Abstract
Apomictic seed development in dandelion (Taraxacum officinale) involves (1) restitutional meiosis (diplospory), (2) egg cell parthenogenesis, and (3) autonomous endosperm development. The question is whether these elements of apomixis are controlled by one single gene or by several independent genes. Five triploid non-apomictic hybrids, obtained in diploid sexual × triploid apomict crosses were characterized using cyto-embryological and genetic methods. Nomarski-differential interference contrast microscopy and the transmission of microsatellite markers and ploidy levels indicated that the hybrids combined elements of the apomictic and the sexual developmental pathway. Hybrids form two complementary groups with respect to the presence or absence of parthenogenesis and autonomous endosperm development. The occurrence of complementary apomixis-recombinants suggests that parthenogenesis and autonomous endosperm development in Taraxacum are regulated independently by different genes. This study also indicates that early embryo development is independent of endosperm formation, but that endosperm is essential for later embryo growth.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sexual seed development in the angiosperms involves the unique process of double fertilization (Nawaschin 1898; Willemse and Van Went 1984). In this process the first generative pollen sperm nucleus (n) fertilizes the egg cell (n), while the second generative pollen sperm nucleus (n) fertilizes the central cell (2n). The zygote (2n) forms the embryo and the fertilized central cell (3n) gives rise to the endosperm, the tissue that nourishes the developing embryo. A relatively small group of angiosperms, the gametophytic apomicts, (Gustaffson 1947a, 1947b; Stebbins 1950) have lost the need for fertilization for embryo development. Instead, an unreduced egg cell develops parthenogenetically into an embryo. This unreduced egg cell may be derived from a somatic cell (apospory) or from an unreduced megaspore (diplospory), the latter produced by either a mitotic-like division or a restitutional meiosis (Nogler 1984; Koltunow 1993). Most gametophytic apomicts still need fertilization of the central cell for endosperm development (pseudogamous apomicts). Autonomous apomicts, however, have abandoned fertilization completely and here also the endosperm develops without fertilization. Autonomous apomixis is relatively common in the Asteraceae (Compositae), but rare in other plant families (Asker and Jerling 1992).
Apomictic offspring are genetically identical to the mother plant. Apomixis fixes additive as well as non-additive genetic variation and is therefore of great interest for plant breeding (Vielle-Calzada et al. 1996). The study of apomixis may also shed light on the problem of the maintenance of sex, which is one of the great unsolved problems in evolutionary biology (Van Dijk and Van Damme 2000). The question of how autonomous apomicts have by-passed meiotic reduction and double fertilization is not only important for the understanding of apomixis, but also for the understanding of the process of sexual reproduction in angiosperms in general.
Three basic developmental elements distinguish the autonomous apomictic reproductive pathway from the sexual reproductive pathway: (1) apomeiosis (the avoidance of meiosis), (2) parthenogenetic embryo development, and (3) autonomous endosperm development. Are these elements controlled by a single gene or by several genes? In Hieracium piloselloides, autonomous apomixis 'as a whole' was shown to inherit as a single dominant trait (Bicknell et al. 2000). This suggests that the complete apomictic pathway in this species is controlled by a single gene or by a tightly linked gene complex. In Erigeron annuus, another Asteraceae species, two unlinked loci have been identified, one for diplospory and another for parthenogenesis and/or autonomous endosperm development (Noyes and Rieseberg 2000). The genetic basis of autonomous apomixis in a third Asteraceae species, the common dandelion, Taraxacum officinale, is however controversial.
Diploid dandelions (2x=16) are sexual, and triploid dandelions (3x=24) are apomictic. Richards (1970, 1973) suggested that apomixis in Taraxacum is controlled by two dominant genes, located on different chromosomes. This model is based mainly on the reports of the loss of apomixis in rare disomics (2n=23) (Sørensen and Gudjonsson 1946; Sørensen 1958). These authors claimed that the loss of a specific chromosome in a triploid apomictic clone resulted in the loss of diplospory. Loss of another, non-homologous, chromosome resulted in the fertilization of unreduced egg cells. The two-locus model has been criticized because of the small sample sizes analyzed and unreliable karyology (Mogie 1988, 1992). Instead, Mogie (1988, 1992) proposed a recessive single locus model for apomixis in Taraxacum. In this model it is assumed that a single recessive gene codes for diplospory, and that this gene has a pleiotropic effect on egg cell parthenogenesis. Mogie's model has received considerable attention in the apomixis literature (e.g., in reviews by Asker and Jerling 1992; Mogie 1992; Koltunow 1993; Sherwood 2001). Experimental data clearly supporting one or the other model have been lacking so far.
We have previously reported on the breakdown of apomixis in crosses between sexual diploid and apomictic triploid dandelions (Tas and van Dijk 1999; Van Dijk et al. 1999). In these crosses, both apomictic and non-apomictic triploid hybrids were produced. The non-apomictic triploid hybrids could be classified into three types (A, B and C), based on the progenies produced in crosses with diploid sexual pollen donors (Van Dijk et al. 1999). Type A hybrids produced only offspring with reduced chromosome numbers. We presumed that type A hybrids were pure sexuals, lacking all elements of apomixis. When crossed with sexual diploid pollen donors, type B hybrids produced a mixture of triploid and tetraploid offspring. This suggested that type B hybrids were diplosporous and had the potential for parthenogenesis, although the presence of tetraploid offspring indicated that some of the unreduced egg cells were fertilized. Based on absence of achene (the one-seeded fruit of Taraxacum) development in type B hybrids in the absence of pollination, we further speculated that type B hybrids lacked autonomous endosperm development. Type C hybrids produced exclusively tetraploid offspring in crosses with sexual diploid pollen donors, suggesting that they were diplosporous, but lacked parthenogenesis. Because of normal achene development in the absence of pollination, it was hypothesized that type C hybrids also possessed autonomous endosperm development.
In the present article the hybrid types B and C are further characterized. Cyto-embryological investigations and detailed progeny analysis indicate that the interpretations outlined above are correct. We conclude that type B and C hybrids are phenotypically complementary apomixis-recombinants. The occurrence of such apomixis-recombinants favors a multi-gene model for control of apomixis in Taraxacum.
Materials and methods
Taraxacum plants were grown in the greenhouse (21°C, 16 h light/15°C, 8 h dark). The origin of the plant material is described in detail in Tas and Van Dijk (1999) and Van Dijk et al. (1999). The plants were vernalized for a period of 2 months at 4°C to induce flowering. After crossing of two diploid sexuals (L2×6-1, cross 3 and L2×17-18, cross 6) with a triploid obligate apomictic pollen donor SE3x-6, four type B plants (H3-1, H3-6, H3-7 and H6-4) and two type C plants (H3-9 and H6-3) were obtained. Seed development was studied with and without cross-pollination (except in H6-4, which died).
To prevent cross-pollination, young buds were covered with small paper bags. Flower buds from type B (H3-1 and H3-7) and C (H6-3) hybrids were collected and fixed in cold Carnoy solution at −1, 0, +1 and +3 days after anthesis. In this time frame parthenogenetic embryo development and autonomous endosperm formation is established in the triploid apomictic pollen donor, SE3×-6. Fixation, gametophyte clearing and Nomarski-differential interference contrast (DIC) microscopy techniques were as described in Van Baarlen et al. (2000). One inflorescence was studied per hybrid/sample date combination; 50–80 florets were studied per capitulum.
The three type B hybrids were crossed with non-related diploid pollen donors. To investigate the segregation of the maternal genotype and the presence of paternal alleles, the 3 type B mother-plants and 24 of their progeny plants were analyzed for five microsatellite loci (Msta60, 61, 64, 72 and 78; Falque et al. 1998). The original diploid pollen donors were no longer available for analysis. However, additional, non-maternal alleles in the progenies were considered to be of paternal origin. DNA was extracted from young leaves according to the protocol of Rogstad (1992). PCR conditions were identical to those described in Falque et al. (1998), with the exception that one of the primers was labeled with the fluorochrome Cy5. PCR products were first checked on a 2% agarose gel and then run on an ALF express II automatic sequencer (Amersham Pharmacia), using a 50 bp size external molecular weight standard (Pharmacia). Microsatellite genotypes were scored in the 'Curve view' mode of the ALFwin Sequence Analyser 2.00.
The ploidy levels of the cross progenies were determined by flow cytometry (Partec Flow Cytometer, Münster, Germany; Tas and van Dijk 1999). In a test series, the relationship between somatic chromosome number and relative DNA content (to an internal diploid standard) was established as 2n =15.38× DNA2n /DNA2× +0.53 (r=0.99; n=42). This formula was used to estimate the chromosome numbers from cytometric data.
Based on microsatellite inheritance and ploidy levels, cross progenies were classified according to Harlan and deWet (1975). In this classification, 2n+0 offspring have arisen apomictically, 2n+n have arisen from the fertilization of an unreduced egg cell, n+0 from a parthenogenetically developing haploid egg cell and n+n offspring have arisen from the fertilization of a reduced egg cell.
Results
Cyto-embryology of non-pollinated type B hybrids
To investigate why type B hybrids did not produce achenes in the absence of cross-pollination, immature, cleared seeds of two type B hybrids (H3-1 and H3-7) were investigated by Nomarski-DIC microscopy. The results are summarized in Fig. 1. At 1 day before anthesis, half of the ovules displayed degenerated embryo sacs. Some degenerated embryo sacs lacked polar nuclei, central cells and egg cells (Fig. 2A). Others had lost cell identity (Fig. 2B) or collapsed completely, with only two endothelial layers visible (Fig. 2C). The frequencies of degenerated embryo sacs corroborated low seed sets in crosses with diploid pollen donors (see below).
Embryo sac development in type B hybrids. The distribution of embryo sac types observed by Nomarski-differential interference contrast (DIC) microscopy in non-pollinated florets ranging from 1 day before anthesis to 3 days after anthesis. The data of H3-1 and H3-7 are pooled. Non-viable embryo sacs were degenerating or empty. Viable embryo sac types: 1 Resting egg cell and undivided central cell; 2 parthenogenetic embryo and undivided central cell; 3 parthenogenetic embryo and 2–8-nucleate, non-cellularized autonomous endosperm. N Number of embryo sacs analyzed
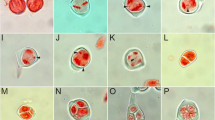
Nomarski-DIC images of embryo sacs of non-pollinated capitula 1 or 3 days after anthesis. A–E Type B hybrids, F type C hybrid. A Empty embryo sac with faint traces of endosperm formation. B Deviant type of embryo sac with large, unidentified cells in the chalazal region (small arrow) and endosperm-like cells in the central region. Note the absence of the egg apparatus in the micropylar region. C Collapsed embryo sac; the arrow points at the 'cavity'. D, E Type B, the same embryo sac at different focal planes. D Globular parthenogenetic embryo. E Non-divided central cell. F Type C. Multi-cellular endosperm with unreduced egg cell. Note that the embryo sac in C has expanded and elongated due to the endosperm growth, whereas the embryo sac in A and B is not expanded and is spherical due to the absence of endosperm. Bars A–C 50 μm, D 100 μm, F 500 μm
Among the apparently viable embryo sacs at 1 day before anthesis, in addition to embryo sacs with a resting egg cell and an undivided central cell nucleus (41%), embryo sacs with parthenogenetic embryos were also observed (9%). In this latter class, the central cell nuclei had not divided and no endosperm had formed, in contrast to the pre-anthesis autonomous endosperm development in natural apomicts grown under the same conditions (Van Baarlen et al. 2002).
At anthesis, the fraction of non-viable embryo sacs had not changed significantly (42%), whereas the percentage of embryo sacs with parthenogenetic embryos had increased to 29%. The fraction with resting egg cells had decreased to 29%. There was no endosperm development observable in the embryo sacs with parthenogenetic embryos.
At 1 and 3 days after anthesis, the fraction of degenerating embryo sacs had increased further to 65 and 72%, respectively. Over 95% of viable embryo sacs showed no traces of autonomous endosperm development. However, a few embryo sacs had undergone division of the central cell resulting in 2–8 endosperm nuclei, but cellularized endosperm was not observed in such embryo sacs. About two-thirds (66%) of viable embryo sacs now contained parthenogenetic embryos. Figure 2D, E shows a globular embryo with a still undivided central cell nucleus. Most parthenogenetic embryos (>90%) just about reached the heart stage. No parthenogenetic embryos developed beyond the heart stage. The embryo sac did not expand properly and remained globular, whereas achenes with mature endosperm are elongated at this stage (compare type B in Fig 2D, E with type C in Fig. 2F).
Progenies from pollinated type B hybrids
When pollinated with sexual diploids, viable seed set in H3-1, 3-6 and 3-7 was 0.14, 0.15 and 0.11, respectively (Van Dijk et al. 1999). To determine the sexual or parthenogenetic origin of these progenies, their ploidy levels were determined. In addition to the 38 progeny plants previously reported in Van Dijk et al. (1999), 33 more plants were analyzed. Figure 3 shows the distribution of the chromosome numbers of the 71 progeny plants. All three type B hybrids produced a mixture of (hypo)-triploid and hyper-triploid offspring after pollination. Hyper-triploid offspring plants must have originated from fertilization, since the type B hybrids themselves were triploid. Fig. 3 shows a high frequency of aneuploid offspring. Because the diploid pollen donor produces regular haploid pollen grains, this must be due to a high frequency of aneuploidy of the egg cells.
(Hypo) triploid offspring plants may have arisen parthenogenetically or sexually. Therefore the multi-locus microsatellite genotypes of thirteen (near) triploid progeny plants and their three mothers were determined. There was no overall segregation of the five-locus heterozygous genotype, indicating an unreduced, diplosporous origin of these triploid progenies (Table 1). Two offspring plants from H3-6 lacked a maternal allele at locus Msta61. The relative DNA contents indicated that these plants were hypo-triploid, lacking one or two chromosomes. None of the 13 (near) triploid offspring plants showed additional alleles that could be of paternal origin. This indicates that the triploid offspring were derived parthenogenetically (2n+0 plants; classification of Harlan and deWet (1975), see Table 1). In contrast, all 11 (near) tetraploid progeny plants showed at least one locus with an additional allele, implying that they were the result of fertilization of an unreduced egg cell (2n+n hybrids).
Based on ploidy levels alone, the fractions of (2n+0) offspring in H3-1, H3-6 and H3-7 were 0.67, 0.67, and 0.74, respectively. The corresponding fractions of (2n+n) were 0.33, 0.33 and 0.26.
Cyto-embryology of non-pollinated type C hybrids
Nomarski-DIC microscopy of non-pollinated inflorescences showed that 2 days before anthesis more than half of the embryo sacs (59%) of non-pollinated type C hybrids already contained two- or four-nucleate endosperm, whereas the rest still had an undivided central cell (n=22). None of the egg cells had divided. At 1 day before anthesis, the resting egg cells were surrounded by endosperm in all 22 ovules examined. Apart from the resting egg cell, morphology of seed development in the type C hybrids was similar to that in the apomictic parent SE3×-6. Between 1 and 3 days after anthesis, the endosperm proliferated and became cellularized. The somatic maternal tissues (integuments) had also expanded, but the egg cell remained in a resting stage (Fig. 2F).
Discussion
The combined microscopic and genetic analyses allow us to draw inferences about the interactions between endosperm and embryo development and the genetic basis of apomixis in Taraxacum.
The cyto-embryological investigations of the non-pollinated ovules reveal that type B hybrids have the capacity for parthenogenesis, but lack the capacity for autonomous endosperm formation. The production of viable apomictic seeds after pollination, as indicated by the microsatellite analysis, can be explained by endosperm development upon fertilization of the central cell. Parthenogenetic embryos are rescued by sexual endosperm. Type B plants are thus pseudogamous offspring from an autonomous apomictic father SE3×-6. As far as we know this is the first report of pseudogamous offspring obtained in a cross with an autonomous apomict.
In the absence of endosperm, parthenogenetic embryos of type B hybrids developed up to the early heart stage, which suggests that endosperm is non-essential for early embryo growth. This corroborates the study of Cooper and Brink (1949), who found that early growth of the embryo and endosperm were not correlated in apomictic T. officinale. These authors describe extreme cases of a 112-celled embryo accompanied by unicellular endosperm and, conversely, of a 256-celled endosperm with a one-celled embryo. Our results indicate however, that endosperm development is essential for further embryo growth and development, as parthenogenetic embryos in type B hybrids did not develop beyond the early heart stage.
Ploidy and microsatellite analyses indicate that when type B hybrids are pollinated, not all unreduced egg cells develop parthenogenetically, but that some are fertilized. This corroborates Nomarski-DIC microscopy observations, which show that, at anthesis, about two-thirds of the viable egg cells had already developed parthenogenetically into embryos, but that one third were still in a resting stage. Apparently, these resting egg cells were fertilized in crosses and produced sexual embryos. Thus penetrance of pathenogenesis of type B hybrids was incomplete.
Type B hybrids are diplosporous, as is evident from the ploidy and microsatellite analysis of the cross progenies. However, ploidy analysis also indicated a high fraction of aneuploid egg cells, which are almost absent in type C hybrids and natural apomicts, including the apomictic father SE3×-6 (Van Dijk et al. 1999). This suggests that nuclear restitution (diplospory) of the type B hybrids is often incomplete. It is known that Taraxacum is intolerant to aneuploidy (Sørensen 1958; Richards 1973). Nomarski-DIC microscopy observations showed that a large fraction of the embryo sacs of type B hybrids degenerated before anthesis. This may be explained by the high frequencies of inviable aneuploid megagametophytes caused by a low penetrance of diplospory.
Type C hybrids produced empty achenes in the absence of pollination and can thus be considered as parthenocarpic (Van Dijk et al. 1999). As some parthenocarpic Arabidopsis mutants form autonomous endosperm (Ohad et al. 1996; Chaudhury et al. 1997; Grossniklaus et al. 1998), we hypothesized that type C hybrids possessed autonomous endosperm development but lacked egg cell parthenogenesis. The Nomarski observations now confirm this hypothesis. Ploidy and microsatellite analysis of the progenies resulting from pollinated type C hybrids had previously established that these plants were diplosporous with high penetrance (Van Dijk et al. 1999).
Both type B and C hybrids possess diplospory, albeit with variable penetrance. Type B hybrids also possess parthenogenesis, but lack endosperm autonomy. Type C hybrids possess endosperm autonomy, but lack parthenogenesis. Therefore, B and C hybrids are phenotypically complementary. It is likely that the recombination of the elements of apomixis occurred during pollen meiosis in the apomictic father plant SE3x-6. This contradicts Mogie's (1988, 1992) single gene model for the control of apomixis in Taraxacum. Richards (1970, 1973) proposed a locus for diplospory and an unlinked locus for parthenogenesis, without specifying the genetic control of autonomous endosperm development. However, the present results suggest that a separate gene for autonomous endosperm development is involved in the genetic control of apomixis in Taraxacum.
References
Asker SE, Jerling L (1992) Apomixis in plants. CRC Press, Boca Raton, Fla.
Bicknell R, Borst NK, Koltunow AM (2000) Monogenic inheritance of apomixis in two Hieracium species with distinct developmental mechanisms. Heredity 84:228–237
Chaudhury AM, Ming L, Miller C, Craig S, Dennis ES, Peacock WJ (1997) Fertilization-independent seed development in Arabidopsis thaliana. Proc Natl Acad Sci USA 94:4223–4228
Cooper DC, Brink RA (1949) The endosperm-embryo relationship in an autonomous apomict, Taraxacum officinale. Bot Gaz 111:139–153
Falque M, Keurentjes J, Bakx-Schotman JMT, Van Dijk PJ (1998) Development and characterization of microsatellite markers in the sexual-apomictic complex Taraxacum officinale (dandelion). Theor Appl Genet 97:283–292
Grossniklaus U, Vielle-Calzada J-P, Hoeppner MA, Gagliano WB (1998) Maternal control of embryogenesis by MEDEA, a polycomb-group gene in Arabidopsis. Science 280:446–450
Gustafsson Å (1947a) Apomixis in angiosperms II. Lunds Univ Årsskr Avd 2 42:71–179
Gustafsson Å (1947b) Apomixis in angiosperms III. Lunds Univ Årsskr Avd 2 43:183–370
Harlan JR, DeWet JMJ (1975) On Ö. Winge and a prayer: The origins of polyploidy. Bot Rev 41:361–390
Koltunow AM (1993) Apomixis: embryo sacs and embryos formed without meiosis or fertilization in ovules. Plant Cell 5:1425–1437
Mogie M (1988) A model for the evolution and control of generative apomixis. Biol J Linn Soc 35:127–153
Mogie M (1992) The evolution of asexual reproduction in plants. Chapman and Hall, London
Nawaschin S (1898) Resultate einer revision der befruchungsvorgänge bei Lilium martagon und Fritillaria tenella. Bull Sci Acad Imp Sci St.-Petersbourg 33:39–47
Nogler GA (1984) Gametophytic apomixis. In: Johri BM (ed) Embryology of angiosperms. Springer, Berlin Heidelberg New York, pp 475–518
Noyes RD, Rieseberg LH (2000) Two independent loci control agamospermy (apomixis) in the triploid flowering plant Erigeron annuus. Genetics 155:379–390
Ohad N, Margossian L, Hsu YC, Williams C, Repetti P, Fischer RL (1996) A mutation that allows endosperm development without fertilization. Proc Natl Acad Sci USA 93:5319–5324
Richards AJ (1970) Eutriploid facultative agamospermy in Taraxacum. New Phytol 69:761–774
Richards AJ (1973) The origin of Taraxacum agamospecies. Bot J Linn Soc 66:189–211
Rogstad SH (1992) Saturated NaCl-CTAB solutions as a means of field preservation of leaves for DNA analyses. Taxon 41:701–708
Sherwood RT (2001) Genetic analysis of apomixis. In: Savidan Y, Carman JG, Dresselhaus T (eds) The flowering of apomixis: from mechanisms to genetic engineering. CIMMYT, IRD, European Commission DG VI (FAIR), Mexico, pp 64–82
Sørensen T (1958) Sexual chromosome-aberrants in triploid apomictic Taraxaca. Bot Tidskr 54:1–22
Sørensen T, Gudjónsson, G (1946) Spontaneous chromosome-aberrants in triploid apomictic Taraxaca. K Dan Vidensk Selsk Biol Skr 4:3–48
Stebbins GL (1950) Variation and evolution in plants. Columbia University Press, New York
Tas ICQ, Van Dijk PJ (1999) Crosses between sexual and apomictic dandelions (Taraxacum). I. The inheritance of apomixis. Heredity 83:707–714
Van Baarlen P, van Dijk PJ, Hoekstra RF, De Jong JH (2000) Meiotic recombination in sexual diploid and apomictic triploid dandelions (Taraxacum officinale L.). Genome 43:827–835
Van Baarlen P, De Jong JH, Van Dijk PJ (2002) Comparative cyto-embryological investigations of sexual and apomictic dandelions (Taraxacum) and their apomictic hybrids. Sex Plant Reprod 15:31–38
Van Dijk PJ, Van Damme JMM (2000) Apomixis-technology and the paradox of sex. Trends Plant Sci 5:81–84
Van Dijk PJ, Tas ICQ, Falque M, Bakx-Schotman JMT (1999) Crosses between sexual and apomictic dandelions (Taraxacum). II. The breakdown of apomixis. Heredity 83:715–721
Vielle-Calzada J-P, Crane CF, Stelly DM (1996) Apomixis. The asexual revolution. Science 274:1322–1323
Willemse MTM, Van Went JL (1984) The female gametophyte. In: Johri BM (ed) Embryology of angiosperms. Springer, Berlin Heidelberg New York, pp 156–196
Acknowledgement
We thank Tanja Bakx-Schotman for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
P.J. van Dijk and P. van Baarlen contributed equally to this paper. Publication 3136 NIOO-KNAW Netherlands Institute of Ecology
Rights and permissions
About this article
Cite this article
van Dijk, P.J., van Baarlen, P. & de Jong, J.H. The occurrence of phenotypically complementary apomixis-recombinants in crosses between sexual and apomictic dandelions (Taraxacum officinale). Sex Plant Reprod 16, 71–76 (2003). https://doi.org/10.1007/s00497-003-0177-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00497-003-0177-5