Abstract
Introduction
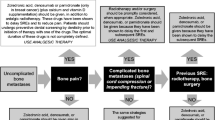
One of the key targets for metastatic cancer cells is the skeleton. Once metastatic cells are established within the bone matrix, skeletal integrity becomes increasingly compromised. Bone lesions lead to various complications, including bone pain, fractures and spinal cord compression.
Mechanisms of bone pain
Bone pain is debilitating and affects quality of life of the patient. In addition, it increases the use of health care resources. Many patients with metastatic bone disease experience substantial bone pain despite state-of-the-art systemic analgesic treatment. Incident pain is the predominant pain syndrome.
Treatment options for bone pain
Typically, this syndrome requires moderate baseline analgesia with increased on-demand doses. Other techniques for treating bone pain, including radiation therapy, neuraxial application of analgesics, nerve blocks and local stabilisation procedures, should be considered. In addition, therapy with bisphosphonates targeting bone-specific pain is an important strategy. This review discusses the various management options for bone pain arising from metastatic bone disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bone metastases are frequent and often very painful consequences of advanced cancer. The incidence of bone metastases varies according to the primary tumour type and can reach 95% in patients with certain cancers (Tables 1 and 2) [16, 22]. In addition to pain, the main complications of bone metastases are pathological fractures, spinal cord compression and hypercalcaemia. In addition to rapid treatment of these complications, maintenance of skeletal integrity is a long-term goal for these patients.
Many patients with metastatic bone disease (MBD) experience bone pain that is often severe and debilitating and require additional physician consultations and inpatient treatment for this symptom [42, 61, 84]. Treatment options for MBD must consider pain palliation, both acute and long term. Research suggests that >20% of patients are resistant to current systemic pain treatment [12, 43, 100]. This review discusses the different treatment options that have been shown to be effective in reducing bone pain in patients with MBD.
Overview of bone lesions
Even after skeletal growth is complete, bone remains metabolically active and continues to remodel throughout adulthood (annual turnover 5–10%) [27]. Bone remodelling requires coordination between bone resorption by osteoclasts and generation of new bone tissue by osteoblasts. Metastatic tumour cells disrupt the balance between osteoblast and osteoclast activity by paracrine secretion of activating factors (i.e. endothelin-1 and parathyroid hormone-related protein) [45]. For several primary tumours, osteolytic lesions are the most common manifestation of secondary disease and result in excessive bone resorption. Osteolytic bone metastases can be associated with bone pain, skeletal fractures, spinal cord compression (with associated neurological symptoms), hypercalcaemia and bone marrow carcinosis.
Dysregulated osteoblast activity results in surplus bone. However, this new bone is of poor quality and fractures easily. Osteoblasts can also upregulate osteoclast activity, which leads to the apparent paradox of bone resorption also increasing as a result of osteoblastic metastasis. Treatment options for osteoblastic lesions should additionally include therapy, such as the bisphosphonates, that targets osteoclasts [7, 34, 74, 80].
Cross-talk exists between invasive malignant cells and osteoclasts. This communication promotes osteoclast recruitment and upregulates their activity. Furthermore, destruction of the bone matrix leads to the release of growth factors that induce tumour cell proliferation [34, 74]. This two-way communication leads to increased tumour burden and skeletal destruction and is commonly called the “vicious circle.”
Detecting and monitoring bone metastases
Several imaging modalities are available to detect bone metastases: plain radiography, skeletal scintigraphy (mainly with technetium), computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography and single photon emission CT. Radionuclide bone scans are particularly suited to screen for and to determine the extent of suspected bone metastases derived from the majority of solid tumours; however, they are not sensitive to all tumours (e.g. multiple myeloma and renal cell carcinoma) or bone marrow involvement. Conventional radiography is generally able to determine the fracture risk at the relevant sites. Typically, initial detection of bone metastases is made using skeletal scintigraphy and confirmed using radiography [32]. Research results in high-risk prostate cancer patients suggest that whole-body MRI is more sensitive at detecting bone metastases than sequential scintigraphy and radiographs [48]; however, MRI is not easily accessible everywhere and is costly. Images providing higher specificity of bone lesions can be obtained using MRI and CT.
One of the current recommendations is to assess metastases thereafter every 2–6months [32]. However, there is no consensus about the validity of the different imaging techniques. Using CT or MRI, it may be possible to visualise a response as soon as 2months after treatment start. In comparison, responses do not typically become evident using conventional radiography before 3–6months.
Bone metastases cause upregulation in bone remodelling. The rate of skeletal turnover can be assessed using several serum and/or urine levels of biomarkers (indicating bone formation or bone resorption) [66]. Two subgroups of biomarkers are defined: substances released by osteoblasts and osteoclasts and markers that indicate the formation–breakdown of collagen.
The most commonly used assays for bone formation are serum tests of bone-specific alkaline phosphatase, osteocalcin and procollagen peptides, which are proteins produced by osteoblasts and released into the bloodstream during bone formation [66]. Bone resorption markers typically measure the breakdown products of collagen, the major protein of bone. These include pyridinoline, deoxypyridinoline, N-telopeptides (NTX) and C-telopeptides of type I collagen cross-links. Measurement of bone turnover biomarkers provides supportive information as to the extent of metastases to bone [66].
Mechanisms of bone pain
Although significant advances are being made in cancer diagnosis and treatment, the mechanisms of bone pain in patients with MBD are poorly understood. In addition to tumour-directed osteoclast-mediated osteolysis and tumour-induced nerve injury, neurochemical and cellular characteristics derived from experimental animal models of bone cancer pain are uniquely different to inflammatory or neuropathic pain [30]. A reorganisation of the central dorsal horn of the spinal cord and nociceptor peripheral sensitisation demonstrated in experimental cancer models the release of substance P, a neurotransmitter synthesised by nociceptors, which in turn activates the neurokinin-1 receptor that is expressed by a subset of spinal cord neurons [30, 83]. In addition, astrocyte hypertrophy, which is associated with decreased expression of glutamate reuptake transporters, increases extracellular levels of excitatory neurotransmitter glutamate and leads to excitotoxicity within the central nervous system [83].
Expression of glial fibrillary acidic protein, an astrocyte-specific cellular protein found in the supporting glial cells of the spinal cord, increases in bone cancer pain [30]. This further increases transmission of nociceptive information, leading to the amplification of the non-noxious input that is perceived as a noxious stimulus. Understanding novel mechanisms of bone pain arising from animal models potentially could change the management of pain associated with MBD; further research is needed.
Treatment options for bone pain from MBD
Once bone metastases have been diagnosed and evaluated, the clinical focus is on effective treatment of skeletal complications, including bone pain. Several strategies have been shown to relieve bone pain in patients with MBD.
Conventional anti-cancer therapy
A common approach to treating bone metastases is to continue therapy targeted against the primary tumour using systemic therapy (i.e. chemotherapy, endocrine therapy or immunotherapy). The ideal systemic therapy will target malignant cells, regardless of where they have migrated within the body, including the skeleton. Treatment with systemic therapy has the potential to reduce tumour burden and bone pain. This approach is not always successful but should be combined with other more focused palliative pain treatment, such as radiotherapy.
Radiotherapy and radionuclide therapy
Radiotherapy is an effective treatment of local metastatic bone pain. Some degree of pain relief occurs in 80–90% of patients within 4weeks. Complete and long-lasting pain relief is seen in approximately 50% of patients [3, 39, 54].
Although palliative doses of radiation are less than those used during a curative course of radiotherapy, a high number of tumour cells are eradicated. Tumour shrinkage will induce osteoblastic repair, leading to partial recovery of bone integrity. External beam irradiation results in ossification in 65–85% of osteolytic metastases [69]. This counters the common misconception that radiation treatment results in a decrease in ossification.
Some patients experience pain relief within 24h of receiving radiotherapy. Furthermore, this effect can be achieved with a single dose of only 4Gy. This rapid response is described before tumour shrinkage occurs and supports the hypothesis that this is not the only mechanism of analgesic effect of radiation therapy. Additional pathways responsible for this early response may include altered activity by inflammatory cells, upregulated osteoclast precursor cell activity and inhibition of release of chemical pain mediators [16, 69].
Several randomised studies have compared single fractions versus multiple fractions of radiotherapy [31, 33, 39, 59, 61, 64, 89, 92, 94].
Meta-analysis of single-fraction radiotherapy versus multifraction radiotherapy for metastatic bone relief and prevention of bone complications showed no difference between the two schedules [90, 99]. A study using meta-analysis for single and multifraction radiotherapy to approximate the outcome of treatment on pain response demonstrated that overall pain response rates were 60% and 59% and complete pain response rates were 34% and 32%, respectively [90]. However, patients treated by single-fraction radiotherapy had a higher re-treatment rate and a two to threefold increase of pathological fracture compared to those treated with a multifractionated schedule (e.g. 5 × 4 or 10 × 3Gy) [33, 90]. The drawbacks to radiotherapy include depression of the immune response, anaemia and tumour flare, which may limit its use [89].
A recent small-scale study provided evidence that a combination of a bisphosphonate (standard-dose ibandronate 6mg) with local radiotherapy worked synergistically. A significant decrease in pain scores was achieved (32/45 patients had a complete pain response, i.e. a pain score of zero) with an overall improvement in performance status [94]. In another study, 35 patients with prostate or breast cancer and suffering from painful bone metastases were treated with zoledronic acid and radiotherapy (30–40Gy over 3–4weeks using conventional dose fractionation). Intensity of bone metabolism was indicated by measuring levels of the markers of bone resorption procollagen-I-propeptide and β-crosslaps before and during therapy. Where high initial values (procollagen-I-propeptide > 190μg/L or β-crosslaps > 0.5μg/L) were measured, complete analgesia was achieved through therapy [96].
Patients with disseminated painful metastases can receive radionuclide treatment (single injection). These compounds accumulate in regions of bone with high metabolic activity, especially in metastatic foci where active bone formation is occurring. Pain relief begins 1–4weeks after the start of treatment and typically continues for up to 18months. Repeat doses are possible. Currently, strontium-89 and samarium-153 are approved for use in Europe and the US, and two rhenium isotopes (186 and 188) are under investigation. A recent Cochrane review compared results of four trials (n = 325 patients) and concluded that there was only weak evidence for a small effect of radioisotopes on pain control (1–6months) [75]. However, a smaller-scale study of 79 patients reported effective pain palliation with strontium-89, samarium-153, rhenium-186 and rhenium-188 [49].
Radium-223 (223Ra) is a new bone-seeking treatment for patients with bone metastases from hormone-refractory prostate cancer and was recently evaluated in a phase II study of 33 patients [65]. The results showed an overall safety profile comparable to conventional isotopes but with less haematologic toxicity: heavily pretreated patients with bone marrow insufficiency and patients with tumour bone marrow infiltration are at risk of relevant haematotoxicity with the use of radionucleotides; caution should be exercised with patients receiving concomitant myelosuppressive chemotherapy. Larger-scale studies are needed to investigate the value of radium-223 on skeletal-related events (SREs) and bone pain in patients with MBD. Haematological tolerability would be particularly beneficial where repeat treatment is required to control bone pain effectively.
Systemic and epidural analgesia
In general, when treating metastatic bone pain, the World Health Organisation (WHO) three-step analgesic ladder can be followed [18]. For mild pain, anti-inflammatory adjuvant analgesics (i.e. paracetamol or acetylsalicylate) are good initial options. Most patients will also require non-steroidal anti-inflammatory drugs, but their use is frequently contraindicated in these patients due to renal impairment.
Many patients will advance to opioid treatment. Patients taking opioids for background pain may also require additional doses (an additional 10–16%) to counter incident pain. Although not currently considered as standard practice, increasing evidence suggests that combination therapy with several opioids may be of value [88]. Management of opioid-related side effects (cognitive impairment, nausea and constipation) should be started prophylactically [55, 85]. Barriers to opiate treatment also include patient concerns, such as the possibility of addiction, additional side effects and the fear that if pain progresses there are no further palliative options.
The dose of opioids required for palliation of movement-related pain is generally high and may be accompanied by significant side effects (sedation, somnolence, depression, cognitive impairment, respiratory depression and constipation) [19, 72]. For localised bone pain in the distal spine that is refractory to systemic palliative therapy, epidural application of analgesics (i.e. local anaesthetics, opioids and others) is an important option to consider. This technique is especially useful in patients who have slow progressive tumours and few, but difficult to treat, bone metastases.
Evaluation of pain management should include patient-reported outcomes (visual analogue scale [VAS], Brief Pain Inventory [BPI]) [62]. Pain syndrome(s) must be characterised by localisation, deteriorating and improving factors, associated symptoms and their impact on the patient’s life. Neurological examination can detect neuropathic pain. For patients with advanced cancer, the risk factors for refractory pain need to be assessed (neuropathic pain, incident pain, psychosocial–spiritual distress and cognitive impairment) [26, 84].
For the treatment of neuropathic pain, systemic opioid treatment is usually efficacious in a majority of patients. Adjuvant analgesics, namely anti-epileptics (pregabalin) and tricyclic anti-depressive agents, also have proven effects for treatment of neuropathic pain, although limited evidence supports their value in cancer patients [20, 25].
Surgery
Often used in conjunction with or prior to other therapy to prevent bone fracture, successful surgical intervention also can reduce bone pain. Conventional techniques for stabilising osteolytic lesions include impregnated acrylic-based bone cements [47], salvage surgery to remove the cancerous area and replace with a prosthesis or bone transplant and surgical insertion of rods and plates. Support frames or surgical fusion of vertebrae can prevent spinal cord compression [51], and laminectomy is also beneficial to increase space around and relieve pressure on the spinal nerves. More recent advances in spinal surgery for metastatic lesions are vertebroplasty and kyphoplasty, both minimally invasive procedures where injections of a cement-like material are made directly into the fractured bone, typically the vertebra. Kyphoplasty includes an additional step to restore bone height and reduce deformity: prior to injection, a balloon is inserted and gently inflated inside the fractured vertebrae [21, 70, 71]. Vertebroplasty and kyphoplasty are very effective at stabilising fractures and providing immediate bone pain relief, with reduced requirement for analgesics and some restoration of mobility in many patients [1, 11, 21, 70, 71]. Clinically asymptomatic cement leakage from the vertebrae is a complication but occurs in the minority of patients treated [71]. These approaches provide mechanical stability to an affected bone; however, biocompatible cement technology is a developing area. To aid in the strengthening of local bone, bisphosphonates have been incorporated into some of these cements [35]. Although this technology is in its infancy, this treatment shows promising effects.
Rationale of targeting bone pain with bisphosphonates
The bisphosphonates are a broad group of compounds, each with a complex pharmacological profile [5, 53]. A key effect of bisphosphonates is to inhibit osteoclast activity, thereby reducing osteolytic destruction of bone and MBD-related complications [30]. Bisphosphonates (e.g. clodronate, ibandronate, pamidronate and zoledronic acid) are the current standard of care for MBD. Inhibiting bone resorption, mechanical stabilisation and increasing the pH of the bone tumour microenvironment, resulting in a decrease of acid-sensing ion channel stimulation, are proposed mechanisms for reducing bone pain using bisphosphonates [30].
The current recommendation is that bisphosphonate therapy is initiated at first diagnosis of bone lesions and should remain ongoing [38] because the clinical benefits of bisphosphonate-based therapy in reducing pathological SREs are well documented [6, 15, 50, 93]. Bisphosphonates also reduce metastatic bone pain and thereby improve quality of life (QoL) [5, 67, 98], with regular dosing typically suppressing pain after 8–12weeks. A systematic review [98] concluded that further data were necessary to recommend bisphosphonates as first-line therapy for metastasis-related bone pain, even though evidence supported bisphosphonate effectiveness in providing some pain relief. Since that time, several investigations of bone pain relief with bisphosphonates have been undertaken, typically as a secondary end point of large-scale clinical trials or in smaller studies of investigational doses.
Standard-dose bisphosphonate therapy for bone pain
Although usually measured as a secondary end point in phase III clinical trials of bisphosphonates, bone pain is often the first manifestation of metastatic disease and negatively impacts QoL and mobility of patients. To date, the efficacy of aminobisphosphonates to reduce bone pain is better than for non-aminobisphosphonates (clodronate), suggesting that this class of compounds should be preferred in patients reporting a high pain score. Table 3 summarises randomised, controlled or large-scale prospective studies that have examined the effect of standard recommended doses of bisphosphonates on metastatic bone pain by primary tumour type.
Prostate cancer
In a pooled analysis of two studies investigating palliation of bone pain in metastatic prostate cancer, pamidronate did not achieve a significant difference versus placebo [86]. In another study, zoledronic acid was compared with placebo and bone pain was defined as a secondary end point. Patients requiring strong opiate analgesics were excluded. The core phase study (15months) was completed by 147 patients; 133 patients entered the extension phase of 9months. The pain increase in the group with zoledronic acid was significantly lower at 3, 9, 21 and 24months [81, 82]. In two smaller placebo-controlled studies of prostate cancer, zoledronic acid also reduced metastatic bone pain [29, 97]. Fulfaro et al. [28] reported that zoledronic acid significantly reduced VAS scores by approximately 50% (P < 0.0005) by 1month. More recently, results from Weinfurt et al. [97] suggest that zoledronic acid reduced bone pain by a clinically meaningful degree (>2 points on the BPI). The analgesic effects of zoledronic acid were particularly notable as more pain-relieving radiotherapy and surgery interventions were received by the control group.
Breast cancer
Although evidence for a reduction in bone pain has been reported for oral clodronate, other trials have reported no palliation of bone pain with this bisphosphonate [40, 46, 91]. This result may reflect that the data were derived from patient populations with different baseline characteristics.
In a combined analysis of two phase III studies, 90mg pamidronate led to a significantly less increase in pain after 24months versus placebo [52]. In a small comparative trial, pamidronate improved pain scores more than oral clodronate over 3months of treatment [41]. In a placebo-controlled registration study in breast cancer patients, zoledronic acid reduced the bone pain index (approximately −0.8 relative to baseline) throughout the whole study from weeks4–52 [44]. These results are supported by a recently published study on patients with progressive bone metastases who were pretreated with oral clodronate or intravenous pamidronate. After a switch to intravenous zoledronic acid, a relevant palliative benefit in sense of pain reduction was demonstrated, especially in patients with a significant drop in urinary bone markers (uNTX) after the switch, where a large decrease in the worst bone pain was recorded [13]. In phase III trials of patients with breast cancer and bone metastases, oral and intravenous formulations of ibandronate reduced bone pain below baseline for up to 2years. In comparison, bone pain gradually increased through the study period with placebo [8, 23]. Oral ibandronate was associated with significantly lower use of analgesics compared with placebo (P = 0.019) and both formulations improved QoL (P < 0.05). As shown with zoledronic acid, switching patients with MBD and breast cancer from oral clodronate or intravenous pamidronate to oral ibandronate led to rapid and significant improvements in metastatic bone pain scores in a phase II study [14].
Multiple myeloma and mixed primary tumour studies
Relief from bone pain in patients with multiple myeloma has been demonstrated with recommended doses of clodronate, pamidronate and zoledronic acid (Table 3) [4, 73, 78]. Ibandronate has also been shown to reduce bone pain in these patients, using a non-standard 2-mg intravenous dose [60]. Other studies have shown that zoledronic acid can relieve metastatic bone pain from various primary tumour types, though some results show that pain increases over time [76, 77].
When selecting bisphosphonate therapy for pain relief, possible side effects should be considered. Patients with MBD typically receive multiple therapies. Additionally, renal impairment is also an issue in elderly patients and patients with multiple myeloma. Depending on the primary tumour, patients with MBD may survive for several years, so the impact that adverse events have on QoL should therefore be minimised. Hypocalcaemia is a common event that can be controlled with dietary calcium and vitamin D. Renal toxicity is reported following high doses and rapid administration of intravenous pamidronate and zoledronic acid and is thought to be caused by damage to the renal tubules [9, 56, 57]. In a phase III clinical trial, decline in renal function was reported in 10.7% of patients receiving intravenous zoledronic acid for bone metastases from multiple myeloma or breast cancer, and some patients receiving zoledronic acid or pamidronate have progressed to renal failure requiring dialysis [2, 10, 56, 102]. However, ibandronate does not seem to have the same effect on renal function, with an incidence of adverse events similar to placebo in phase III trials of patients with breast cancer and bone metastases for up to 4years of treatment [68].
Osteonecrosis of the jaw (ONJ) is another serious side effect associated with aminobisphosphonate, and diagnosis requires the presence of exposed bone for at least 6weeks [58, 63, 79]. Main symptoms include oral mucosal ulcerations, soft tissue infection and pain. The pathogenesis of ONJ is not fully understood yet, although it has been suggested to result from bisphosphonate interference with bone remodelling or anti-angiogenic effects. Current evidence suggests that the incidence of ONJ is related to duration of exposure and the type of bisphosphonate used [24, 101]. For this reason, the benefit of long-term use of bisphosphonates has to be weighed carefully against the risk of ONJ occurrence. Further studies are required to determine the relative incidence of this condition with the different bisphosphonates.
Loading-dose bisphosphonate therapy for bone pain
Evidence suggests that standard-dose bisphosphonates reduce metastatic bone pain to a greater or lesser degree depending on the drug used, primary tumour burden and bone pain. It is likely to take at least 4–12weeks after start of bisphosphonate therapy before a significant decline in bone pain is recorded. In addition to standard recommended doses, several studies have evaluated the analgesic potential of intensive bisphosphonate therapy for the relief of metastatic bone pain. Results to date have been promising. For example, an early trial in prostate cancer patients where intravenous clodronate was administered as a loading dose (300mg/day for 8days) followed by standard high-dose oral clodronate 1,600mg/day demonstrated palliation of pain [36]. Mean pain score significantly declined (P < 0.001) in 64/85 patients, and 22% became pain free without the need for additional analgesics. The first analgesic effects of clodronate were seen on day4 of parenteral therapy. Because of severe side effects and poor tolerance, this regimen was discontinued. High doses of intravenous pamidronate were also found to be beneficial in symptomatic relief for patients with MBD [17]. A single 120-mg, 2-h infusion of pamidronate was administered to 86 patients with progressive bone metastases from breast, prostate or other carcinoma in two separate studies. Both studies showed approximately 25% reduction in the overall pain score based upon a questionnaire and WHO performance status. The authors also found that clinical benefit was greatest for patients with slowly progressive disease and only modest increases in the rate of bone resorption.
More intensive dosing schedules for bisphosphonates have been limited (e.g. due to low bioavailability with oral formulations and renal toxicity with intravenous therapy). However, recent reports have suggested that intensive bisphosphonate treatment is possible for some intravenous formulations that can then be followed by long-term standard therapy. Phase II studies demonstrate that ibandronate has a marked analgesic effect in patients with moderate to severe metastatic bone pain from various tumour types, including bladder, prostate and kidney cancer, when administered as 6-mg infusions on three consecutive days. Bone pain scores were significantly reduced within days (compared with the 4–12weeks with standard dose; 6mg infused every 3–4weeks), and many patients were pain free at the end of study [37, 95].
Conclusion
Metastatic bone pain is a debilitating problem for patients with advanced cancer. A variety of treatment options to reduce bone pain are available to physicians, although choosing and prescribing a suitable treatment for each individual is not always easy. Fortunately, not only can several therapies be used sequentially without reducing the efficacy of future treatment, they can also be used concomitantly. Recent advances in surgical techniques for painful vertebral compression and the potential to combine such approaches with other interventions, such as radiotherapy, are improving available options for patients. Treatment schedules designed to specifically provide relief from bone pain in addition to other SREs provide promise for future therapeutic algorithms for patients with bone metastases.
Unfortunately, many patients with MBD still experience bone pain despite recommended doses of opioids (according to the WHO analgesic ladder), highlighting the need for improved symptom management. Bisphosphonate therapy, which targets the underlying skeletal metastases to improve bone integrity, has proved particularly successful in patients with moderate to severe bone pain and has reduced the need for analgesic consumption and palliative radiotherapy in clinical trials. Skeletal-related efficacy and safety profiles (renal toxicity, hypocalcaemia and ONJ) should be considered, especially during long-term use of bisphosphonates. Another question remains as to which dosing schedule is the best for bisphosphonates intended for bone pain relief. Standard dosing provides long-term reductions in bone pain for up to 2years but takes several weeks to become maximal. Rapid relief of bone pain with loading-dose bisphosphonates may represent an additional treatment option for patients with MBD. There are not enough data yet from large clinical trials, but this approach seems to be effective and well tolerated and may be particularly beneficial for patients who are receiving standard analgesia but still have treatment-resistant (breakthrough) pain and pain on movement, but further research is required.
References
Anselmetti GC, Corgnier A, Debernardi F, Regge D (2005) Treatment of painful compression vertebral fractures with vertebroplasty: results and complications. Radiol Med (Torino) 110(3):262–272
Banerjee D, Asif A, Striker L, Preston RA, Bourgoignie JJ, Roth D (2003) Short-term, high-dose pamidronate induced acute tubular necrosis: the postulated mechanisms of bisphosphonate nephrotoxicity. Am J Kidney Dis 41(5):E18 doi:10.1016/S0272-6386(03)00373-1
Bates T (1992) A review of local radiotherapy in the treatment of bone metastases and cord compression. Int J Radiat Oncol Biol Phys 23(1):217–221
Berenson JR, Lichenstein A, Porter L, Dimopoulos MA, Bordoni R, George S et al (1996) Efficacy of pamidronate in reducing skeletal events in patients with advanced multiple myeloma. N Engl J Med 334(8):488–493 doi:10.1056/NEJM199602223340802
Body JJ (1999) Bisphosphonates for metastatic bone pain. Support Care Cancer 7(1):1–3 doi:10.1007/s005200050213
Body JJ (2001) Bisphosphonates in the treatment of metastatic breast cancer. J Mammary Gland Biol Neoplasia 6(4):477–485 doi:10.1023/A:1014795216669
Body JJ, Mancini I (2002) Bisphosphonates for cancer patients: why, how and when? Support Care Cancer 10(5):399–407 doi:10.1007/s005200100292
Body JJ, Diel IJ, Bell R, Pecherstorfer M, Lichinitser MR, Lazarev AF et al (2004) Oral ibandronate improves bone pain and preserves quality of life in patients with skeletal metastases due to breast cancer. Pain 111(3):306–312 doi:10.1016/j.pain.2004.07.011
Body JJ, Pfister T, Bauss F (2005) Preclinical perspectives on bisphosphonate renal safety. Oncologist 10(suppl 1):3–7 doi:10.1634/theoncologist.10-90001-3
Chang JT, Green L, Beitz J (2003) Renal failure with the use of zoledronic acid. N Engl J Med 349(17):1676–1679 doi:10.1056/NEJM200310233491721
Cheung G, Chow E, Holden L, Vidmar M, Danjoux C, Yee AJ et al (2006) Percutaneous vertebroplasty in patients with intractable pain from osteoporotic or metastatic fractures: a prospective study using quality-of-life assessment. Can Assoc Radiol J 57(1):13–21
Cleeland CS, Janjan NA, Scott CB, Seiferheld WF, Curran WJ (2000) Cancer pain management by radiotherapists: a survey of radiation therapy oncology group physicians. Int J Radiat Oncol Biol Phys 47(1):203–208 doi:10.1016/S0360-3016(99)00276-X
Clemons MJ, Dranitsaris G, Ooi WS, Yogendran G, Sukovic T, Wong BY et al (2006) Phase II trial evaluating the palliative benefit of second-line zoledronic acid in breast cancer patients with either a skeletal-related event or progressive bone metastases despite first-line bisphosphonate therapy. J Clin Oncol 24(30):4895–4900 doi:10.1200/JCO.2006.05.9212
Clemons M, Dranitsaris G, Ooi W, Cole DE (2008) A phase II trial evaluating the palliative benefit of second-line oral ibandronate in breast cancer patients with either a skeletal related event (SRE) or progressive bone metastases (BM) despite standard bisphosphonate (BP) therapy. Breast Cancer Res Treat 108(1):79–85 doi:10.1007/s10549-007-9583-y
Coleman RE (2001) Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev 27(3):165–176 doi:10.1053/ctrv.2000.0210
Coleman RE (2004) Bisphosphonates: clinical experience. Oncologist 9(suppl. 4):14–27 doi:10.1634/theoncologist.9-90004-14
Coleman RE, Purohit OP, Vinholes JJ, Zekri J (1997) High dose pamidronate: clinical and biochemical effects in metastatic bone disease. Cancer 80(Suppl 8):1686–1690 doi:10.1002/(SICI)1097-0142(19971015)80:8+<1686::AID-CNCR20>3.0.CO;2-V
Dalton JA, Youngblood R (2000) Clinical application of the World Health Organization analgesic ladder. J Intraven Nurs 23(2):118–124
Davis MP, Walsh D (2004) Epidemiology of cancer pain and factors influencing poor pain control. Am J Hospice Palliat Care 21(2):137–142 doi:10.1177/104990910402100213
Davis MP, Walsh D, Lagman R, LeGrand SB (2005) Controversies in pharmacotherapy of pain management. Lancet Oncol 6(9):696–704 doi:10.1016/S1470-2045(05)70317-X
De Negri P, Tirri T, Paternoster G, Modano P (2007) Treatment of painful osteoporotic or traumatic vertebral compression fractures by percutaneous vertebral augmentation procedures: a nonrandomized comparison between vertebroplasty and kyphoplasty. Clin J Pain 23(5):425–430 doi:10.1097/AJP.0b013e31805593be
Diel IJ (2001) Bisphosphonates in the prevention of bone metastases: current evidence. Semin Oncol 28(4):75–80 (suppl 11)doi:10.1053/sonc.2001.25429
Diel IJ, Body JJ, Lichinitzer MR, Kreuser ED, Dornoff W, Gorbunova VA, Budde M, Bergström B, MF 4265 Study Group (2004) Improved quality of life after long-term treatment with the bisphosphonate ibandronate in patients with metastatic bone disease due to breast cancer. Eur J Cancer 40(11):1704–1712 doi:10.1016/j.ejca.2004.03.025
Dimopoulos MA, Kastritis E, Anagnostopoulos A, Melakopoulos I, Gika D, Moulopoulos LA et al (2006) Osteonecrosis of the jaw in patients with multiple myeloma treated with bisphosphonates: evidence of increased risk after treatment with zoledronic acid. Haematologica 91(7):968–971
Eisenberg E, River Y, Shifrin A, Krivoy N (2007) Antiepileptic drugs in the treatment of neuropathic pain. Drugs 67(9):1265–1289 doi:10.2165/00003495-200767090-00003
Ernst DS, Tannock IF, Winquist EW, Venner PM, Reyno L, Moore MJ, et al (2003) Randomized, double-blind, controlled trial of mitoxantrone/prednisone and clodronate versus mitoxantrone/prednisone and placebo in patients with hormone-refractory prostate cancer and pain. J Clin Oncol 21(17):3335–3342
Fernandez-Tresguerres-Hernandez-Gil I, Alobera-Gracia MA, del-Canto-Pingarron M, Blanco-Jerez L (2006) Physiological bases of bone regeneration II. The remodeling process. Med Oral Patol Oral Cir Bucal 11:E151–E157
Fulfaro F, Arcara C, Badalamenti G et al (2003) The use of zoledronic acid in the treatment of painful bone metastases from prostate cancer. J Clin Oncol 22(suppl):428 abstract 1721
Fulfaro F, Leto G, Badalamenti G, Arcara C, Cicero G, Valerio MR et al (2005) The use of zoledronic acid in patients with bone metastases from prostate carcinoma: effect on analgesic response and bone metabolism biomarkers. J Chemother 17(5):555–559
Goblirsch MJ, Zwolak PP, Clohisy DR (2006) Biology of bone cancer pain. Clin Cancer Res 12(Suppl 20):6231s–6235s doi:10.1158/1078-0432.CCR-06-0682
Haddad P, Wong RK, Pond GR, Soban F, Williams D, McLean M et al (2005) Factors influencing the use of single vs multiple fractions of palliative radiotherapy for bone metastases: a 5-year review. Clin Oncol (R Coll Radiol) 17(6):430–434 doi:10.1016/j.clon.2005.03.012
Hamaoka T, Madewell JE, Podoloff DA, Hortobagyi GN, Ueno NT (2004) Bone imaging in metastatic breast cancer. J Clin Oncol 22(14):2942–2953 doi:10.1200/JCO.2004.08.181
Hartsell WF, Scott CB, Bruner DW, Scarantino CW, Ivker RA, Roach M 3rd et al (2005) Randomized trial of short- versus long-course radiotherapy for palliation of painful bone metastases. J Natl Cancer Inst 97(11):798–804
Harvey HA, Cream LR (2007) Biology of bone metastases: causes and consequences. Clin Breast Cancer 7(suppl 1):S7–S13
Healey JH, Shannon F, Boland P, DiResta GR (2003) PMMA to stabilize bone and deliver antineoplastic and antiresorptive agents. Clin Orthop Relat Res (415 suppl):S263–S275 doi:10.1097/01.blo.0000093053.96273.ee
Heidenreich A, Hofmann R, Engelmann U (2001) The use of bisphosphonate for the palliative treatment of painful bone metastasis due to hormone refractory prostate cancer. J Urol 165(1):136–140 doi:10.1097/00005392-200101000-00033
Heidenreich A, Body J, Bergström B (2004) Relief from severe metastatic bone pain with intensive ibandronate dosing. Support Care Cancer 12:407 (A-127)
Hillner BE, Ingle JN, Chlebowski RT, Gralow J, Yee GC, Janjan NA, American Society of Clinical Oncology et al (2003) American Society of Clinical Oncology 2003 update on the role of bisphosphonates and bone health issues in women with breast cancer. J Clin Oncol 21(21):4042–4057 doi:10.1200/JCO.2003.08.017
Hoskin PJ (2003) Bisphosphonates and radiation therapy for palliation of metastatic bone disease. Cancer Treat Rev 29(4):321–327 doi:10.1016/S0305-7372(03)00013-6
Hurst M, Noble S (1999) Clodronate: a review of its use in breast cancer. Drugs Aging 15(2):143–166 doi:10.2165/00002512-199915020-00007
Jagdev SP, Purohit OP, Heatley S, Herling C, Coleman RE (2001) Comparison of the effects of intravenous pamidronate and oral clodronate on symptoms and bone resorption in patients with metastatic bone disease. Ann Oncol 12(10):1433–1438 doi:10.1023/A:1012506426440
Janjan N (2001) Bone metastases: approaches to management. Semin Oncol 28(4):28–34 (suppl 11) doi:10.1053/sonc.2001.25444
Johnson G, Gralow J, Benson P (2004) Use of bisphosphonates for the palliative management of metastatic bone pain in the US. Support Care Cancer 12:400 (A-105)
Kohno N, Aogi K, Minami H, Nakamura S, Asaga T, Iino Y et al (2005) Zoledronic acid significantly reduces skeletal complications compared with placebo in Japanese women with bone metastases from breast cancer: a randomized, placebo-controlled trial. J Clin Oncol 23(15):3314–3321 doi:10.1200/JCO.2005.05.116
Kozlow W, Guise TA (2005) Breast cancer metastasis to bone: mechanisms of osteolysis and implications for therapy. J Mammary Gland Biol Neoplasia 10(2):169–180 doi:10.1007/s10911-005-5399-8
Kristensen B, Ejlertsen B, Groenvold M, Hein S, Loft H, Mouridsen HT (1999) Oral clodronate in breast cancer patients with bone metastases: a randomized study. J Intern Med 246(1):67–74 doi:10.1046/j.1365-2796.1999.00507.x
Kuehn KD, Ege W, Gopp U (2005) Acrylic bone cements: mechanical and physical properties. Orthop Clin North Am 36(1):29–39 doi:10.1016/j.ocl.2004.06.011
Lecouvet FE, Geukens D, Stainier A, Jamar F, Jamart J, d’Othée BJ et al (2007) Magnetic resonance imaging of the axial skeleton for detecting bone metastases in patients with high-risk prostate cancer: diagnostic and cost-effectiveness and comparison with current detection strategies. J Clin Oncol 25(22):3281–3287 doi:10.1200/JCO.2006.09.2940
Liepe K, Kotzerke J (2007) A comparative study of 188Re-HEDP, 186Re-HEDP, 153Sm-EDTMP and 89Sr in the treatment of painful skeletal metastases. Nucl Med Common 28(8):623–630 doi:10.1097/MNM.0b013e32825a6adc
Lipton A (2003) Bisphosphonates and metastatic breast carcinoma. Cancer 97(suppl 3):848–853 doi:10.1002/cncr.11123
Lipton A (2003) Bone metastases in breast cancer. Curr Treat Opt Oncol 4(2):151–158 doi:10.1007/s11864-003-0016-9
Lipton A, Theriault RL, Hortobagyi GN, Simeone J, Knight RD, Mellars K et al (2000) Pamidronate prevents skeletal complications and is effective palliative treatment in women with breast carcinoma and osteolytic bone metastases: long-term follow-up of two randomized, placebo-controlled trials. Cancer 88(5):1082–1090 doi:10.1002/(SICI)1097-0142(20000301)88:5<1082::AID-CNCR20>3.0.CO;2-Z
LoRusso P (2001) Analysis of skeletal-related events in breast cancer and response to therapy. Semin Oncol 28(4):22–27 (suppl 11) doi:10.1053/sonc.2001.25437
Maher EJ (1992) The use of palliative radiotherapy in the management of breast cancer. Eur J Cancer 28(2–3):706–710 doi:10.1016/S0959-8049(05)80131-5
Marinangeli F, Ciccozzi A, Leonardis M, Aloisio L, Mazzei A, Paladini A et al (2004) Use of strong opioids in advanced cancer pain: a randomized trial. J Pain Symptom Manage 27(5):409–416 doi:10.1016/j.jpainsymman.2003.10.006
Markowitz GS, Appel GB, Fine PL, Fenves AZ, Loon NR, Jagannath S et al (2001) Collapsing focal segmental glomerulosclerosis following treatment with high-dose pamidronate. J Am Soc Nephrol 12(6):1164–1172
Markowitz GS, Fine PL, Stack JI, Kunis CL, Radhakrishnan J, Palecki W et al (2003) Toxic acute tubular necrosis following treatment with zoledronate (Zometa). Kidney Int 64(1):281–289 doi:10.1046/j.1523-1755.2003.00071.x
Marx RE, Sawatari Y, Fortin M, Broumand V (2005) Bisphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: risk factors, recognition, prevention, and treatment. J Oral Maxillofac Surg 63(11):1567–1575 doi:10.1016/j.joms.x2005.07.010
McQuay HJ, Collins SL, Carroll D, Moore RA (2000) Radiotherapy for the palliation of painful bone metastases. Cochrane Database Syst Rev (2):CD001793
Menssen HD, Sakalová A, Fontana A, Herrmann Z, Boewer C, Facon T et al (2002) Effects of long-term intravenous ibandronate therapy on skeletal-related events, survival and bone resorption markers in patients with advanced multiple myeloma. J Clin Oncol 20(9):2353–2359 doi:10.1200/JCO.2002.02.032
Mercadante S (1997) Malignant bone pain: pathophysiology and treatment. Pain 69(1–2):1–18 doi:10.1016/S0304-3959(96)03267-8
Mercadante S (2006) Scoring the effect of radiotherapy for painful bone metastases. Support Care Cancer 14(9):967–969 doi:10.1007/s00520-006-0036-7
Migliorati CA, Casiglia J, Epstein J, Jacobsen PL, Siegel MA, Woo SB (2005) Managing the care of patients with bisphosphonate-associated osteonecrosis: an American Academy of Oral Medicine position paper. J Am Dent Assoc 136(12):1658–1668
Nielsen OS, Bentzen SM, Sandberg E, Gadeberg CC, Timothy AR (1998) Randomized trial of single dose versus fractionated palliative radiotherapy of bone metastases. Radiother Oncol 47(3):233–240 doi:10.1016/S0167-8140(98)00011-5
Nilsson S, Franzén L, Parker C, Tyrrell C, Blom R, Tennvall J et al (2007) Bone-targeted radium-223 in symptomatic, hormone-refractory prostate cancer: a randomised, multicentre, placebo-controlled phase II study. Lancet Oncol 8(7):587–594 doi:10.1016/S1470-2045(07)70147-X
Palma MA, Body JJ (2005) Usefulness of bone formation markers in breast cancer. Int J Biol Markers 20(3):146–155
Pavlakis N, Schmidt R, Stockler M (2005) Bisphosphonates for breast cancer. Cochrane Database Syst Rev (3):CD003474, Jul 20
Pecherstorfer M, Rivkin S, Body JJ, Diel I, Bergström B (2006) Long-term safety of intravenous ibandronic acid for up to 4years in metastatic breast cancer: an open-label trial. Clin Drug Investig 26(6):315–322 doi:10.2165/00044011-200626060-00002
Perez CA, Brady LW, Halperin EC, Schmidt-Ullrich RK (2004) Principles and practice of radiation oncology, 4th edn. Lippencott, Williams and Wilkins, Philadelphia
Pflugmacher R, Schleicher P, Schroder RJ, Melcher I, Klostermann CK (2006) Maintained pain reduction in five patients with multiple myeloma 12months after treatment of the involved cervical vertebrae with vertebroplasty. Acta Radiol 47(8):823–829 doi:10.1080/02841850600812728
Pflugmacher R, Beth P, Schroeder RJ, Schaser KD, Melcher I (2007) Balloon kyphoplasty for the treatment of pathological fractures in the thoracic and lumbar spine caused by metastasis: one-year follow-up. Acta Radiol 48(1):89–95 doi:10.1080/02841850601026427
Ripamonti C, Fulfaro F (2000) Malignant bone pain: pathophysiology and treatments. Curr Rev Pain 4(3):187–196
Robertson AG, Reed NS, Ralston SH (1995) Effect of oral clodronate on metastatic bone pain: a double-blind, placebo-controlled study. J Clin Oncol 13(9):2427–2430
Roodman GD (2004) Mechanisms of bone metastasis. N Engl J Med 350(16):1655–1664 doi:10.1056/NEJMra030831
Roque M, Martinez MJ, Alonso P, Catala E, Garcia JL, Ferrandiz M (2003) Radioisotopes for metastatic bone pain. Cochrane Database Syst Rev (4):CD003347
Rosen LS, Gordon D, Tchekmedyian NS, Yanagihara R, Hirsh V, Krzakowski M, et al (2004) Long-term efficacy and safety of zoledronic acid in the treatment of skeletal metastases in patients with nonsmall cell lung carcinoma and other solid tumors: a randomized, Phase III, double-blind, placebo-controlled trial. Cancer 100(12):2613-2621
Rosen LS, Gordon D, Tchekmedyian S, Yanagihara R, Hirsh V, Krzakowski M et al (2003) Zoledronic acid versus placebo in the treatment of skeletal metastases in patients with lung cancer and other solid tumors: a phase III, double-blind, randomized trial—the zoledronic acid lung cancer and other solid tumors study group. J Clin Oncol 21(16):3150–3157 doi:10.1200/JCO.2003.04.105
Rosen LS, Gordon D, Kaminski M et al (2007) Zoledronic acid versus pamidronate in the treatment of skeletal metastases in patients with breast cancer or osteolytic lesions of multiple myeloma: a phase III, double-blind, comparative trial. Cancer J 7:377–387
Ruggiero SL, Mehrotra B, Rosenberg TJ, Engroff SL (2004) Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofac Surg 62(5):527–534 doi:10.1016/j.joms.2004.02.004
Russell RG, Croucher PI, Rogers MJ (1999) Bisphosphonates: pharmacology, mechanisms of action and clinical uses. Osteoporos Int 9(suppl 2):S66–S80 doi:10.1007/PL00004164
Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L, Zoledronic Acid Prostate Cancer Study Group et al (2002) A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst 94(19):1458–1468
Saad F, Karakiewicz P, Perrotte P (2005) The role of bisphosphonates in hormone-refractory prostate cancer. World J Urol 23(1):14–18 doi:10.1007/s00345-004-0472-2
Sabino MAC, Mantyh PW (2005) Pathophysiology of bone cancer pain. J Support Oncol 3(1):15–24
Serafini AN (2001) Therapy of metastatic bone pain. J Nucl Med 42(6):895–906
Skaer TL (2004) Practice guidelines for transdermal opioids in malignant pain. Drugs 64(23):2629–2638 doi:10.2165/00003495-200464230-00002
Small EJ, Smith MR, Seaman JJ, Petrone S, Kowalski MO (2003) Combined analysis of two multicenter, randomized, placebo-controlled studies of pamidronate disodium for the palliation of bone pain in men with metastatic prostate cancer. J Clin Oncol 21(23):4277–4284 doi:10.1200/JCO.2003.05.147
Strang P, Nilsson S, Brändstedt S, Sehlin J, Borghede G, Varenhorst E, Bandman U, Borck L, Englund G, Selin L (1997) The analgesic efficacy of clodronate compared with placebo in patients with painful bone metastases from prostatic cancer. Anticancer Res 17(6D):4717–4721
Strasser F (2005) Promoting science in a pragmatic world: not (yet) time for partial opioid rotation. Support Care Cancer 13(10):765–768 doi:10.1007/s00520-005-0855-y
Sugawara Y, Kajihara M, Semba T, Ochi T, Fujii T, Mochizuki T (2005) Healing focal “flare” phenomenon after radiotherapy in a bone metastasis from bladder cancer. Clin Nucl Med 30(10):672–673 doi:10.1097/01.rlu.0000178028.13072.36
Sze WM, Shelley M, Held I, Mason M (2004) Palliation of metastatic bone pain: single fraction versus multifraction radiotherapy—a systematic review of the randomised trials. Cochrane Database Syst Rev (2):CD004721
Tubiana-Hulin M, Beuzeboc P, Mauriac L, Barbet N, Frenay M, Monnier A et al (2001) Double-blinded controlled study comparing clodronate versus placebo in patients with breast cancer bone metastases (Article in French). Bull Cancer 88(7):701–707
Vakaet LA, Boterberg T (2004) Pain control by ionizing radiation of bone metastasis. Int J Dev Biol 48(5–6):599–606 doi:10.1387/ijdb.041817lv
Van Poznak CH (2002) The use of bisphosphonates in patients with breast cancer. Cancer Control 9(6):480–489
Vassiliou K, Kalogeropoulou C, Christopoulos CH, Solomou EK, Kardamakis D (2006) Treating bone metastases from solid tumors with a combination of ibandronate and radiotherapy: clinical evaluation and radiological assessment. Bone 38(3):S85 (suppl 1) abstract 110 doi:10.1016/j.bone.2006.01.056
von Moos R, Cathomas R, Egli F, Inauen R (2006) Loading-dose ibandronate rapidly reduces metastatic bone pain. Ann Oncol 17(suppl 9):78 abstract 928P
Wagner W (2007) Procollagen-I-propeptide and β-crosslaps are prognostic markers for pretherapeutic estimation of treatment success of combined radio- and bisphosphonate therapy in patients with bone metastases—a phase-II study. J Clin Oncol 25(18S):Abstract 9096
Weinfurt KP, Anstrom KJ, Castel LD, Schulman KA, Saad F (2006) Effect of zoledronic acid on pain associated with bone metastasis in patients with prostate cancer. Ann Oncol 17(6):986–989 doi:10.1093/annonc/mdl041
Wong R, Wiffen PJ (2002) Bisphosphonates for the relief of pain secondary to bone metastases. Cochrane Database Syst Rev (2):CD002068
Wu JS, Wong RKS, Lloyd NS, Johnston M, Bezjak A, Whelan T, Supportive Care Guidelines Group of Cancer Care Ontario (2004) Radiotherapy fractionation for the palliation of uncomplicated painful bone metastases—an evidence-based practice guideline. BMC Cancer 4:71 doi:10.1186/1471-2407-4-71
Yau V, Chow E, Davis L, Holden L, Schueller T, Danjoux C (2004) Pain management in cancer patients with bone metastases remains a challenge. J Pain Symptom Manage 27(1):1–3 letter to the editor doi:10.1016/j.jpainsymman.2003.10.003
Zarychanski R, Elphee E, Walton P, Johnston J (2006) Osteonecrosis of the jaw associated with pamidronate therapy. Am J Hematol 81(1):73–75 doi:10.1002/ajh.20481
Anonymous (2007) Zoledronic acid (Zometa) [prescribing information]. Novartis Pharmaceuticals Corporation, East Hanover
Acknowledgment
Professional editorial assistance for this manuscript was provided by Gardiner-Caldwell US and funded by an unrestricted educational grant by Roche.
Conflict of interest statement
Dr. Roger von Moos has a consultant or advisory relationship with Roche and Novartis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
von Moos, R., Strasser, F., Gillessen, S. et al. Metastatic bone pain: treatment options with an emphasis on bisphosphonates. Support Care Cancer 16, 1105–1115 (2008). https://doi.org/10.1007/s00520-008-0487-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-008-0487-0