Abstract
Goals of work
We report on the routine use of the NCCN Distress Thermometer and the Functional Assessment of Cancer Therapy—Brain (FACT-Br) to assess patient distress and quality of life in GBM patients. The purpose of this study was to examine the relationship between patient quality of life and distress.
Materials and methods
Data from 50 GBM patients presenting to a neuro-oncology clinic were evaluated. Descriptive statistics and correlations between the distress score and the FACT-Br subscale scores were generated.
Main results
The mean distress score was 2.15 (std 2.66), and 28.9% of brain tumor patients identified a distress score of 4 or above. The mean FACT-Br total was 127.34 (std 21.29), with patients scoring lowest in the EWB (18.95 std 4.4) and FWB (15.06 std 6.80) subscales. No differences between demographic groups were identified with regard to distress or quality of life. Statistically significant correlations were identified between the distress score and the SWB (R = −0.46, P = 0.001) and EWB (R = −0.56, P = 0.001) subscales of the FACT-Br. Fifty percent of participants who did not complete the FACT-Br reported clinically significant distress, but this did not differ significantly from participants who completed it.
Conclusions
Assessment of distress in brain tumor patients provides clinically relevant information and suggests interventions that may support quality of life. Further research is needed to explore the relationship between distress and quality of life. Current approaches to measuring quality of life in brain tumor patients may systematically undersample patients with advanced illness or significant psychosocial distress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glioblastoma multiforme is the most common type of malignant primary brain tumor and accounts for 25% of cases. It most commonly affects adults aged 50 to 70 years and is seen more often in men than women (ratio 3:2) [25]. This type, considered to be a Grade IV astrocytoma, spreads quickly and rapidly invades brain tissue. These high-grade gliomas are difficult to cure and the recurrence rate even after surgery and high dose radiation therapy remains high [4]. Brain cancer is an illness that is particularly devastating to patients and families: patients frequently experience neurological and psychiatric disruption of their personhood; patients tend to be younger, and are therefore more likely to be in an age group where they are wage-earners and care-givers.
Despite advances in therapies that offer improved survival [21, 23], the clinical course of most primary brain cancers is one of progressive deterioration followed by death. The median survival of patients with glioblastoma multiforme (GBM) treated with the combination of radiotherapy and temozolomide is 14.6 months [23]. The 2-year survival rate for this combination therapy is 26.5%. Maintaining patient quality of life and minimizing patient distress are significant therapeutic goals in this population.
The literature gives some insight into factors associated with quality of life in brain tumor patients. Disease progression is consistently associated with deterioration in quality of life (4, 5). The negative impact of disease progression on quality of life appears most evident in the QOL domains of physical functioning, social functioning, and role functioning. This impact may be mediated to some extent by neurological function. Osoba et al. examined the impact of neurological dysfunction on HRQOL in high-grade glioma and found that among patients who were dependent for activities of daily living, the QOL domains of role and physical functioning were significantly negatively impacted, as were global quality of life scores [16]. In a study with the purpose of identifying determinants of quality of life in patients with recurrent high-grade glioma, researchers utilized the Functional Living Index—Cancer (FLIC) QOL self evaluation. Higher FLIC scores were associated with better cognition, better physical performance and better mood [6], suggesting a role for both neurological and physical function as determinants of quality of life.
The literature also provides evidence of the impact of symptom burden on quality of life. Physical symptoms and side effects of treatment are identified by patients as their number one unmet supportive care need [10]. Fatigue, which is identified by general populations as their most troubling symptom [20], is a frequent and burdensome symptom among brain cancer patients. The disease process itself contributes to fatigue, and brain tumor patients are highly likely to receive treatments, such as cranial irradiation, that are associated with high levels of fatigue. Fatigue has been noted in a number of studies in high-grade-glioma patients [1, 19]. Depression has a high prevalence among patients with brain cancer, with depressive symptoms in one study being reported by 69 (95%) of 73 participants [5]. Cognitive impairment is common among patients with high-grade glioma, and is identified by patients as a highly troubling symptom that is also associated with functional decline [16]. Sleep disturbance has been reported at a very high prevalence among brain cancer patients. In one study examining a mixed population of primary brain tumor patients, 100% of 73 participants reported sleep disturbance [5]. There is increasing recognition that symptoms do not occur in isolation in brain tumor patients, and that there appear to be predictable clusters of symptoms that occur at a level higher than would be expected to occur randomly. The literature includes descriptions of symptom clusters that include the symptoms of depression, fatigue, sleep disturbance, and cognitive impairment [5].
Functional status has been linked with quality of life in a number of studies. The finding that inpatient rehabilitation efforts that improve functional status do not significantly improve quality of life raises some important issues in this population [8]. Decline in functional status is clearly associated with disease progression, and quality of life also declines with disease progression. It may not be possible to separate these factors during an intervention study [7].
As noted above, depression and anxiety are highly prevalent among brain tumor patients. In recent years the NCCN has advocated screening for symptoms of psychological distress among all cancer patients. This recommendation is based on evidence in the literature that cancer care providers do not do well at identifying psychological distress when it is not intentionally screened for [17, 18]. The prevalence of symptoms of psychological distress is generally higher than the prevalence of anxiety or depression meeting diagnostic criteria, and recent publications indicate that high levels of psychological distress occurs among brain tumor patients. Among a sample of 60 adult patients with primary brain tumors, 63% reported experiencing elevated levels of stress on the ten-item Perceived Stress Scale [13]. Use of the Distress Thermometer, a single-item instrument that compares favorably with longer instruments, also allows patients to identify factors that they feel are contributing to their distress [9]. This feature of the instrument may be particularly useful in populations such as brain tumor patients where there is little evidence to support interventions to reduce distress or improve quality of life.
Psychological distress has a significant impact on quality of life. In symptom cluster analysis, depression explained 26% of the variance in quality of life, and 56% of the variance in functional status in brain cancer patients [5]. Similarly, Pelletier et al. found that the presence of depressive symptoms was the most significant predictor of quality of life in a sample of brain tumor patients [19]. This study did not utilize a discreet instrument for psychosocial distress, but utilized emotional distress as measured by the FACT-Br. Researchers in this study found that emotional distress increased with length of survival. The relationship between psychological distress and quality of life in primary brain tumor patients has not been extensively examined in the literature, but these findings suggest that effective interventions to reduce psychological distress could help support patient quality of life in brain tumor patients. The purpose of the present study is twofold: (1) to describe the relationship between psychological distress and quality of life in brain tumor patients; (2) to identify patient-reported attributions for psychological distress. An enhanced understanding of the role of psychological distress in overall quality of life may give insight into the development of interventions to support quality of life in these patients.
Materials and methods
Participants and procedures
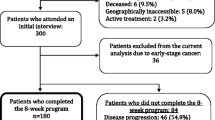
All study activities were reviewed and approved by the Institutional Review Board at the University of Alabama at Birmingham (UAB). The participants were drawn from a convenience sample of all primary brain tumor patients who were seen at the Neuro-oncology clinic at UAB between January 2007 and April 2007. Only the first visit each patient had during the study period was included in this cross-sectional analysis. Screening for psychological distress and surveillance of quality of life are components of routine clinical care in this clinic. Medical records from 320 consecutive patients who presented to the neuro-oncology clinic at UAB between January 2007 and April 2007 were assessed. Additional inclusion criteria were: (1) histologically confirmed glioblastoma multiforme (2) legal age (3) ability to complete self-report instruments. Seventy-five individuals were identified as being eligible for inclusion in the study.
Study instruments
The distress thermometer
The single-item distress thermometer was developed by Roth et al. as a rapid means to screen for distress in cancer patients (Roth). It has been adapted by the National Comprehensive Cancer Network (NCCN) as an element of the Clinical Practice Guidelines for Distress Management. The Distress Thermometer has an 11-point range (0–10), with the endpoints identified as “no distress” and “extreme distress”. The operating characteristics of the Distress Thermometer as a screen for psychological distress were evaluated in a multi-center study involving 380 participants that compared the use of this instrument to longer screening instruments, the 14-item Hospital Anxiety and Depression Scale (HADS) and an 18-item version of the Brief Symptom Inventory (BSI-18). Receiver operating characteristic (ROC) curve analysis demonstrated area under the curve estimates indicative of the accuracy of the distress thermometer relative to the HADS (0.80) and the BSI (0.78). The distress thermometer score of 4 was established as the cutoff that demonstrated optimal sensitivity and specificity using either the HADS or the BSI as the criterion, with a sensitivity of 0.77 and specificity of 0.68 using the HADS as the criterion, and similar values (0.7, 0.7) utilizing the BSI. The version of the distress thermometer recommended in the NCCN Distress Management Guidelines also includes a problem list consisting of 32 problems that are grouped into five categories (practical problems, family problems, emotional problems, spiritual/religious concerns, and physical problems). The problem list asks patients to identify factors that have contributed to their distress in the past week.
The functional assessment of cancer therapy—brain (FACT-Br)
The FACT-Br is a quality of life instrument consisting of the Functional Assessment of Cancer general version (FACT-G) with an additional subscale specific for brain tumor patients. The FACT-G consists of 33 questions assessing five domains including physical well-being (PWB, seven items), social/family well-being (SWB, seven items), relationship with physician (two items), emotional well-being (EWB, five items), and functional well-being (FWB, 7 items). The brain subscale consists of 15 items that assess areas of concern such as neurocognitive function and functional ability and mobility demonstrated in the instrument development process to measure facets of quality of life important to brain cancer patients and distinct from those assessed by the FACT-G. The psychometric properties of the FACT-Br were evaluated and the instrument was found to maintain the good psychometric properties of the FACT-G with convergent validity (r = 0.5) with similar measures. Test–retest reliability demonstrated high correlations at a 1-week time frame (r, 0.78; P < 0.001). Participants who were unable to complete the instrument were excluded from the validation studies [24].
Statistics
Descriptive statistics (i.e., frequencies, proportions, and t tests) were used to characterize the demographics of the population. The Wilcoxon rank sum test was used to compare distress and quality of life scores by race and gender. Spearman rank correlation coefficients were calculated to determine the relationship between the Distress Thermometer score and the FACT-Br subscales. A chi-square test was used to determine whether a distress score of greater than 4 was associated with failure to complete the FACT-Br.
Results
Overall, data from 75 patients with GBM who presented to the neuro-oncology clinic at UAB between January 2007 and April 2007 were analyzed. Population demographics are described in Table 1. Of these 75, 25 patients were eliminated from data analysis because they had an incomplete FACT-Br and five patients were eliminated due to proxy completion of the FACT-Br. Demographic information on patients who did not complete the FACT-Br are also presented in Table 1. They did not differ significantly from the patients who did complete the FACT-Br.
Quality of life
The average FACT-Br total score for this population was 127.33 (std 27.2). For the 50 participants in the study, the brain subscale score (mean 48.8; std14.3), social well-being (mean 23.3; std 4.7), physical well-being (mean 21.2; std 5.9), emotional well-being (mean19.0; std 4.4), and functional well-being (mean 15.1; std 6.8). The two racial groups and the two gender groups were compared with respect to all of these measures and no statistically significant differences were identified.
Distress scores
Among the 50 patients who individually and fully completed both assessment tools, the mean distress score was 2.15 (std 2.66). No differences in mean distress score were identified for age, gender, or race. Twenty-eight percent of patients indicated a distress score of 4 or greater, indicating a clinically significant level of distress. Among the 28% who reported elevated distress scores, only two patients documented a distress level of 8 or higher.
Analysis of attributions identified by patients as sources of distress was conducted to identify the most frequently reported issues within the domains denoted in the DT. The top two attributions in each of four domains, practical/logistical, psychological/emotional, symptom related, and functional, are reported in Fig. 1.
Associations between quality of life and distress
The relationship between quality of life scores and distress was evaluated through examination of correlations between quality of life total and subscale results and the DT score. Moderately strong and statistically significant inverse correlations between the total distress score and the Social Well-being (SWB) subscale of the FACT-Br (R = −0.46, P = 0.001) and the Emotional Well-being (EWB) subscale(R = −0.56. P < 0.001) and a lesser degree of correlation with the Physical subscale (−0.23, P = 0.134) was identified. The Functional Well-being (FWB; −0.27, P = .070) and BR subscale (−0.27, P = .069) demonstrated trends toward significance (Table 2).
Incomplete assessments
A substantial number of the eligible participants failed to complete either the DT and the FACT-Br, or the FACT-Br. No instances were identified where a patient completed the FACT-Br but not the Distress Thermometer. Twenty-five of the 75 potentially eligible subjects (33%) provided data inadequate for inclusion. Twenty people had FACT-Brs with an inadequate degree of completion for scoring, and five people had FACT-Brs with indication of proxy completion. Within this group of twenty five, seven participants also did not complete the DT. Among the twenty patients who had an incomplete FACT-Br, twelve patients completed the distress thermometer portion of the survey and six of these (50%) documented a score of 4 or greater. Of the six patients reporting clinically significant distress, three patients indicated a distress level of 8 or greater. Two of the five patients who were eliminated due to proxy completion of the FACT-Br indicated a significant distress level with a score of 4 or greater.
Exploratory comparison of proportions of individuals with elevated distress scores in the groups that submitted a Complete, Incomplete, or Proxy-complete FACT-Br did not identify statistically significant group differences.
Discussion
Quality of life and distress are important considerations in patients with malignant brain tumors. Without therapy, patients die from this brain tumor within 3 months. With extensive debulking surgery, radiation, and chemotherapy, median survival is 12 months [22]. These patients often present with neurological signs or deficits that reflect the location and neurochemical activity of the tumor. These can include slowed cognition, personality changes, mood changes, and a deficit in concentration or memory [14]. The side effects of the currently available therapies further exacerbate these neuropsychiatric symptoms. The routine, concurrent use of the FACT-Br and Distress thermometer in the clinical care of these patients has allowed the neuro-oncology team to clinically monitor response to treatments. Analysis of these data allows evaluation of the association between distress and quality of life among patients with GBM, yields insight into factors that contribute to patient’s level of distress, and underscores the limitations of standard approaches to quality of life assessment in this population.
The quality of life scores in this population were fairly well-preserved, with total and subscale scores substantially higher than those reported in other trials in populations of patients with high-grade gliomas [1]. Factors that have been demonstrated in other studies to have an association with quality of life include duration and stage of illness, and presence of depression [3]. This sample is primarily comprised of persons who had been diagnosed within the past year, and is also a fairly young sample, and these characteristics may have influenced findings. Preserved quality of life in this sample, however, may reflect the rigorous methods to exclude incomplete and proxy-completed FACT-Brs, as most studies do not comment in the methods on their approach to proxy-completed forms in particular. Another factor that may have influenced QOL reporting in this population is the fact that these instruments were utilized as a component of routine clinical care in neuro-oncology clinic, and were not associated with a specific study protocol. Patients may consider and report quality of life differently when the instrument is utilized as a routine component of care. It is not possible with the data available in this study to evaluate other factors, such as the presence of a caregiver in the patient’s home, that might have an important impact on quality of life.
The mean Distress Thermometer score in this population was somewhat lower than what has been reported in other studies of high-grade-glioma patients [12]. We hypothesized that the exclusion of participants who had failed to complete the FACT-Br may have led to the systematic exclusion of patients with higher levels of distress, but comparison of distress among the participants who completed the FACT-Br with those who did not, did not identify statistically significant group differences. It is also noteworthy that this sample was relatively young, with a mean age of 53.3. There were higher rates of completion for the Distress Thermometer than for the FACT-Br, suggesting that this was a less burdensome instrument for the patients to complete. These findings underscore the challenges of adequately assessing the factors that contribute to quality of life in this population, which is characterized by functional and communication impairment relatively early in the disease trajectory. Preliminary work indicates that single-item quality of life assessment or caregiver proxy assessment may improve response rates in this population [2, 15]. Further research to explore whether assistive technologies such as computer based assessment or interview approach to instrument completion improves response rates in this population is called for.
The relationship between Distress Thermometer scores and FACT-Br scores demonstrated the predicted inverse relationship. This relationship was stronger for quality of life scores in the SWB and EWB subscales, but did not achieve statistical significance in other subscales. The trends observed in the FWB and BR subscales may be important, and should be re-evaluated in a larger study. Further study is needed to determine whether interventions aimed at factors contributing to patient distress have potential to preserve quality of life. The areas most frequently reported by patients as factors that contribute to distress underscore the need for a multi-disciplinary approach to caring for brain tumor patients. For example, a brain tumor team social worker could address issues related to transportation and insurance and financial issues, a team psychologist could address depression and work with patients to reframe thinking about cognitive losses to diminish associated distress, a team nutritionist or speech pathologist could assist with issues associated with eating, and a palliative specialist could assist as needed with symptom management. Current limitations to employing a multi-disciplinary approach to the care of brain tumor patients include a reimbursement structure that makes such approaches to care financially unsustainable. In addition, the limited capacity of patients with brain tumor to engage with interventions, even those that they identify as potentially beneficial, has been noted in other studies and must be considered in subsequent research [11].
The strengths of the current study include the use of data that reflect the routine assessment of quality of life and distress instruments in a neuro-oncology clinic to provide a cross-sectional description of these constructs in clinic patients. The findings presented here suggest that interventions to reduce psychological distress may have a role in preserving quality of life for brain tumor patients, at least with regard to psychological and social well-being. A methodologically rigorous approach to patient self-report on the FACT-Br ensured that the instrument was utilized in a manner consistent with validation studies, but also increased the number of exclusions from this study. It was felt that this level of rigor was necessary in an initial report on the relationship between the constructs of distress and quality of life in this population. Efforts were made to describe excluded participants and report on the level of distress among those excluded due to incomplete FACT-Br’s. It is difficult to consider the role of disease progression with regard to distress and quality of life in this cross-sectional report. Duration of illness was assessed using date of diagnosis as a reference point. It may be more meaningful in this population to utilize date of death as a referent point in assessing quality of life, but such an approach was not feasible given that a large number of participants remained in active treatment at the conclusion of data analysis. The relatively small sample size presents a threat to generalizability of these findings to other populations of GBM patients. The continued routine use of assessment of quality of life and distress in the neuro-oncology clinic will enable reporting of robust longitudinal data to provide a more precise understanding of the trajectory and mediators of quality of life in this population.
References
Brown PD, Ballman KV, Rummans TA, Maurer MJ, Sloan JA, Boeve BF, Gupta L, Tang-Wai DF, Arusell RM, Clark MM, Buckner JC (2006) Prospective study of quality of life in adults with newly diagnosed high-grade gliomas. J Neurooncol 76:283–291 doi:10.1007/s11060-005-7020-9
Brown PD, Decker PA, Rummans TA, Clark MM, Frost MH, Ballman KV, Arusell RM, Buckner JC (2008) A prospective study of quality of life in adults with newly diagnosed high-grade gliomas: comparison of patient and caregiver ratings of quality of life. Am J Clin Oncol 31:163–168 doi:10.1097/COC.0b013e318149f1d3
Brown PD, Maurer MJ, Rummans TA, Pollock BE, Ballman KV, Sloan JA, Boeve BF, Arusell RM, Clark MM, Buckner JC (2005) A prospective study of quality of life in adults with newly diagnosed high-grade gliomas: the impact of the extent of resection on quality of life and survival. Neurosurgery 57:495–504 discussion 495–504 doi:10.1227/01.NEU.0000170562.25335.C7
Fine HA, Dear KB, Loeffler JS, Black PM, Canellos GP (1993) Meta-analysis of radiation therapy with and without adjuvant chemotherapy for malignant gliomas in adults. Cancer 71:2585–2597 doi:10.1002/1097-0142(19930415)71:8<2585::AID-CNCR2820710825>3.0.CO;2-S
Fox SW, Lyon D, Farace E (2007) Symptom clusters in patients with high-grade glioma. J Nurs Scholarsh 39:61–67 doi:10.1111/j.1547-5069.2007.00144.x
Giovagnoli AR, Silvani A, Colombo E, Boiardi A (2005) Facets and determinants of quality of life in patients with recurrent high grade glioma. J Neurol Neurosurg Psychiatry 76:562–568 doi:10.1136/jnnp.2004.036186
Huang ME, Wartella J, Kreutzer J, Broaddus W, Lyckholm L (2001) Functional outcomes and quality of life in patients with brain tumours: a review of the literature. Brain Inj 15:843–856 doi:10.1080/02699050010013653
Huang ME, Wartella JE, Kreutzer JS (2001) Functional outcomes and quality of life in patients with brain tumors: a preliminary report. Arch Phys Med Rehabil 82:1540–1546 doi:10.1053/apmr.2001.26613
Jacobsen PB, Donovan KA, Trask PC, Fleishman SB, Zabora J, Baker F, Holland JC (2005) Screening for psychologic distress in ambulatory cancer patients. Cancer 103:1494–1502 doi:10.1002/cncr.20940
Janda M, Steginga S, Dunn J, Langbecker D, Walker D, Eakin E (2008) Unmet supportive care needs and interest in services among patients with a brain tumour and their carers. Patient Educ Couns 71:251–258 doi:10.1016/j.pec.2008.01.020
Jones LW, Guill B, Keir ST, Carter K, Friedman HS, Bigner DD, Reardon DA (2007) Using the theory of planned behavior to understand the determinants of exercise intention in patients diagnosed with primary brain cancer. Psychooncology 16:232–240 doi:10.1002/pon.1077
Keir ST, Calhoun-Eagan RD, Swartz JJ, Saleh OA, Friedman HS (2008) Screening for distress in patients with brain cancer using the NCCN’s rapid screening measure. Psychooncology 17:621–625
Keir ST, Guill AB, Carter KE, Friedman HS (2006) Stress and intervention preferences of patients with brain tumors. Support Care Cancer 14:1213–1219 doi:10.1007/s00520-006-0087-9
Laack NN, Brown PD, Ivnik RJ, Furth AF, Ballman KV, Hammack JE, Arusell RM, Shaw EG, Buckner JC (2005) Cognitive function after radiotherapy for supratentorial low-grade glioma: a North Central Cancer Treatment Group prospective study. Int J Radiat Oncol Biol Phys 63:1175–1183 doi:10.1016/j.ijrobp.2005.04.016
Locke DE, Decker PA, Sloan JA, Brown PD, Malec JF, Clark MM, Rummans TA, Ballman KV, Schaefer PL, Buckner JC (2007) Validation of single-item linear analog scale assessment of quality of life in neuro-oncology patients. J Pain Symptom Manage 34:628–638 doi:10.1016/j.jpainsymman.2007.01.016
Osoba D, Aaronson NK, Muller M, Sneeuw K, Hsu MA, Yung WK, Brada M, Newlands E (1997) Effect of neurological dysfunction on health-related quality of life in patients with high-grade glioma. J Neurooncol 34:263–278 doi:10.1023/A:1005790632126
Passik SD, Donaghy KB, Theobald DE, Lundberg JC, Holtsclaw E, Dugan WM (2000) Oncology staff recognition of depressive symptoms on videotaped interviews of depressed cancer patients: implications for designing a training program. J Pain Symptom Manage 19:329–338 doi:10.1016/S0885-3924(00)00137-8
Passik SD, Dugan W, McDonald MV, Rosenfeld B, Theobald DE, Edgerton S (1998) Oncologists’ recognition of depression in their patients with cancer. J Clin Oncol 16:1594–1600
Pelletier G, Verhoef MJ, Khatri N, Hagen N (2002) Quality of life in brain tumor patients: the relative contributions of depression, fatigue, emotional distress, and existential issues. J Neurooncol 57:41–49 doi:10.1023/A:1015728825642
Portenoy R, Thaler H, Kornblith A, Lepore J, Friedlander-Klar H, Kiyasu E, Sobel K, Coyle N, Kemeny M, Norton L (1994) The Memorial Symptom Assessment Scale: an instrument for the evaluation of symptom prevalence, characteristics and distress. Eur J Cancer 30A:1326–1336 doi:10.1016/0959-8049(94)90182-1
Robe PA, Martin D, Albert A, Deprez M, Chariot A, Bours V (2006) A phase 1-2, prospective, double blind, randomized study of the safety and efficacy of sulfasalazine for the treatment of progressing malignant gliomas: study protocol of ISRCTN45828668. BMC Cancer 6:29 doi:10.1186/1471-2407-6-29
Salmaggi A, Silvani A, Merli R, Caroli M, Tomei G, Russo A, Riva M, Marchioni E, Imbesi F (2008) Multicentre prospective collection of newly diagnosed glioblastoma patients: update on the Lombardia experience. Neurol Sci 29:77–83 doi:10.1007/s10072-008-0865-x
Stupp R, Dietrich PY, Ostermann Kraljevic S, Pica A, Maillard I, Maeder P, Meuli R, Janzer R, Pizzolato G, Miralbell R, Porchet F, Regli L, de Tribolet N, Mirimanoff RO, Leyvraz S (2002) Promising survival for patients with newly diagnosed glioblastoma multiforme treated with concomitant radiation plus temozolomide followed by adjuvant temozolomide. J Clin Oncol 20:1375–1382 doi:10.1200/JCO.20.5.1375
Weitzner MA, Meyers CA, Gelke CK, Byrne KS, Cella DF, Levin VA (1995) The Functional Assessment of Cancer Therapy (FACT) scale. Development of a brain subscale and revalidation of the general version (FACT-G) in patients with primary brain tumors. Cancer 75:1151–1161 doi:10.1002/1097-0142(19950301)75:5<1151::AID-CNCR2820750515>3.0.CO;2-Q
Wrensch M, Minn Y, Chew T, Bondy M, Berger MS (2002) Epidemiology of primary brain tumors: current concepts and review of the literature. Neuro-oncol 4:278–299 doi:10.1215/15228517-4-4-278
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kvale, E.A., Murthy, R., Taylor, R. et al. Distress and quality of life in primary high-grade brain tumor patients. Support Care Cancer 17, 793–799 (2009). https://doi.org/10.1007/s00520-008-0551-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-008-0551-9