Abstract
Goal of work
The aim of the study was to investigate the incidence of herpes simplex virus-1 (HSV-1) infection in mucositis during head and neck cancer radiotherapy.
Patients and methods
Sixty patients with malignant head and neck tumor, eligible to receive radiotherapy, who were referred to the Dental Oncology Unit, entered the study. Sixteen patients (26.6%) received concomitant chemotherapy. Mucositis was recorded weekly. Smears taken from the ulcers of mucositis grade 2, or 3, or 4 were stained with Papanicolaou and alkaline phosphatase/antialkaline phosphatase immunocytochemical method to identify HSV-1.
Main results
Forty-eight of all 60 patients developed ulcerative mucositis. Smear was available from 29 of 48 patients with ulcerations. HSV-1 infection was identified in 14 of 29 smears available (48.2%). Mucositis healed or was reduced after 1 week of antiviral treatment in 11 of those 14 HSV-1-positive patients; 3 patients responded to 1 g/day of valacyclovir, 7–2 g/day, and 1 patient responded to i.v. acyclovir. Ulcerations recurred after quitting antivirals. Three patients did not respond to 1 g/day of valacyclovir. No HSV-1-negative patient responded to acyclovir (P=0.000).
Conclusion
HSV-1 was isolated from 14 of 29 available smears taken from 48 patients with ulcerative mucositis. The incidence of HSV-1 infection during radiotherapy was estimated as being 14 of all 48 patients at risk (29.1%). Healing or reduction in the grade of mucositis after antivirals in HSV-1 positive patients, combined with the negative response to antivirals in HSV-1 negative patients, denoted that HSV-1 infection was a component of ulcerative radiation mucositis in those HSV-1-positive patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
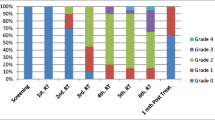
Oral radiation-induced mucositis is an important, almost inevitable, costly and dose-limiting complication of radiotherapy (RT) for head and neck malignancies [3, 18, 28]. Nearly all patients (90–97%) receiving RT alone or with concomitant chemotherapy will experience some degree of mucositis [3, 27]. According to severity, mucositis is scored in four grades. Grade 1 is characterized by a diffuse erythema, and patient can take solid diet. Mucositis grade 2 is characterized by erythema and small foci of ulcers, while patient can take soft diet. Mucositis grades 3 and 4 are considered as severe, equal to painful ulcers extending on more than half of the oral mucosa, while patient can take liquids only or alimentation may not be possible. Severe mucositis is the most debilitating side effect during RT.
The frequency and severity of mucositis are influenced by numerous treatment- and patient-related risk factors. Treatment-related factors are type of irradiation, volume of irradiated tissue, daily and total dose, and duration of RT. Patient-related factors are age, gender, poor oral health, nutritional status, oral microflora, inflammation, and salivary gland function [1, 3]. About 34–43% of patients who receive conventional RT, alone or in combination with chemotherapy, for head and neck malignancies may experience severe, grade 3–4 mucositis. Severe mucositis compromises patient’s quality of life and is associated with increases in the frequency of hospitalization, total parenteral nutrition use, and interruptions of therapy, with adverse effect on tumor control. An overall incidence of 9–19% of RT or radiochemotherapy interruptions due to severe mucositis has been reported [27].
Oral radiation-and chemotherapy-induced mucositis remains an incompletely understood phenomenon.
In the past, mucositis was considered to be the consequence of local viral or bacterial infection. Later on, mucositis was seen as being the sole consequence of the nonspecific cytotoxic effects of radiation and chemotherapy on the basal epithelial stem cells in the mucosa.
Mucositis is now viewed as a panmucosal phase. It appears to be the consequence of a series of biological events that begin in the submucosa and target the epithelium [25, 26]. During these events, NF-κB and other transcription factors are activated by RT, proinflammatory cytokines are produced and apoptosis and cell injury follows. The dynamic biological cascade results in mucosal injury, often manifested as ulceration. A fibrinous exudate, teeming with bacteria, also referred to as a pseudomembrane, covers the ulcers. Cell wall products, from the colonizing bacteria, penetrate into the submucosa, where they activate infiltrating macrophages to pour out additional cytokines. The bacterial colonization of the ulcerations may lead to secondary local or systemic infection. Local mucosal infection, being superimposed on mucositis, will aggravate the condition.
Herpes simplex virus-1 (HSV-1) is also an important activator of NF-κB [25]. Thus, the presence of HSV-1 within lesions of mucositis would aggravate, through NF-κB activation, the grade of mucositis. In addition, HSV-1 (in case of infection) exerts its effects on the epithelial cells of the oral mucosa and leads to the formation of an intraepithelial vesicle. Mucosal vesicles are rapidly ruptured, and an ulcer, covered by a fibrinous exudate, is formed [14]. That herpetic ulcer, being superimposed on the ulcers of mucositis, would further add to the severity of mucositis.
Current management of mucositis consists primarily of infection prevention or treatment, once infection has occurred, and palliation [22]. The most common oral mucosal infection, reported during head and neck RT, is oral candidiasis, while HSV-1 infection during head and neck RT has been addressed in few studies [5, 15, 17, 20, 21], with conflicting results.
Bubley et al. [5] reported a significantly decreased incidence of HSV-1-positive cultures in oral radiation- or chemotherapy-induced “mucositis” or “stomatitis” lesions in patients with head and neck malignancies receiving acyclovir prophylaxis, without, however, any clinical benefit observed for the patient. All patients of the study had to be seropositive for HSV.
Redding et al. [20] found no positive viral culture in 18 patients receiving head and neck RT. Cultures were taken when frank oral ulceration was present. No antibody titers for HSV were determined.
Oakley et al. [17] reported two cases of HSV reactivation, as a cause of oral ulcerations, during head and neck RT, with positive response to antiviral treatment, and Nicolatou-Galitis et al. [15] reported five HSV-1-positive cultures of 14 patients with radiation-induced oral ulcerations. An immediate positive clinical response was observed after antiviral treatment. Riel-Romero and Baumann [21] reported a case of herpes simplex encephalitis during RT for pontine glioma. The above three studies suggested that RT might play a role in HSV reactivation. The need for further clinical research into the reactivation and infection of orofacial HSV during RT was pointed out.
In contrast to the sparse and conflicting reports of HSV-1 infection during RT, HSV-1 infection has been a well-studied viral infection during immunosuppressive chemotherapy and hematopoietic stem cell transplantation. It has been found as an important component of oral ulcerative chemotherapy-induced mucositis, with an incidence of 40–60% [7, 12, 13, 23, 24]. HSV infection exacerbates or even causes oral ulcerations after immunosuppressive cancer chemotherapy. Furthermore, in Schubert et al. study [24], where oropharyngeal HSV infection occurred in 37% of patients after allogeneic bone marrow transplantation for hematological malignancy, a significant increase in the incidence of infection between patients conditioned with radiation as opposed to those without radiation was found.
Viral reactivation, and not primary infection, was documented in most cases in the above reports.
The virus persists, after primary infection [6, 10, 19], within infected cells in a repressed, latent state in the trigeminal ganglion, until reactivated. By middle adulthood, more than 90% of the population of poor socioeconomic status and more than 50% of the Western industrialized middle-class populations have antibodies to HSV-1. At autopsy, HSV-1 could be detected in as many as 53% of trigeminal ganglia [19].
The exact manner in which reactivation occurs remains undefined. Factors such as emotional and somatic stress, ultraviolet light, dental extraction, surgery, and immunosuppression have been implicated in the reactivation process [6, 7, 10–13, 24]. Few data have indicated that RT may also play a role in the reactivation of HSV-1 when radiation encompasses the trigeminal ganglion [15, 17, 21]. Importantly, when the oral cavity is included in the radiation field, the viral ulcers, in the case of HSV-1 infection, are similar to and cannot be differentiated from the ulcers of ulcerative radiation mucositis. Both types of ulcers are painful, are covered with fibrinous exudate or pseudomembrane, and can be located on any mucosal surface. Viral ulcers may be superimposed on the lesions of ulcerative radiation mucositis, adding to the severity of mucositis. The incidence of HSV-1 infection and its contribution to the severity of radiation mucositis has been poorly documented [15, 17].
In the present prospective, cohort study, the incidence of HSV-1 infection within the lesions of oral ulcerative radiation mucositis was investigated. The study was initiated based on the few literature data [15, 17, 21] and on personal empirical observations, where a clinical beneficial response of ulcerative radiation mucositis to antiviral treatment was often encountered. Oral mucosal evaluation included the assessment of oral mucositis, the assessment of oral candidiasis, the laboratory verification of HSV-1 infection within the ulcerative lesions of mucositis, and the response to antiviral treatment.
The study included all registered 60 patients who were referred between January 2004 to April 2005, from the Radiotherapy Departments of four Athens hospitals to the Dental Oncology Unit, Department of Oral Pathology and Surgery, School of Dentistry, University of Athens, for the standard oral mucosal and dental care, before the initiation and during the course of RT.
Patients and methods
Patients and eligibility criteria
Sixty consecutive patients, referred from the cancer centers of four Athens hospitals, with malignant head and neck tumor, eligible to receive RT were included in the study. No patient was excluded. A total of 25 of the 60 patients had been included in our previous study on oral candidiasis [16]. General blood tests and liver and renal function were within normal limits. Karnofsky performance status ranged between 80 and 100%.
Thirty-one patients received 100 mg/day of fluconazole antifungal prophylaxis administered per os, after lunch, from the initiation to the completion of RT. The decision for those patients to receive antifungal prophylaxis was made by the radiotherapist of each patient (based on the high risk of the development of candidiasis during RT, shown in our Unit and from literature data). Six of those patients were included in the fluconazole prophylaxis patient cohort described before [16].
Twenty-nine patients received antifungal therapy for 1 week (100 mg/day of fluconazole administered per os), after lunch, upon the development of candidiasis. Nineteen of those patients were also included in our previous patient cohort [16].
All patients were thoroughly informed about their disease and the treatment they would receive. All patients agreed and gave their consent for the above, written to the Radiotherapy Centers and orally to the Dental Oncology Unit. Since January 2005, all patients have signed consent forms to the Dental Oncology Unit, too. Standard oral mucosal and dental care was introduced to all patients.
Hypothesis and study end point
If HSV-1 infection was, at times, a component of oral ulcerative radiation mucositis lesions and was playing a role in the severity of mucositis, then (a) cytologic smears taken from the ulcerative lesions should show the characteristic viral cytopathic epithelial changes when stained with Papanicolaou method, while immunocytochemistry should identify the presence of HSV-1 in the cells, and (b) the ulcers should, at least partially, heal after antiviral treatment.
The objectives of the study were (a) to document viral cytopathic epithelial changes in smears taken from lesions of ulcerative mucositis and to disclose the presence of HSV-1 in the smears thus assessing the incidence of HSV-1 infection during RT, and (b) to evaluate any clinical healing of the ulcers, during the course of RT, after the antiviral treatment, showing, by this way, the aggravation of radiation mucositis by HSV-1 infection. The reduction of the grade of mucositis after 1 week of antiviral treatment was defined as positive response and healing.
Radiotherapy
Patients were irradiated with a 6-MV linear accelerator. Thirty-four patients received radical and 26 patients received postoperative radiotherapy (RT). The primary tumor and draining lymphatics were treated with parallel opposed fields. Supraclavicular and low neck nodes were treated with an anterior field.
The daily and the total radiation dose are shown in Table 1. The lateral field doses were reduced after 40–43 Gy to avoid overdosage to the spinal cord. The regional nodes were irradiated to a total dose of 45–61 Gy, depending on the nodal stage. Concomitant chemotherapy, including 2 cycles of cisplatinum (100 mg/m2 on days 1 and 28 of RT) and 5-fluorouracil (800 mg/m2 on days 1–5 and 28–32 of RT), was administered to 16 patients. Table 1 shows patient characteristics, tumor diagnosis, and type and dose of RT, assessed by antifungal prophylaxis group.
Oral clinical evaluation
Patients were examined weekly, and oral mucosal evaluation was performed by the oral medicine specialist. Mucosal evaluation, performed routinely in our Unit, included:
-
1.
The scoring of oral mucositis, which was recorded according to EORTC/RTOG criteria.
-
2.
The definitive diagnosis of oral pseudomembranous candidiasis. The three criteria used for the diagnosis of candidiasis were the ones published previously [15, 16], i.e., clinical presumptive diagnosis of candidiasis (criterion 1) and positive direct microscopic observation of Candida organisms in the smear (criterion 2; positive Candida carriage). Presumptive diagnosis of candidiasis was made when easily removable, mostly painless, whitish pseudomembranes were observed. Both positive clinical and laboratory findings had to be verified by positive response to antifungal treatment (criterion 3).
-
3.
The presumptive diagnosis of HSV-1 infection, which was evaluated according to the clinical criteria reported previously [15]. Those criteria included (a) abrupt appearance of severe, extensive ulcers and/or (b) early initiation of ulceration, within the first 2 weeks of RT.
Viral laboratory investigation
Cytologic smears were taken, with a brush, from the ulcers of patients who developed ulcerative mucositis grades 2, 3, and 4. Eight glass slides with specimens were prepared from each patient. Four specimens of each patient were fixed with 96% alcohol and were stained with Papanicolaou stain [2, 8].
Four specimens with smears from each patient were air-dried for 2 h after preparation and fixed for 10 min in acetone–methanol 1:1, wrapped airtight and stored at −70°C until used. Immunocytochemical staining was performed by use of alkaline phosphatase/antialkaline phosphatase (APAAP) staining at room temperature [9]. Smears were placed in buffer bath (Tris buffer, pH 7.6) for 5 min and then incubated for 35 min with rabbit polyclonal antibody (antiherpes simplex virus type 1, Diagnostic Biosystems, USA) at a dilution of 1:50. After washing in buffer, smears were incubated with rabbit antimouse immunoglobulins diluted in 1:200 followed by the APAAP complex (BioGenex, USA). Visualization was achieved by a final incubation in chromogen (alkaline phosphatase substrate) containing naphthol AS-MX phosphate, Fast Red TR, and levamisole (BioGenex). The resulting preparations were counterstained with Mayer’s hematoxyline and mounted in warmed aqueous medium. Nonimmune mouse serum was used for negative controls in place of the primary antibody. Known positive controls were included. In all smears, the percentage of positive cells was determined by counting 500 cells in randomly selected areas. Immunoreactivity was evaluated as negative (trace staining) and positive (moderate to strong staining).
Smears for viral cytology have been routinely performed in the Dental Oncology Unit since January 2004 as medically necessary diagnostic procedures, in patients receiving head and neck RT when they develop ulcerative mucositis.
Statistical analysis
Chi-squared test was employed to compare the group of patients who received antifungal prophylaxis with those who did not receive antifungal prophylaxis during RT, with respect to baseline characteristics, incidence of mucositis and incidence of interruptions due to severe mucositis, incidence of candidiasis, as well as antiviral treatment conduct and viral cytology outcome. Loss of weight, age, and daily and total dose of RT were assessed by Mann–Whitney U test. Fisher’s exact test was used to compare the response to empirical antiviral therapy between the groups of patients with ulcerative mucositis, who were cytologically found HSV-1-positive vs HSV-1-negative, and the incidence of HSV-1 infection during RT in relation to concomitant chemotherapy. All statistical tests were two-sided, and level of statistical significance was set at 5%.
Results
Radiotherapy
Forty-seven of all 60 patients completed radiotherapy (RT) within the preplanned time. Four patients interrupted RT due to a heart problem, a cold, a burn neck, and a reduction of white blood cells. Nine patients interrupted RT due to severe mucositis. Patients’ liver and renal function remained within normal limits at the end of RT.
Oral mucositis
Forty-eight patients developed grade 2, 3, and 4 mucositis at different times throughout RT. Twelve patients did not develop ulcerations during RT, depending on the different patient- and treatment-related risk factors [1, 3]. Seven patients (22.5%) of the antifungal prophylaxis group and 16 patients (55.1%) of the group without antifungal prophylaxis completed RT with severe, grade 3, mucositis, as is shown in Table 2. The difference in the incidence of severe mucositis at the end of RT between the two groups, with antifungal prophylaxis vs no antifungal prophylaxis, was found significant (p=0.009). Two patients (6.5%) from the antifungal prophylaxis group and seven patients (24.1%) without antifungal prophylaxis interrupted RT as a result of severe mucositis.
The difference of RT interruptions due to severe mucositis between the group of patients with antifungal prophylaxis as opposed to the patients without antifungal prophylaxis was found to be of marginal statistical significance (p=0.055).
Oral candidiasis
No case of candidiasis was documented in patients with antifungal prophylaxis as opposed to 12 cases of pseudomembranous candidiasis observed in the patients without antifungal prophylaxis. The prevention of candidiasis in the antifungal prophylaxis group was significant (p=0.000).
HSV-1 laboratory investigation
Cytologic smears were available from 29 of all 48 patients with ulcerative mucositis. Smear was not available in the rest (19 patients) of 48 patients with ulcerative mucositis, due to medical–technical difficulties (location of the ulceration on the soft palate and strong reflex of the patient did not allow to rub the brush on that area of the mucosa).
From those 29 patients who were available for smear, 14 received antifungal prophylaxis and 15 did not. Smear with APAAP method identified the presence of HSV-1 in 14 of 29 patients (48.2%; Table 2). As is shown in Table 2, 13 smears stained positive with both Papanicolaou stain and immunocytochemical method (Figs. 1,2, 3 and 4), and 1 smear stained with the immunocytochemical APAAP method only.
Typical cytopathology for herpetic infection is seen, with epithelial multinucleation, nuclear molding, and ground glass appearance. Papanicolaou stain, ×500. Smear taken from the patient of Fig. 1
Immunocytochemical staining was positive for the presence of herpes simplex virus-1 (HSV-1). Alkaline phosphatase/antialkaline phosphatase (APAAP) method, ×500. Smear taken from the patient of Fig. 1
The incidence of viral infection during head and neck RT was assessed as 29.1% (14 positive smears of all 48 patients who developed ulcerative, grade 2, 3, or 4 mucositis).
Chemotherapy was not found to significantly contribute to the development of HSV-1 infection, as 8 of all 29 patients who were investigated for the presence of HSV-1 had received concomitant chemotherapy; of those 8 patients, only 3 developed HSV-1 infection (P=0.682).
No statistically significant difference was also observed in viral cytology between the patients who had received antifungal prophylaxis as opposed to the ones who had not received prophylaxis (P=0.573; Table 2), indicating that candidiasis is not a predisposing factor for HSV-1 infection.
Table 3 shows, in detail, the tumor diagnosis, the age, the presence of severe mucositis at the end of RT and the interruptions of RT due to severe mucositis, the administration of concomitant chemotherapy, the antiviral treatment, and the response to it, in the 14 patients who were found HSV-1-positive. Three of those 14 patients (patient nos. 11, 13, and 14) had interrupted RT for 5–7 days before the administration of the antiviral treatment. As it is shown in Table 3, 11 of 14 HSV-1-positive patients showed reduction in the grade of mucositis after they had received antiviral treatment. None of HSV-1-negative patients who had received empirical antiviral treatment showed healing of mucositis. The difference was statistically significant (P=0.000).
Mucositis was recurring after quitting the antiviral treatment or after reducing the dose from 2 to 1 g of valacyclovir per day.
Empirical antiviral treatment
Upon presumptive diagnosis of viral infection, according to the clinical criteria described in “Patients and methods,” 34 of all 48 patients who developed ulcerative mucositis received empirical antiviral treatment (Table 2). The empirical antiviral treatment was always administered after a cytology smear was taken while waiting for the virology results from the laboratory. No significant difference was observed in the number of patients with empirical antiviral therapy, in each group of patients with antifungal prophylaxis or without (P=0.181).
Sixteen of those 34 patients who received antivirals showed a favorable clinical response with healing of mucositis, from grade 3 or 4 to grade 2 or 1 (Figs. 1 and 2), after 1 week of antiviral treatment.
Side effects after valacyclovir treatment were as follows: Two patients quitted valacyclovir, after 1 day of administration; one due to a stomach ache and one due to vomiting. One patient (the patient in Figs. 1 and 2) showed urine retention after receiving valacyclovir 2 g/day for 1 week. Urination was normalized after quitting valacyclovir. One patient showed a skin rash after 2 g of valacyclovir; rash regressed after reducing the dose to 1.5 g.
Discussion
HSV-1 infection has been well documented as an important component of chemotherapy-induced ulcerative mucositis, complicating as many as 50% of the cases of severe, grades 3 and 4, mucositis [4, 7, 12, 13, 23, 24]. Especially with relation to the location of the ulcers, Bergmann et al. [4], in their series of patients with acute myeloid leukemia, receiving remission induction chemotherapy, concluded that intraoral ulcers excluding the soft palate are most often due to infection with HSV, whereas ulcers located on the soft palate have a nonherpetic etiology.
In contrast, in radiation-induced mucositis, during head and neck RT, HSV-1 infection has been addressed in few studies [5, 15, 17, 20] with conflicting results. Redding et al. [20] found no HSV-positive culture in 18 smears taken from the oral radiation-induced ulcerations.
Bubley et al. [5] reported a significantly higher incidence of HSV-positive cultures, but no clinical benefit was observed in relation to acyclovir administration. In addition, patients with negative HSV cultures did develop orofacial vesicular lesions at the vermillion border (which are known to be typical herpes labialis lesions). On the other hand, Oakley et al. [17] and Nicolatou-Galitis et al. [15] reported two and five cases, respectively, of HSV infection complicating oral ulcerative mucositis during RT, with positive response to antiviral treatment.
In the present prospective study, 60 patients were followed during head and neck RT; 48 of those patients developed oral radiation-induced ulcerative mucositis. Twenty-nine smears were available, taken from 29 patients who developed oral ulcerations located on the anterior part of the oral cavity. Fourteen of those 29 smears were found HSV-1-positive, and an incidence of 14 HSV-1-positive out of 48 patients at risk with ulcerations (29.1%) was estimated.
The difficulty and the inability to take smears from 19 patients with ulcerations located on the soft palate was considered as a limitation of the study. At this point, however, we would like to mention Bergmann’s et al. report [4], where ulcers located on the soft palate had a nonherpetic etiology. According to that report, we could postulate that the ulcers on the soft palate in the 19 patients who were not evaluated, were, most possibly, HSV negative, and the incidence of the virus was not far higher from the 29.1%.
Mucositis was reduced to a lower grade after 1 week of 1 or 2 g/day of valacyclovir in 11 out of 14 patients. Mucositis worsened to a higher grade after quitting or reducing the dose of the antivirals in the 11 patients. On the other hand, none of the HSV-1-negative patients showed healing or reduction in the grade of mucositis after empirical antiviral treatment. The difference was significant (P=0.000). The above clinical response of radiation mucositis to antivirals denoted that HSV-1 infection had aggravated the lesions of ulcerative radiation-induced mucositis. The results of the present study are also in accordance to the current model of mucositis [25, 26], where HSV-1, through the activation of NF-κB, may aggravate mucosal injury.
In relation to the reported discrepancies on the isolation of HSV from oral ulcerative radiation mucositis, technical difficulties in viral culture might be an explanation (e.g., the inactivation or washing out of the virus by the common, hourly use of mouthwashes by the patient during RT). Similar difficulties were also observed in our laboratory trying to culture the virus from ulcerative mucositis lesions, although the patients had obviously and immediately responded to antiviral treatment (unpublished observations).
On the contrary, cytology with Papanicolaou stain [2] is a simple and cheap technique and is not influenced by the mouthwash. A limitation of Papanicolaou-stained cytology is the reported 5% false-negative results [2]. In deed, one of our specimens was found negative with Papanicolaou, and the virus was isolated using the APAAP method.
The absence of clinical response to acyclovir, as reported by Bubley et al. [5], in patients with positive viral cultures might be related to the dosage scheme (acyclovir administered every 12 h and not every 4 h, in a dose of 1.6 g/day).
Chemotherapy was not found to be an important predisposing factor for HSV-1 infection during RT in the present study. The above finding is in agreement with our previous pilot study of HSV-1 infection [15], where, again, chemotherapy was not a significant contributing factor for HSV-1 infection during RT.
Candidiasis was not found to be a predisposing factor for HSV-1 infection either. No significant differences were observed either in the administration of the empirical antiviral treatment or in positive viral cytology between the patients who received antifungal prophylaxis vs the ones who did not (Table 2; P=0.181 and P=0.573, respectively).
Whether herpetic infection in our patients appeared as a primary or as a secondary infection after reactivation of a latent virus is not known since no antibody titers for HSV-1 were determined. The above lack of investigation on the patients’ viral serology was considered as a weakness of the study. We could, however, assume that HSV-1 infections in our patients appeared after reactivation of the virus, which was in latency within the gasserion ganglion of the trigeminal nerve. The above assumption is based on the age of our patients, combined with the absence of clinical herpetic gingivitis, which is a characteristic of the primary infection.
Trigeminal ganglion was included, in all our cases, in the radiation field, indicating that RT might have played a role in the presumed reactivation of a latent virus. The psychosomatic stress of the cancer patient and the local trauma, caused directly to the oral mucosa by the radiation, might have also triggered the reactivation of the virus.
Although the effect of antifungal prophylaxis on the severity of mucositis and RT interruptions was not included in the aim of the present investigation, a statistical analysis of the above factors was performed since 31 of our 60 patients had received antifungal prophylaxis and only 25 of all 60 had been included in our previous study on antifungal prophylaxis [16]. A significantly lower incidence of severe mucositis at the end of RT was seen in the patients with antifungal prophylaxis, while the reduced number of interruptions in the same group was of marginal statistical significance. However, the significantly lower median total dose of RT in the group of patients with antifungal prophylaxis could have accounted, at least in part, for the reduced incidence of severe mucositis and RT interruptions.
In conclusion, HSV-1 infection was found with an incidence of 29.1% and was observed to aggravate radiation-induced oral ulcerative mucositis. Further studies are needed to evaluate the dose, duration, and any possible side effects of the antivirals during the course of RT. The use of antiviral prophylaxis could be also evaluated in a future study, in HSV-1-seropositive patients receiving head and neck RT, given the high incidence of the infection and the difficulty in clinical differential diagnosis from radiation mucositis.
References
Barasch A, Peterson DE (2003) Risk factors for ulcerative oral mucositis in cancer patients: unanswered questions. Oral Oncol 39:91–100
Barrett AP, Buckley DJ, Greenberg ML et al (1986) The value of exfoliative cytology in the diagnosis or oral herpes simplex infection in immunosuppressed patients. Oral Surg Oral Med Oral Pathol 62:175–178
Berger AM, Fall-Dickinson (2005) Oral complications. In: DeVita VT, Hellman S, Rosenberg SA (eds) Cancer principles and practice of oncology, 7th edn. Lippincott Williams & Wilkins, Philadelphia, pp 2523–2524
Bergmann OJ, Ellermann-Eriksen S, Mogesen SC, Ellegaard J (1995) Acyclovir given as prophylaxis against oral ulcers in acute myeloid leukemia: randomized, double blind, placebo controlled trial. BMJ 310:1169–1172
Bubley GJ, Chapman B, Chapman SK, Crumpacker CS, Schnipper LE (1989) Effect of acyclovir on radiation- and chemotherapy-induced mouth lesions. Antimicrob Agents Chemother 33:862–865
Buddingh GJ, Schrum DI, Lanier JC et al (1953) Studies of the natural history of herpes simplex infections. Pediatrics 11:595–609
Carrega G, Castagnola E, Canessa A et al (1994) Herpes simplex virus and oral mucositis in children with cancer. Support Care Cancer 2:266–269
Cohen PR (1994) Tests for detecting herpes simplex virus and varicella-zoster virus infections. Dermatol Clin 12:51–68
Cordell JL, Falini B, Erber WN et al (1984) Immuno-enzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes). J Histochem Cytochem 32:219–229
Corey L, Spear PG (1986) Infections with herpes simplex viruses. First of two parts. N Engl J Med 314:686–691
Eisen D (1998) The clinical characteristics of intraoral herpes simplex virus infection in 52 immunocompetent patients. Oral Surg Oral Med Oral Pathol 86:432–437
Epstein JB, Sherlock C, Page JL et al (1990) Clinical study of herpes simplex virus infection in leukemia. Oral Surg Oral Med Oral Pathol 70:38–43
Greenberg M, Cohen S, Boosz B, Friedman H (1987) Oral herpes simplex virus infections in patients with leukemia. J Am Dent Assoc 114:483–486
Neville BW, Damm DD, Allen CM, Bouquot JE (1995) Oral and maxillofacial pathology. Saunders, Philadelphia, p 185
Nicolatou-Galitis O, Dardoufas K, Markoulatos P et al (2001) Oral pseudomembranous candidiasis, herpes simplex virus-1 infection, and oral mucositis in head and neck cancer patients receiving radiotherapy and granulocyte-macrophage colony-stimulating factor (GM-CSF) mouthwash. J Oral Pathol Med 30:471–480
Nicolatou-Galitis O, Velegraki A, Sotiropoulou-Lontou A et al (2005) Effect of fluconazole antifungal prophylaxis on oral mucositis in head and neck cancer patients receiving radiotherapy. Support Care Cancer (Epub ahead of print)
Oakley C, Epstein JB, Sherlock CH (1997) Reactivation of oral herpes simplex virus. Implications for clinical management of herpes simplex virus recurrence during radiotherapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 84:272–278
Ohrn KEO, Wahlin YB, Sjoden OP (2001) Oral status during radiotherapy and chemotherapy: a descriptive study of patient experiences and the occurrence of oral complications. Support Care Cancer 9:247–257
Pevenstein SR, Williams RK, McChesney D et al (1999) Quantitation of latent varicella-zoster virus and herpes simplex virus genomes in human trigeminal ganglia. J Virol 12:10514–10518
Redding SW, Luce EB, Boren MW (1990) Oral herpes simplex virus infection in patients receiving head and neck radiation. Oral Surg Oral Med Oral Pathol 69:578–580
Riel-Romero RMS, Baumann RJ (2003) Herpes simplex encephalitis and radiotherapy. Pediatr Neurol 29:69–71
Rubenstein EB, Peterson DE, Schubert M et al (2004) Clinical practice guidelines for the prevention and treatment of cancer therapy-induced oral and gastrointestinal mucositis. Cancer 100(9 Suppl):2026–2046
Saral R, Burns WH, Laskin OL, Santos GW (1981) Acyclovir prophylaxis of herpes-simplex-virus infections. N Engl J Med 305:63–67
Schubert MM, Peterson DE, Flournoy N et al (1990) Oral and pharyngeal herpes simplex virus infection after allogeneic bone marrow transplantation: analysis of factors associated with infection. Oral Surg Oral Med Oral Pathol 70:286–293
Sonis ST (2002) The biologic role for nuclear factor-kappaB in disease and its potential involvement in mucosal injury associated with anti-neoplastic therapy. Crit Rev Oral Biol Med 13:380–389
Sonis ST (2004) A biological approach to mucositis. J Support Oncol 2:21–36
Trotti A, Bellm LA, Epstein JB et al (2003) Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: a systematic literature review. Radiother Oncol 66:253–262
Wijers OB, Levendag PC, Harms ERE et al (2001) Mucositis reduction by selective elimination of oral flora in irradiated cancers of the head and neck: a placebo-controlled double-blind randomized study. Int J Radiat Oncol Biol Phys 50:343–352
Acknowledgements
We wish to thank Professor Aristea Velegraki for the laboratory verification of mycology smears. The reagents for the mycology laboratory investigation were kindly donated by Pfizer Hellas. GlaxoSmithKline kindly supported part of the costs of the investigation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nicolatou-Galitis, O., Athanassiadou, P., Kouloulias, V. et al. Herpes simplex virus-1 (HSV-1) infection in radiation-induced oral mucositis. Support Care Cancer 14, 753–762 (2006). https://doi.org/10.1007/s00520-005-0006-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-005-0006-5