were entered and managed using REDCap electronic data capture tools hosted at the University of California San Diego (UCSD) Clinical and Translational Research Institute [33]. IBM® SPSS® Statistics software version 23.0 was used for all analyses.
Results
We mailed informational letters to 71 eligible patients, 9 of whom declined participation (Fig. 1). There was no significant difference in baseline characteristics between those who participated and those who declined (E-Table 1). Most of the 62 participants had a history of tobacco exposure (58 patients, 94%), clinical stage I–II disease (51 patients, 82%), and lobectomy or stereotactic body radiotherapy as the primary modality for curative-intent treatment (50 patients, 81%). The median time from completion of treatment was 19 months (interquartile range 4–44) (Table 1).
All participants completed the 6MWT and PRO assessments. The overall mean 6MWD was low (335 m, 65% predicted) and most patients (35, 57%) had impaired functional EC (Table 2). Sixteen patients (26%) stopped or paused during the 6MWT due to symptom limitation (7 due to pain, 6 dyspnea, 2 fatigue, and 1 imbalance). The cancer-specific QoL as assessed by the mean EORTC-QLQ-C30 summary score was 77 (range 26 to 99 on a scale of 0 to 100). The most common abnormal subscales, defined as raw symptom score > mean reference value [34], was pain (33 patients, 53%) and the least common was nausea/vomiting (13 patients, 21%). More than half of patients had abnormal dyspnea (36, 58%), pain in arms or shoulders (33, 53%), and pain in other parts (36, 58%) as assessed by the EORTC-QLQ-LC13 (Table 3), and sleep quality (Table 4).
The 6MWD m correlated moderately well with the cancer-specific QoL summary score (correlation coefficient = 0.45, p < 0.001). In UVAs (Table 5), in addition to functional EC (Fig. 2a), HF, obstructive sleep apnea (OSA), psychiatric illness, DLCO % predicted, and surgical treatment were also significantly associated with cancer-specific QoL. In MVAs (Table 6) starting with all baseline clinical characteristics with p < 0.15 from UVAs, the 6MWD was independently associated with cancer-specific QoL (partial R2 = 0.20, p = 0.001).
Scatter plots of functional EC and cancer-specific QoL and selected functional subscales. Legend: scatter plots showing correlations between functional EC (6MWD) with a cancer-specific QoL (EORTC-QLQ-C30 Summary Score), b physical function (EORTC-QLQ-C30 Physical Function), and c social function (EORTC-QLQ-C30 Social Function) subscales. 6MWD 6-min walk distance, EC exercise capacity, EORTC-QLQ-C30 European Organization for Research and Treatment of Cancer QoL Questionnaire Core 30, QoL quality of life
Additional analyses of the functional subscales showed that in UVAs the 6MWD was associated with the physical function (R2 = 0.44, p < 0.001) and social function (R2 = 0.18, p = 0.001) domains of cancer-specific QoL (Fig. 2b, c). In MVAs, the 6MWD was an independent predictor of the physical function (partial R2 = 0.45, p < 0.001) and social function (partial R2 = 0.17, p = 0.001) domains of cancer-specific QoL (Tables 7 and 8). Psychiatric illness was also found in MVAs to be independently associated with cancer-specific QoL and the physical and social function domains of cancer-specific QoL (Tables 6, 7, and 8).
Baseline clinical characteristics significantly associated with the 6MWD in UVAs included age (R2 = 0.09, p = 0.02), hyperlipidemia (R2 = 0.08, p = 0.03), DLCO % predicted (R2 = 0.15, p = 0.002), and surgical treatment (R2 = 0.16, p = 0.001). In MVAs, in a model (overall R2 = 0.38, p < 0.001) that also contained surgical treatment, age (partial R2 = 0.12, p = 0.007), hyperlipidemia (partial R2 = 0.07, p = 0.04), and DLCO % predicted (partial R2 = 0.16, p = 0.002) were significantly associated with the 6MWD. In one-way ANOVAs, there were significant differences in the 6MWD (p = 0.003) and cancer-specific QoL (p = 0.02) between the three most common curative-intent treatment modalities (lobectomy, SBRT, chemoradiation). In post hoc analyses, there was a significant difference in 6MWD and cancer-specific QoL in the lobectomy compared to SBRT groups (+ 118 m, p = 0.002 and + 14, p = 0.02, respectively), but not between lobectomy compared to chemoradiation (+ 85 m, p = 0.19, and + 3.4, p = 1.0) or SBRT compared to chemoradiation (− 33 m, p = 1.0, and − 10, p = 0.39).
In exploratory assessments using other PROs, more than half of patients had abnormal scores on the EQ-VAS (43 patients, 69%) and PSQI (45, 73%) (Table 4). Exploratory UVAs showed significant associations (with correction for seven comparisons) between the 6MWD and the EQ-5D index (R2 = 0.12, p = 0.04), EQ-VAS (R2 = 0.15, p = 0.01), and UCSD SOBQ (R2 = 0.14, p = 0.02), but not PSQI, BFI, or HADS.
Discussion
In a cross-sectional study of lung cancer survivors who previously completed curative-intent treatment, approximately 60% had functional exercise limitation. Overall, functional EC accounted for 20% of the variance in cancer-specific QoL.
Exercise capacity evaluation in lung cancer is most commonly performed to risk-stratify patients being considered for anatomic lung cancer resection surgery [13, 14]. We previously reviewed the utility of exercise testing outside of the preoperative evaluation context, including in postresection lung cancer survivors [18], and identified that the 6MWT, cardiopulmonary exercise test (CPET), and stair-climb test have been used in this group of patients. In the largest study involving the 6MWT, Deslauriers and coworkers [36] assessed functional EC in 100 lung cancer patients at least 5 years postpneumonectomy and found that the 6MWD was 83% of the predicted values in these patients; only 19 of 91 patients (10%) had lower than expected 6MWD. Since our review, Cavalheri and coworkers [37] assessed EC using the 6MWT and CPET in a cross-sectional study of lung cancer survivors who completed curative-intent treatment 4–10 weeks previously and found that, compared to age-and gender-matched healthy controls, there were statistically significant differences in ECs as reflected by the 6MWD and VO2peak. Ten of 22 patients (45%) had 6MWD below the lower limit of normal (LLN) and 15 of 21 patients (71%) had VO2peak below the LLN.
In contrast to most studies to date involving EC evaluation in postcurative intent treated lung cancer patients, where the primary interest lies in characterizing the differences in EC associated with treatment, our study is a cross-sectional study highlighting the prevalence of exercise limitation in these patients. Similar to the study by Cavalheri and coworkers [37], our study reports a prevalence of exercise limitation of at least 50% in a sample size that contains almost three times the number of patients. In the study by Deslauriers and coworkers [36], only 10% of postpneumonectomy patients had impaired functional EC as reflected by the 6MWD. However, one must be cautious in comparing results from these previous studies with ours due to differences in patient selection (postcombined modality vs. postpneumonectomy) and time elapsed since treatment (weeks/months vs. years). In our study, time since completion of treatment was not significantly associated with functional EC or cancer-specific QoL, possibly due to a small sample size or adequate health recovery after a minimum of one month following completion of treatment. In MVAs, the primary curative-intent mode of treatment was not significantly associated with functional EC or cancer-specific QoL, possibly due to nonrandom treatment selection or small sample size.
Similar to that reported in the systematic review by the European Respiratory Society/ATS [38] on the measurement properties of field walking tests in chronic respiratory disease, the 6MWD was moderately correlated (correlation coefficient 0.31 to 0.70) with the PROs included in our study. To the best of our knowledge, our study is the first to analyze the relationship between the 6MWD and cancer-specific QoL using the novel and validated composite EORTC-QLQ-C30 summary score [25]. Additional analyses demonstrate that similar to a previous study involving 56 patients with stage I–IV lung cancer [39], the 6MWD was significantly associated with the physical function domain of the EORTC-QLQ-C30. These results contrast with another study involving 20 patients with stage I-IIIB NSCLC [40] which showed no significant association between the 6MWD and the physical function domain of the EORTC-QLQ-C30. This difference in results may well be due to the small sample size (20 patients) included in that study.
Many of the patients included in our study had comorbidities including COPD and HF that also could limit cardiopulmonary health and EC. Our additional analyses did not demonstrate significant associations between these comorbidities and the 6MWD, suggesting that untreated/unoptimized cardiopulmonary disease was not prevalent in these patients. Interventions to improve functional EC in these patients, therefore, could possibly improve cancer-specific QoL without titration of medications (e.g., inhalers, diuretics) to optimize cardiopulmonary health. These findings should not lessen the importance of medically optimizing these comorbidities in the clinical setting. The lack of association between comorbidities and the 6MWD may also be due to a small sample size. The significant associations between psychiatric illness and cancer-specific QoL and physical function highlight the importance of the management of concomitant psychiatric disorders in lung cancer patients. Our exploratory analyses also highlight the important associations between dyspnea and fatigue with functional EC and could serve as secondary endpoints in future studies aimed at improving EC in lung cancer survivors.
Lung cancer is the second-most commonly diagnosed cancer in the US [41]. Historically, the majority of lung cancer cases are diagnosed at an advanced stage when curative-intent treatment is not possible. However, there is an expected increase in the number of cases of lung cancer diagnosed at an earlier stage due to the findings of the National Lung Screening Trial [42] and practice guideline recommendations supporting the role of low-dose computed tomography (LDCT) screening for lung cancer in high-risk patients. A recent analysis shows that LDCT can lead to more early-stage lung cancers being detected [43]. The American Cancer Society projects that by 2026, more than 673,000 lung cancer survivors will be living in the US [2]. Current evidence supports the utility of physical activity and exercise in improving health in posttreatment cancer survivors [44], though the evidence is not as consistent in the lung cancer population [45]. This may be due to factors such as differences in comorbidities and treatment-related effects in lung cancer compared with other cancer populations, variations in study design (e.g., patient selection, type of physical activity, intensity, duration), measured outcomes, and sample sizes. Our study describes exercise limitations and highlights the importance of EC evaluation for curative-intent treated lung cancer survivors. The mean 6MWD of the patients enrolled in our study is similar to a cohort [46] of patients undergoing pulmonary rehabilitation. These findings may present an opportunity for healthcare providers and systems to intervene to improve health and QoL through exercise-based interventions in these patients.
Our study has several strengths. First, all exercise testing and PRO assessments were conducted in person by one observer which optimized the quality and consistency of the data obtained. Second, we analyzed data using a prespecified validated exposure (functional EC) [21, 22] and outcome (cancer-specific QoL) [25] to minimize chance bias. Third, a comprehensive list of baseline characteristics was included in the data collected, much of which (e.g., COPD, HF, OSA, and psychiatric illness) was confirmed for accuracy using available lung function test results, echocardiography reports, and clinical documentation from sleep and psychiatric specialists. Finally, a combination of prespecified, additional, and exploratory UVAs and MVAs enabled us to interpret results which can facilitate future studies and/or clinical practice.
Our study also has some limitations. First, we did not assess other components of cardiopulmonary/physical fitness such as VO2peak and muscle strength which may also be improved with exercise training and contribute significantly to cancer-specific QoL. Second, the cross-sectional design limits our ability to draw conclusions about temporal relationships; it is possible that poor cancer-QoL led to functional EC limitations in some patients and not vice versa. In addition, it is not possible to determine whether the functional EC and cancer-specific QoL limitations were preexisting or related to lung cancer or its treatment. Third, the small sample size may limit our study’s power to detect associations between important predictors of functional EC and cancer-specific QoL. Finally, the findings may not be generalizable due to selection bias towards survivors and the high prevalence of comorbidities in a predominantly male veteran patient population with early stage lung cancer recruited from a single VA Health System center.
Important future work on the role of physical activity/exercise in lung cancer survivors may include assessment of barriers and facilitators of exercise, development, and implementation of effective exercise programs to improve physical activity and exercise capacity, patient-reported outcomes and clinical outcomes, and their cost-effectiveness analyses. Investigations of the physiobiological changes associated with exercise in these patients may be equally important. In newly diagnosed lung cancer patients undergoing curative-intent therapy, exercise may also have role in cancer rehabilitation to decrease treatment related morbidity, increase cancer treatment options, and improve physical and psychological health outcomes [47].
We conclude that in a cross-sectional study of lung cancer patients postcurative intent treatment, impaired functional EC was prevalent in > 50% of patients, and functional EC was independently associated with cancer-specific QoL. Exercise-based interventions aimed at improving functional EC may improve cancer-specific QoL in these patients.
Abbreviations
- 6MWD:
-
Six-minute walk distance
- 6MWT:
-
Six-minute walk test
- ATS:
-
American Thoracic Society
- BFI:
-
Brief Fatigue Inventory
- CI:
-
Confidence interval
- CPET:
-
Cardiopulmonary exercise test
- COPD:
-
Chronic obstructive pulmonary disease
- DLCO :
-
Diffusion capacity of the lung for carbon monoxide
- EC:
-
Exercise capacity
- EORTC-QLQ-C30:
-
European Organization for Research and Treatment of Cancer QoL Questionnaire Core 30
- EORTC-QLQ-LC13:
-
European Organization for Research and Treatment of Cancer QoL Questionnaire Lung Cancer Module 13
- EQ-5D/VAS:
-
EuroQoL-5 Dimensions/visual analogue scale
- FEV1 :
-
Forced expiratory volume in 1 s
- HADS:
-
Hospital Anxiety and Depression Scale
- HF:
-
Heart failure
- LDCT:
-
Low-dose computed tomography
- LLN:
-
Lower limit of normal
- MVA:
-
Multivariable linear regression analysis
- NSCLC:
-
Nonsmall cell lung cancer
- OSA:
-
Obstructive sleep apnea
- PRO:
-
Patient-reported outcome
- PSQI:
-
Pittsburgh Sleep Quality Index
- QoL:
-
Quality of life
- TLC:
-
Total lung capacity
- SBRT:
-
Stereotactic body radiotherapy
- TNM:
-
Tumor node metastasis
- UCSD:
-
University of California San Diego
- UCSD SOBQ:
-
University California San Diego Shortness of Breath Questionnaire
- US:
-
United States
- UVA:
-
Univariable linear regression analysis
- VASDHS:
-
VA San Diego Healthcare System
- VO2peak :
-
Peak oxygen consumption
References
Dinan MA, Curtis LH, Carpenter WR, Biddle AK, Abernethy AP, Patz EF Jr, Schulman KA, Weinberger M (2012) Stage migration, selection bias, and survival associated with the adoption of positron emission tomography among medicare beneficiaries with non-small-cell lung cancer, 1998-2003. J Clin Oncol 30:2725–2730
American Cancer Society Cancer treatment and survivorship, facts and figures 2016-2017. (Last Updated: 2016)
Agostini P, Cieslik H, Rathinam S, Bishay E, Kalkat MS, Rajesh PB, Steyn RS, Singh S, Naidu B (2010) Postoperative pulmonary complications following thoracic surgery: are there any modifiable risk factors? Thorax 65:815–818
Ha D, Choi H, Zell K, Raymond DP, Stephans K, Wang XF, Videtic G, McCarthy K, Minai OA, Mazzone PJ (2014) Association of impaired heart rate recovery with cardiopulmonary complications after lung cancer resection surgery. J Thorac Cardiovasc Surg 149:1168–1173
Win T, Groves AM, Ritchie AJ, Wells FC, Cafferty F, Laroche CM (2007) The effect of lung resection on pulmonary function and exercise capacity in lung cancer patients. Respir Care 52:720–726
Carver JR, Shapiro CL, Ng A, Jacobs L, Schwartz C, Virgo KS, Hagerty KL, Somerfield MR, Vaughn DJ, ASCO Cancer Survivorship Expert Panel (2007) American Society of Clinical Oncology clinical evidence review on the ongoing care of adult cancer survivors: cardiac and pulmonary late effects. J Clin Oncol 25:3991–4008
Didier KD, Ederer AK, Reiter LK, Brown M, Hardy R, Caldwell J, Black C, Bemben MG, Ade CJ (2017) Altered blood flow response to small muscle mass exercise in cancer survivors treated with adjuvant therapy. J Am Heart Assoc 6:2. https://doi.org/10.1161/JAHA.116.004784
Ederer AK, Didier KD, Reiter LK, Brown M, Hardy R, Caldwell J, Black CD, Larson RD, Ade CJ (2016) Influence of adjuvant therapy in cancer survivors on endothelial function and skeletal muscle deoxygenation. PLoS One 11:e0147691
Islam KM, Jiang X, Anggondowati T, Lin G, Ganti AK (2015) Comorbidity and Survival in Lung Cancer Patients. Cancer Epidemiol Biomark Prev 24:1079–1085
Kenny PM, King MT, Viney RC, Boyer MJ, Pollicino CA, McLean JM, Fulham MJ, McCaughan BC (2008) Quality of life and survival in the 2 years after surgery for non small-cell lung cancer. J Clin Oncol 26:233–241
Sarna L, Evangelista L, Tashkin D, Padilla G, Holmes C, Brecht ML, Grannis F (2004) Impact of respiratory symptoms and pulmonary function on quality of life of long-term survivors of non-small cell lung cancer. Chest 125:439–445
Gralla RJ, Hollen PJ, Msaouel P, Davis BV, Petersen J (2014) An evidence-based determination of issues affecting quality of life and patient-reported outcomes in lung cancer: results of a survey of 660 patients. J Thorac Oncol 9(1243-1248):22
Brunelli A, Kim AW, Berger KI, Addrizzo-Harris DJ (2013) Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: diagnosis and management of lung cancer, 3rd ed.: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 143:e166S–e190S
Brunelli A, Charloux A, Bolliger CT, Rocco G, Sculier JP, Varela G, Licker M, Ferguson MK, Faivre-Finn C, Huber RM, Clini EM, Win T, De Ruysscher D, Goldman L, European Respiratory Society and European Society of Thoracic Surgeons joint task force on fitness for radical therapy (2009) ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemo-radiotherapy). Eur Respir J 34:17–41
Lim E, Baldwin D, Beckles M, Duffy J, Entwisle J, Faivre-Finn C, Kerr K, Macfie A, McGuigan J, Padley S, Popat S, Screaton N, Snee M, Waller D, Warburton C, Win T, British Thoracic Society, Society for Cardiothoracic Surgery in Great Britain and Ireland (2010) Guidelines on the radical management of patients with lung cancer. Thorax 65(Suppl 3):iii1–ii27
Kasymjanova G, Correa JA, Kreisman H, Dajczman E, Pepe C, Dobson S, Lajeunesse L, Sharma R, Small D (2009) Prognostic value of the six-minute walk in advanced non-small cell lung cancer. J Thorac Oncol 4:602–607
Ha D, Stephens K, Choi H, Zell K, Wang X, Minai O, Raymond D, Videtic G, Mazzone P (2015) Heart rate recovery and survival in patients undergoing stereotactic body radiotherapy for treatment of early-stage lung cancer. JRSBRT 3(3):193–201
Ha D, Mazzone PJ, Ries AL, Malhotra A, Fuster M (2016) The utility of exercise testing in patients with lung cancer. J Thorac Oncol 11:1397–1410
Brunelli A, Xiume F, Refai M, Salati M, Marasco R, Sciarra V, Sabbatini A (2007) Evaluation of expiratory volume, diffusion capacity, and exercise tolerance following major lung resection: a prospective follow-up analysis. Chest 131:141–147
von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, STROBE Initiative (2007) The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 147:573–577
Schmidt K, Vogt L, Thiel C, Jager E, Banzer W (2013) Validity of the six-minute walk test in cancer patients. Int J Sports Med 34:631–636
Nakagawa T, Chiba N, Saito M, Sakaguchi Y, Ishikawa S (2014) Clinical relevance of decreased oxygen saturation during 6-min walk test in preoperative physiologic assessment for lung cancer surgery. Gen Thorac Cardiovasc Surg 62:620–626
ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories (2002) ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 166:111–117
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC (1993) The European Organization for Research and Treatment 23 of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85:365–376
Giesinger JM, Kieffer JM, Fayers PM, Groenvold M, Petersen MA, Scott NW, Sprangers MA, Velikova G, Aaronson NK, EORTC Quality of Life Group (2016) Replication and validation of higher order models demonstrated that a summary score for the EORTC QLQ-C30 is robust. J Clin Epidemiol 69:79–88
Bergman B, Aaronson NK, Ahmedzai S, Kaasa S, Sullivan M (1994) The EORTC QLQ-LC13: a modular supplement to the EORTC Core Quality of Life Questionnaire (QLQ-C30) for use in lung cancer clinical trials. EORTC Study Group on Quality of Life. Eur J Cancer 30A:635–642
Shaw JW, Johnson JA, Coons SJ (2005) US valuation of the EQ-5D health states: development and testing of the D1 valuation model. Med Care 43:203–220
Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ (1989) The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 28:193–213
Eakin EG, Resnikoff PM, Prewitt LM, Ries AL, Kaplan RM (1998) Validation of a new dyspnea measure: the UCSD Shortness of Breath Questionnaire. University of California, San Diego. Chest 113:619–624
Mendoza TR, Wang XS, Cleeland CS, Morrissey M, Johnson BA, Wendt JK, Huber SL (1999) The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer 85:1186–1196
Zigmond AS, Snaith RP (1983) The hospital anxiety and depression scale. Acta Psychiatr Scand 67:361–370
Enright PL, Sherrill DL (1998) Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med 158:1384–1387
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG (2009) Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42:377–381
Scott NW, Fayers PM, Aaronson NK, Bottomley A, de Graeff A, Groenvold M, Gundy C, Koller M, Peterson MA, Sprangers MAG (2008) EORTC QLQ-C30 reference values. EORTC Quality of Life Group
Martinez FJ, Foster G, Curtis JL, Criner G, Weinmann G, Fishman A, MM DC, Benditt J, Sciurba F, Make B, Mohsenifar Z, Diaz P, Hoffman E, Wise R, NETT Research Group (2006) Predictors of mortality in patients with emphysema and severe airflow obstruction. Am J Respir Crit Care Med 173:1326–1334
Deslauriers J, Ugalde P, Miro S, Deslauriers DR, Ferland S, Bergeron S, Lacasse Y, Provencher S (2011) Long-term physiological consequences of pneumonectomy. Semin Thorac Cardiovasc Surg 23:196–202
Cavalheri V, Jenkins S, Cecins N, Gain K, Phillips M, Sanders LH, Hill K (2015) Impairments after curative intent treatment for non-small cell lung cancer: a comparison with age and gender-matched healthy controls. Respir Med 109:1332–1339
Singh SJ, Puhan MA, Andrianopoulos V, Hernandes NA, Mitchell KE, Hill CJ, Lee AL, Camillo CA, Troosters T, Spruit MA, Carlin BW, Wanger J, Pepin V, Saey D, Pitta F, Kaminsky DA, McCormack MC, MacIntyre N, Culver BH, Sciurba FC, Revill SM, Delafosse V, Holland AE (2014) An official systematic review of the European Respiratory Society/American Thoracic Society: measurement properties of field walking tests in chronic respiratory disease. Eur Respir J 44:1447–1478
Granger CL, Holland AE, Gordon IR, Denehy L (2015) Minimal important difference of the 6-min walk distance in lung cancer. Chron Respir Dis 12:146–154
Granger CL, Denehy L, Parry SM, Martin J, Dimitriadis T, Sorohan M, Irving L (2015) Which field walking test should be used to assess functional exercise capacity in lung cancer? An observational study. BMC Pulm Med 15:89
Siegel RL, Miller KD, Jemal A (2016) Cancer statistics, 2016. CA Cancer J Clin 66:7–30
National Lung Screening Trial Research Team, Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, Gareen IF, Gatsonis C, Marcus PM, Sicks JD (2011) Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 365:395–409
Wang Z, Hu Y, Wang Y, Han W, Wang L, Xue F, Sui X, Song W, Shi R, Jiang J (2016) Can CT screening give rise to a beneficial stage shift in lung cancer patients? Systematic review and meta-analysis. PLoS One 11:e0164416
Speck RM, Courneya KS, Masse LC, Duval S, Schmitz KH (2010) An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv 4:87–100
Bade BC, Thomas DD, Scott JB, Silvestri GA (2015) Increasing physical activity and exercise in lung cancer: reviewing safety, benefits, and application. J Thorac Oncol 10:861–871
Soler X, Diaz-Piedra C, Ries AL (2013) Pulmonary rehabilitation improves sleep quality in chronic lung disease. COPD 10:156–163
Silver JK, Baima J (2013) Cancer prehabilitation: an opportunity to decrease treatment-related morbidity, increase cancer treatment options, and improve physical and psychological health outcomes. Am J Phys Med Rehabil 92:715–727
Acknowledgements
We would like to thank Dr. Florin Vaida for assistance in the statistical analyses in this study, Dr. Michael Gould and Dr. Lyudmila Bazhenova for manuscript feedback and edits, the Division of Pulmonary, Critical Care and Sleep Medicine and the Clinical and Translation Research Institute Clinical Research Enhancement through Supplemental Training Program at UCSD for financial and scholarship support, Svetlana Sheinkman for maintaining an ongoing list of lung cancer patients at the VASDHS, Christine Miller for assistance with patient recruitment, and staff of the Pulmonary Function Laboratory at the VASDHS for assistance with patient scheduling and 6-min walk test assessments.
Funding
Duc Ha, MD was supported directly by a Postdoctoral Fellowship, PF-17-020-01-CPPB, from the American Cancer Society; and Institutional National Research Service Award 1T32HL134632-01, from the NHLBI; and indirectly by the NCI through the NIH Loan Repayment Program for Extramural Clinical Research. Scott Lippman, MD was supported by the Specialized Cancer Center Support Grant, 3P30CA023100-31, from the NCI. Mark Fuster, MD was supported by the Lung Cancer Discovery Award, LCD-400697, from the American Lung Association; and R01-HL107652, from the NHLBI; and Sleep and Cancer Grant, from the ResMed Foundation. Data management was supported by the University of California San Diego Clinical and Translational Research Institute, grant number UL1TR001442.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOCX 18 kb)
Rights and permissions
About this article
Cite this article
Ha, D., Ries, A.L., Mazzone, P.J. et al. Exercise capacity and cancer-specific quality of life following curative intent treatment of stage I–IIIA lung cancer. Support Care Cancer 26, 2459–2469 (2018). https://doi.org/10.1007/s00520-018-4078-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-018-4078-4