Abstract
The concept of a pollen tube attractant was proposed in the late nineteenth century when pollen tubes were found to grow toward excised pistil tissues on medium. Since then, for about 140 years, plant biologists have tried to identify the pollen tube attractants. However, no molecule has been convincingly demonstrated to be the true attractant that actually controls the navigation of pollen tubes in the pistil. The past decade has seen substantial progress in this field in terms of our understanding of the various mechanisms of pollen tube guidance. It was suggested that diffusible pollen tube attractants might provide localized signals that affect the directional growth of the pollen tube, especially in the last phase of guidance by the target female gametophyte. Here, we review the mechanisms of pollen tube guidance, with special focus on the gametophytic guidance and the attractant. The necessary and appropriate conditions required by the true attractant will be discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pollen tube guidance is the mechanism whereby the pistil tissue helps in navigating the pollen tube (male gametophyte) from the stigma to the target embryo sac (female gametophyte). The pollen tube grows inside the pistil without losing its way and finally arrives at the micropylar end of the embryo sac, where the surface diameter is narrow and close to that of the pollen tube. Chemoattractants for the pollen tube have been assumed to control such directional growth of the pollen tube in the pistil, and many plant biologists have tried to identify the attractants. In classical studies, biochemical and histochemical properties of putative attractants were reported using pistil tissues and their extracts from various species (Mascarenhas and Machlis 1962a; Reger et al. 1992), and one candidate, the calcium ion, was identified as the chemoattractant molecule (Mascarenhas and Machlis 1962b, 1964). There was an increasing gradient of the calcium concentration along the pistil, which was consistent with the classical hypotheses that a single attractant mediated the guidance from the stigma to the ovule. The external calcium ion is, however, a molecule necessary for the tip growth of the pollen tube, and it was argued that the assay systems could not discriminate between attraction and growth stimulation (Heslop-Harrison 1987). Since mechanical control by the architecture of the pistil tissue can also explain the pollen tube guidance, classical studies could not determine whether the attractant really existed.
What property is required for the presumed attractant? The presumed attractant may diffuse, form a gradient, and cause directional growth of the pollen tube toward the increasing gradient. It is now widely accepted that multiple stage-specific signals mediate pollen tube guidance (Johnson and Lord 2006). Search for attractant molecules in sporophytic tissues have identified several candidates of attractants, as well as molecules that perform other essential roles in pollen tube guidance, including growth stimulation and reorientation (Johnson and Lord 2006). In sporophytic tissues, presumed attractants do not trap pollen tubes at the highest concentration. Relation between a concentration gradient of each potential attractant and guidance still remains unclear.
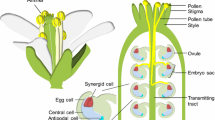
Studies in this decade, however, have shown that such an attractant definitely exists in the last phase of pollen tube guidance by the female gametophyte (Higashiyama et al. 2003; Johnson and Lord 2006). Genetic studies using mutants of Arabidopsis defective in embryo sac development have shown that the female gametophyte governs the pollen tube guidance to the ovule (Hülskamp et al. 1995; Ray et al. 1997; Shimizu and Okada 2000). An in vitro system using Torenia fournieri, in which the embryo sac protrudes from the micropyle of the ovule, showed that the female gametophyte clearly attracted the pollen tubes using a diffusible signal (Higashiyama et al. 1998; Fig. 1a). The two synergid cells proved to be the origin of the signal, as identified by a laser ablation experiment in the in vitro Torenia system (Higashiyama et al. 2001), and later by the myb98 mutant of Arabidopsis (Kasahara et al. 2005). This attractant (or attractants) derived from the synergid cell is expected to be identified as the true attractant. Here, we review the mechanisms of gametophytic pollen tube guidance, with special focus on the attractant derived from the synergid cell. Characteristics and candidates for the attractant derived from the synergid cell will be discussed to provide understanding of the necessary and appropriate conditions required by the true attractant.
Various in vitro systems. a In vitro Torenia system. A protruding embryo sac (arrowhead) provides ready access for viewing pollen tube reception (arrow) in Torenia. Such protruding embryo sacs facilitate manipulation of both embryo sac cells and pollen tubes (see text and Higashiyama et al. 1998, for details). b Dark-field image of pollen tubes of Torenia emerging from both ends of the cut style. Pollen grains were inserted into the middle of a cut style and cultured on the medium for the in vitro Torenia system. Note that pollen tubes emerge not only from the correct end (the end close to the ovary) but also from the left side (the end close to the stigma) at similar frequencies. A similar experiment was originally performed in lily by Iwanami (1959). c An in vitro system for Arabidopsis. Pollen tube attraction to the micropyle of the ovule is shown, as described by Palanivelu and Preuss (2006). Arrows indicate attracted pollen tubes and arrowheads denote embryo sacs. Bar 20 μm in a and c and 500 μm in b
Mechanisms of gametophytic pollen tube guidance
Comparison to sporophytic guidance
Before entering the ovary, pollen tube guidance is governed by the sporophytic cells of the pistil. That pollen tubes can grow through the stigma and style even in a pistil that lacks a female gametophyte (e.g., Hülskamp et al. 1995) has been demonstrated in a semi-in vitro system using a cut pistil in which the ovary has been removed (e.g., Higashiyama et al. 1998), or in plants in which megasporogenesis occurs after pollination (e.g., Sogo and Tobe 2005). These results suggest that the female gametophyte is not necessary for pollen tube guidance from the stigma to the base of the style in many species.
Not only chemotropic guidance but also mechanical guidance appears to be involved in the sporophytic guidance. For example, the lily style has been suggested to mechanically guide the pollen tubes because of its hollow architecture (Iwanami 1959). When pollen grains were placed onto the top end of a cut style, germinated pollen tubes emerged from the bottom end. In contrast, when pollen grains were placed at the bottom end of a cut style, germinated pollen tubes emerged from the top end of the style, growing in the “wrong” direction. When pollen grains were inserted into the middle of the stylar canal, germinated pollen tubes emerged from both ends of the style at equal frequency. The style of Torenia is also of the hollow type, and the same phenomenon occurs as found in the lily (Fig. 1b). These results suggest that no directional signal exists in lily or Torenia styles and pollen tubes growing straight emerge from the opposite end of the entrance, although the possibility cannot be excluded that this is due to limited or impaired competency of the pollen tube to receive the directional signal. In the tobacco style, which is of the solid type, glycosylated transmitting tissue-specific (TTS) proteins (arabinogalactan proteins; AGPs) are involved in pollen tube guidance (Cheung et al. 1995; Wu et al. 1995). Debate, however, is ongoing as to whether TTS proteins control the directional growth of the pollen tube or only sustain the elongation of the pollen tube (Higashiyama and Inatsugi 2006). Growth stimulation by the transmitting tissue was suggested in a no transmitting tract (ntt) mutant of Arabidopsis, which was defective in the development of the transmitting tract. Pollen tubes in the ntt pistil grew more slowly and were shorter, and fertilization efficiency was reduced in the lower half of the ovary (Crawford et al. 2007). Interestingly, pollen tubes at the top of ovary frequently grew toward the sides of the ovary chamber, suggesting that the transmitting tract of Arabidopsis is required for rapid apical-to-basal growth of the pollen tube in the pistil.
The stigma often appears to attract germinating pollen tubes in vitro. The lily stigma contains exudates and displays strong activity to attract (reorient) pollen tubes from lily species but not those of tobacco. Chemocyanin, a 9.9-kDa basic plantacyanin, was identified biochemically as the responsible molecule for chemotropism in lily (Kim et al. 2003), and chemocyanin protein was expressed most abundantly in the stigma and style. The chemotropic activity of chemocyanin increased when combined with stigma–stylar cysteine-rich adhesin (SCA), and the chemocyanin fraction alone showed minimal activity at 0.23 μg/μl and full activity at 0.69 μg/μl. However, neither binding activity with copper nor involvement in redox reactions has been shown for chemocyanin. The activity of recombinant chemocyanin expressed in other systems has not been reported.
Plantacyanin is a blue, copper cell wall protein that is often capable of redox reactions. In Arabidopsis, only one plantacyanin gene exists, which shows 51.9% identity and 86.8% similarity to lily chemocyanin at the amino acid level (Dong et al. 2005). Plantacyanin was expressed in various tissues, including the style of the pistil, and plantacyanin protein was most abundantly localized to the transmitting tract in the pistil tissues. Overexpression of plantacyanin caused aberrant growth of wild-type pollen tubes on the papilla cell in about half of the tubes. In these plants, the pollen tubes made several turns before growing into the style, but otherwise no differences were observed in pollen tubes emerging from the cut end of the style between the wild type and the overexpressor. No line has been found with a T-DNA insertion in the exon of plantacyanin, and the ability of purified plantacyanin of Arabidopsis to attract the pollen tube has not been demonstrated in vitro; thus, it is yet to be determined whether the plantacyanins of both lily and Arabidopsis actually govern the directional growth of the pollen tube in the pistil. It would be interesting to find whether a concentration gradient of plantacyanins is formed in the exudates of lily and in each papilla cell of Arabidopsis.
In contrast, on the stigma of tobacco with lipid-rich exudates, and on the dry stigma of Arabidopsis, an appropriate water gradient (flow) has also been suggested to be the directional cue (Lush et al. 1998; Wolters-Arts et al. 1998). Pollen grains of tobacco in unsaturated triacylglycerides directionally grew toward the water, but this did not occur if they were in saturated triacylglycerides. Such a water gradient might be a redundant signal available together with other chemoattractants on the stigma.
After entering the ovary, guidance of pollen tube requires the presence of a target female gametophye, as described below, but sporophytic tissues may also contribute to the guidance in the ovary (reviewed in Johnson and Lord 2006). GABA is a candidate for the sporophytic guidance cue, and forms a concentration gradient in the pistil and is highest at the inner integument of the ovule (Palanivelu et al. 2003). POLLEN–PISTIL INTERACTION 2 (POP2) of Arabidopsis encodes a transaminase that degrades GABA. The GABA level increased several tens of times in the pistil of a pop2 mutant, and the pollen tubes of the pop2 mutant were sensitive to higher concentrations of GABA. As a result, pollen tube guidance in the ovary was impaired when the pop2 mutant was self-pollinated. Neither the ability of GABA to attract pollen tubes nor pollen tube guidance in a pistil with low level of GABA has been demonstrated.
Signaling in gametophytic guidance
Pollen tube guidance to the ovule is governed by the target female gametophyte in Arabidopsis (Hülskamp et al. 1995; Ray et al. 1997; Shimizu and Okada 2000) and in the in vitro Torenia system (Higashiyama et al. 1998). In TL1 of Arabidopsis, a heterozygote of the reciprocal translocation, half of the female gametophyte is genetically unbalanced to degenerate during ovule formation (Ray et al. 1997). Guidance by the female gametophyte can be genetically divided into two steps: from the surface of the septum to the funiculus, and from the entrance of the micropyle to the embryo sac. In magatama (maa) mutants, in which a delay occurred in the development of the female gametophyte, pollen tubes grew on the funiculus but lost their way at the entrance to the micropyle (Shimizu and Okada 2000). An interesting correlation between the embryo sac development and pollen tube guidance has also been reported in the intermittent pollen tube growth of alders (Sogo and Tobe 2005).
The mechanism of funicular guidance is unknown: some attractant might be emitted directly from the developing and mature female gametophyte, or some signal from the female gametophyte may evoke attraction in ovular sporophytic cells indirectly. Micropylar guidance, however, was suggested to be governed by the synergid cell. MYB98, a transcription factor specifically expressed in the synergid cell and trichomes of the leaves, was shown to be necessary for both the organization of the filiform apparatus of the synergid cell and micropylar pollen tube guidance (Kasahara et al. 2005; Punwani and Drews 2008, this issue). The synergid cell of Arabidopsis is likely to emit some diffusible signal because in an in vitro system, pollen tubes cultured on a medium are attracted to the micropyle of the ovule (Palanivelu and Preuss 2006; Fig. 1c), although the possibility of a contribution from the sporophytic cells along the micropyle, such as secretion of GABA (Palanivelu et al. 2003), cannot be excluded.
In the in vitro Torenia system, pollen tubes are directly attracted to the micropylar end of the protruding embryo sac, which the filiform apparatus of the synergid cells occupies (Higashiyama et al. 1998). Pollen tubes precisely control their direction of growth in the medium and need not contact either ovular sporophytic cells of the ovule or another region of the embryo sac (Fig. 2 and Movie S1). Once attracted, pollen tubes never leave the embryo sac and often form narrow coils on the surface of the embryo sac before entering the sac. Moreover, when an ovule attracting a pollen tube is moved by micromanipulation, the pollen tube still moves toward the micropylar end of the embryo sac (Fig. 3 and Movie S2). Such behavior of pollen tubes suggests that some diffusible attractant is derived from the micropylar end of the embryo sac, and the tubes are trapped at the highest concentration of the attractant. The source of the attractant was identified by laser ablation as the two synergid cells (Higashiyama et al. 2001). Although the attractant is suggested to diffuse precisely from the filiform apparatus of the synergid cell (Figs. 2, 3, Movies S1 and S2), contribution of the ovular sporophytic cells to the pollen tube attraction cannot be excluded in the in vitro Torenia system. For example, another diffusible signal may be derived from the ovular sporophytic cells to attract the pollen tube into the range of precise attraction by the synergid cell.
Pollen tube attraction in the in vitro Torenia system. Two pollen tubes are attracted to the micropylar end of a protruding embryo sac. In the in vitro Torenia system, most pollen tubes do not enter the embryo sac smoothly, and often more than two pollen tubes are attracted (Higashiyama et al. 1998). Blocking of supernumerary pollen tubes seems to occur after pollen tube discharge in this system. The movie file of this sequence is provided as a supplemental material (Movie S1). Time indicated in each panel is mm:ss. Bar 30 μm
Course of pollen tube elongation toward the embryo sac of a manipulated ovule. An ovule attracting a pollen tube in the in vitro Torenia system was inserted with a glass needle and manipulated with a micromanipulator. Note that the pollen tube is precisely following the micropylar end of the embryo sac. The movie file of this sequence is provided as a supplemental material (Movie S2). Time indicated in each panel is mm:ss. Bar 30 μm
After fertilization in T. fournieri, pollen tube attraction ceases, despite the persistence of one synergid cell (Higashiyama et al. 2001). Some active mechanism must halt the attraction, such as the attractant being no longer being secreted, degradation of the attractant, and/or repulsion of the additional pollen tubes. Nitric oxide (NO) was shown to work as a repellent of lily pollen tubes in vitro (Prado et al. 2004). In Arabidopsis, live imaging of wild-type ovules suggested that blocking the penetration of supernumerary pollen tubes occurs just after the first pollen tube enters the micropyle (Rotman et al. 2003; Palanivelu and Preuss 2006). The mechanism of blocking is impaired in sirene (sir; Rotman et al. 2003) and feronia (fer; Huck et al. 2003) mutants, which are defective in the same receptor-like kinase gene expressed in the synergid cell (Escobar-Restrepo et al. 2007).
The stigma/style tissue has been suggested as contributing to the competency of the pollen tube in response to the directional signal from the synergid cell of Torenia (Higashiyama et al. 1998) and Arabidopsis (Palanivelu and Preuss 2006). Such a function of the stigma/style tissue was partly retained by the stigma tissue (papillae) alone or diffusible molecules from the stigma/style tissue (Palanivelu and Preuss 2006), or by the style tissue alone (Higashiyama T, unpublished data), although the frequency of competent pollen tubes was reduced in such cases. The molecular mechanism underlying this phenomenon is unknown but of great interest.
The relationship between gametophytic pollen tube guidance and the development of the sperm cell in the pollen tube has been previously discussed (Lord and Russell 2002). In the in vitro Torenia system, this relationship is unclear because division of the generative cell always occurs before the emergence of pollen tubes from the cut end of the style (Higashiyama T., unpublished data). Neither pollen tube attraction at earlier stages nor inhibition of the division of the generative cell has been reported in Torenia. In Arabidopsis, the cdka;1 mutant, defective in one of the cyclin-dependent kinases (CDKs), produces only one sperm cell (generative-like cell), but a defect in pollen tube guidance has not been reported (Nowack et al. 2006). The duo1 mutant, defective in a MYB transcription factor required for sperm cell formation (Rotman et al. 2005), and the generative cell specific 1 (gcs1) mutant, defective in a plasma membrane protein of the sperm cell required for fertilization (Mori et al. 2006), appear normal in pollen tube guidance. In contrast, the hapless 2 (hap2) mutants, alleles of GCS1, showed impaired pollen tube guidance; in mutant pollen tubes, attraction to the female gametophyte decreased by about half, and abnormal growth path and growth arrest were observed in the ovary (von Besser et al. 2006). We await elucidation of the relationship between gametophytic pollen tube guidance and the development of sperm cells. Fluorescent probes using HISTONE3 (H3) variants, which first enabled us to perform live imaging of double-fertilization processes (Ingouff et al. 2007), would also be helpful such analysis.
The attractant(s) in gametophytic pollen tube guidance
Characteristics of the pollen tube attractant(s) derived from the synergid cell
The synergid cell is likely to attract the pollen tube in flowering plants, as judged from the similarities in structure and its function in accepting the pollen tube (Higashiyama 2002; Punwani and Drews 2008, this issue). However, involvement of the synergid cell in pollen tube attraction has been confirmed in T. fournieri (Higashiyama et al. 2001), Arabidopsis (Kasahara et al. 2005), and Scrophulariaceae species closely related to T. fournieri, including T. baillonii, T. concolor, Lindernia crustacea, and L. micrantha (Higashiyama et al. 2006). Here, we focus on the properties of the attractant derived from the synergid cell of these species.
The maximum distance of the attraction appears to be ∼200 μm in the in vitro Torenia system (Higashiyama et al. 1998) and ∼100 μm (from the micropylar end of the synergid cell to the entrance of the micropyle) in Arabidopsis (Kasahara et al. 2005). In the in vitro system of Arabidopsis, pollen tubes that grew within ∼100 μm of an unfertilized ovule often made a sharp turn toward an ovule (Fig. 1c; Palanivelu and Preuss 2006). Such short-range attraction is consistent with the mathematical estimation that 1.2–9.3 mm should be the maximum distance of attraction when a single, unbound molecule makes a concentration gradient and when a tube tip, 5–10 μm in diameter, can sense a 1–2% difference across the pollen tube (103–104 difference in total; Lush 1999). The chemoattractant protein for axon guidance, netrin, is effective over a distance of ∼300 μm in vitro (Kennedy et al. 1994). The attractant derived from the synergid cell is suggested to navigate pollen tubes in close proximity to the embryo sac, with a very high accuracy. In T. fournieri, two synergid cells attract more pollen tubes than a single synergid cell, implying that the distance of attraction is longer in the former case (Higashiyama et al. 2001). As estimated mathematically, the rate of production in the attractant might limit the maximum distance of attraction, especially when this rate is low (Goodhill 1997).
One characteristic of the attractant derived from the synergid cell is species preferentiality. The in vitro Torenia system is suitable for analyzing the species difference of the attractant because the attractant from the synergid cell spreads directly through the medium, and thus the possibility of sporophytic cells contributing along the path can be excluded. Plant species having a protruding embryo sac and suitable for cultivation, such as the in vitro T. fournieri system, were surveyed (Higashiyama et al. 2006). Plant species having protruding embryo sacs are phylogenetically divergent, suggesting parallel evolution. Some species of Philadelphus, Thesium, Galium, Utricularia, Lindernia (Vandellia), and Torenia have a protruding embryo sac (Maheshwari 1950). However, Torenia and the closely related genus Lindernia are suitable for cultivation, and T. fournieri, T. baillonii, T. concolor, L. crustacea, and L. micrantha have been used to examine species differences in the attractant. Laser ablation of both the synergid cells of these plant species stopped pollen tube attraction, suggesting their involvement in pollen tube attraction (Higashiyama et al. 2006).
When T. fournieri ovules were mixed with those of another species, and the pollen tubes grown, the pollen tubes preferentially tended to grow toward the embryo sac of their own species. In the most divergent combination, T. fournieri and L. micrantha, the pollen tubes grew specifically toward the embryo sac of their own species. Moreover, even when ovules were positioned with their embryo sacs facing toward the sacs of the other species, the pollen tubes still specifically grew toward the embryo sac of their own species, and interference between their respective attraction signals was not observed. These results suggest that the attraction is species-preferential, and that each species uses a different molecule(s) (not an order-specific attractant; Higashiyama et al. 2006). Such species preferentiality likely contributes to the reproductive barrier during in vivo crossing (Higashiyama et al. 2006). The frequencies of targeting the ovules of Arabidopsis consistently decrease as the target plant species diverges in the in vitro system (Palanivelu and Preuss 2006), as well as in the in vivo crossing (Shimizu and Okada 2000; Hall et al. 2002). Interestingly, pollen tubes of Mimulus hybridas, which is a Scrophulariaceae species that has diverged considerably from T. fournieri (Wolfe and dePamphilis 1998; Albach et al. 2005), could arrive at the embryo sac of T. fournieri when crossed with the pistil of T. fournieri, suggesting that the pollen tube of M. hybridas can sense the attractant of T. fournieri (Kikuchi et al. 2007). A genome project for Mimulus guttatus is now in progress (http://www.mimulusevolution.org/), which may help to shed light on this phenomenon.
These pollen tube attraction results appear to be inconsistent with the hypothesis that the calcium ion might be the attractant of the pollen tube, or more precisely, the attractant derived from the synergid cell (reviewed in Higashiyama 2002). When the concentration of calcium in the medium was increased to 20 mM in the in vitro Torenia system, which is the maximum concentration that supports pollen tube growth, pollen tube attraction by the synergid cell still occurred (Higashiyama et al. 2006). Thus, the calcium ion may not be the sole attractant derived from the synergid cell. The calcium ion plays multiple roles during plant fertilization (Dumas and Gaude 2006; Hepler et al. 2006). High concentrations of calcium in the synergid cell might be required for pollen tube–synergid cell interactions and/or fertilization processes (Higashiyama 2002; Punwani and Drews 2008, this issue). Species preferentiality observed in closely related species suggests that the attractant molecule has evolved rapidly. It is likely that the attractant is a molecule synthesized in the synergid cell, such as a peptide or protein. Lower plants, including algae, sometimes use hydrocarbons as sex pheromones, which are not specific at the species or genus level and mislead gametes of other species (Sekimoto 2005).
Other properties of the attractant derived from the synergid cells are that the secretion appears to be developmentally regulated in Arabidopsis (Palanivelu and Preuss 2006) and Torenia (Higashiyama T., unpublished data) and the onset of attraction is correlated with the development of the synergid cell. Heat-killing ovules stops the attraction in both Torenia (Higashiyama et al. 1998) and Arabidopsis (Palanivelu and Preuss 2006), but it has not been determined whether the attractant is heat-labile or if active secretion is necessary to maintain the gradient of the attractant. The molecular size of the attractant is unknown, but Palanivelu and Preuss (2006) estimated it to be <85 kDa based on diffusion rates of fluorescein-conjugated dextrans.
It is unknown whether the attractant derived from the synergid cell is a singular substance or a number of substances working redundantly or in a complex. No mutant specifically defective in synthesis of a single attractant has been reported, implying the possibility that attractant substances might be redundant. On the other hand, reverse genetic analysis of gametophytic genes such as MYB98 suggest that the forward genetic analysis has not been saturated yet.
Candidates for the pollen tube attractant(s) secreted from the synergid cell
Genes related to the attraction of pollen tubes that are expressed in the synergid cell have been investigated (Punwani and Drews 2008, this issue). Zea mays EGG APPARATUS 1 (ZmEA1), which is expressed more abundantly in the synergid cells, is one candidate for being such an attractant, and was discovered using an EST analysis of a cDNA library of the egg cell (Márton et al. 2005). ZmEA1, a small protein of 94 amino acids predicted to be a plasma membrane protein, is the first identified female gametophytic protein related to pollen tube guidance. However, it is still unknown if purified ZmEA1 has the ability to attract pollen tubes. Complex histochemical properties of the filiform apparatus and micropylar exudates (Huang and Russell 1992) imply the possibility that other molecules, not only the attractant, are secreted from the synergid cell.
ZmEA1 was reported to be a member of the EA1-like (EAL) family, a large family of genes carrying the EA box (Gray-Mitsumune and Matton 2006). Five EAL genes were found to be expressed in various tissues of Arabidopsis, including the flower, but not specifically in the female gametophyte. Relation of ZmEA1 with these EAL genes is discussed in this issue by Márton and Dresselhaus (2008).
Except for ZmEA1, no other candidate has been proposed as being the attractant derived from the synergid cell. Genes expressed in the synergid cell (summarized in Punwani and Drews 2008, this issue) as determined by an analysis of a cDNA library for the isolated female gametophytic cells (e.g., Márton et al. 2005; Yang et al. 2006), microarray analysis (e.g., Steffen et al. 2007), or reverse genetic analysis (e.g., Kasahara et al. 2005) might provide insights into not only the chemoattractant but also the molecular basis of pollen tube attraction by the synergid cell. Large-scale forward genetic analyses are also in progress (Johnson et al. 2004; Pagnussat et al. 2004).
The necessary and appropriate conditions for the true attractant
As discussed above, some molecule(s) synthesized in the synergid cell may be the true attractant and the guidance cue that navigates the directional growth of the pollen tube in the ovary. The study on pollen tube attractants has a long history, but no true attractant has yet been identified. What are the necessary and appropriate conditions for the true attractant? One suggestion is that the candidate molecule, purified using some expression system or total synthesis, has the ability to attract the pollen tube, although it is difficult to demonstrate this ability convincingly. One difference between the in vitro Torenia system and classical in vitro systems is that the pollen tube is trapped at the highest concentration of the attractant in Torenia (Higashiyama and Inatsugi 2006; Fig. 2 and Movie S1). Pollen tubes can follow the source of the attractant precisely (Fig. 3 and Movie S2), and if one could demonstrate such an attraction using the purified molecule, it would be convincing. The other suggestion is that a specific deletion in the attractant molecule(s) makes pollen tubes lose their way. Even if a molecule has the ability to attract pollen tubes in vitro, it is necessary to show that the molecule is actually localized to the corresponding site and works as the guidance cue.
If these two conditions were clearly confirmed for a molecule, that molecule would be the true attractant. However, these two conditions have not been yet been met. For example, some antisense RNA and RNAi lines of ZmEA1 are defective in pollen tube guidance (Márton et al. 2005), but the method used to attract pollen tubes is unknown. Chemocyanin has the ability to attract pollen tubes (Kim et al. 2003), but a molecular analysis for deletion or knockdown of chemocyanin in lily is still difficult. These analyses are hampered by difficulties associated with plant species but of great interest to be achieved. Search for other candidates are also continuing. As discussed above, analysis of genes expressed in the synergid cell is in progress, and the attractant derived from the synergid cell might be the peptide (protein) that rapidly evolves. We believe that these known candidates and/or novel molecules will be identified as the true attractant in the near future.
Conclusions
Substantial progress in this decade has elucidated several mechanisms involved in pollen tube guidance. Our next goal should be to identify the true attractant and its receptor, which has been a goal of plant scientists for more than a century. The properties of the attractant derived from the synergid cell have been characterized and a candidate, ZmEA1, has also been reported. Judging from the large species differences in the attractant, genes expressed in the synergid cell would provide insights into the molecular mechanism of pollen tube attraction. We expect the true attractant to soon be identified.
References
Albach DC, Meudt HM, Oxelman B (2005) Piecing together the “new” plantaginaceae. Am J Bot 92:297–315
Cheung AY, Wang H, Wu HM (1995) A floral transmitting tissue-specific glycoprotein attracts pollen tubes and stimulates their growth. Cell 82:383–393
Crawford BCW, Ditta G, Yanofsky MF (2007) The NTT gene is required for transmitting-tract development in carpels of Arabidopsis thaliana. Curr Biol 17:1101–1108
Dong J, Kim ST, Lord EM (2005) Plantacyanin plays a role in reproduction in Arabidopsis. Plant Physiol 138:778–789
Dumas C, Gaude T (2006) Fertilization in plants: is calcium a key player? Semin CellDev Biol 17:244–253
Escobar-Restrepo JM, Huck N, Kessler S, Gagliardini V, Gheyselinck J, Yang WC, Grossniklaus U (2007) The FERONIA receptor-like kinase mediates male-female Interactions during pollen tube reception. Science 317:656–660
Goodhill GJ (1997) Diffusion in axon guidance. Eur J Neurosci 9:1414–1421
Gray-Mitsumune M, Matton DP (2006) The EGG APPARATUS 1 gene from maize is a member of a large gene family found in both monocots and dicots. Planta 223:618–625
Hall AE, Fiebig A, Preuss D (2002) Beyond the Arabidopsis genome: opportunities for comparative genomics. Plant Physiol 129:1439–1447
Hepler PK, Lovy-Wheeler A, McKenna ST, Kunkel JG (2006) Ions and pollen tube growth. Plant Cell Monogr 3:47–69
Heslop-Harrison J (1987) Pollen germination and pollen-tube growth. Int Rev Cytol 107:1–78
Higashiyama T (2002) The synergid cell: attractor and acceptor of the pollen tube for double fertilization. J Plant Res 115:149–160
Higashiyama T, Inatsugi R (2006) Comparative analysis of biological models used in the study of pollen tube growth. Plant Cell Monogr 3:265–286
Higashiyama T, Kuroiwa H, Kawano S, Kuroiwa T (1998) Guidance in vitro of the pollen tube to the naked embryo sac of Torenia fournieri. Plant Cell 10:2019–2031
Higashiyama T, Yabe S, Sasaki N, Nishimura Y, Miyagishima S, Kuroiwa H, Kuroiwa T (2001) Pollen tube attraction by the synergid cell. Science 293:1480–1483
Higashiyama T, Kuroiwa H, Kuroiwa T (2003) Pollen-tube guidance: beacons from the female gametophyte. Curr Opin Plant Biol 6:36–41
Higashiyama T, Inatsugi R, Sakamoto S, Sasaki N, Mori T, Kuroiwa H, Nakada T, Nozaki H, Kuroiwa T, Nakano A (2006) Species preferentiality of the pollen tube attractant derived from the synergid cell of Torenia fournieri. Plant Physiol 142:481–491
Huang BQ, Russell SD (1992) Female germ unit: organization, isolation, and function. Int Rev Cytol 140:233–293
Huck N, Moore JM, Federer M, Grossniklaus U (2003) The Arabidopsis mutant feronia disrupts the female gametophytic control of pollen tube reception. Development 130:2149–2159
Hülskamp M, Shneitz K, Pruitt RE (1995) Genetic evidence for a long-range activity that directs pollen tube guidance in Arabidopsis. Plant Cell 7:57–64
Ingouff M, Hamamura Y, Gourgues M, Higashiyama T, Berger F (2007) Distinct dynamics of HISTONE3 variants between the two fertilization products in plants. Curr Biol 17:1032–1037
Iwanami Y (1959) Physiological studies of pollen. J Yokohama City Univ 116:1–137
Johnson MA, Lord E (2006) Extracellular guidance cues and intracellular signaling pathways that direct pollen tube growth. Plant Cell Monogr 3:223–242
Johnson MA, Besser KV, Zhou Q, Smith E, Aux G, Patton D, Levin JZ, Preuss D (2004) Arabidopsis hapless mutations define essential gametophytic functions. Genetics 168:971–982
Kasahara RD, Portereiko MF, Sandaklie-Nikolova L, Rabiger DS, Drews GN (2005) MYB98 Is required for pollen tube guidance and synergid cell differentiation in Arabidopsis. Plant Cell 17:2981–2992
Kennedy TE, Serafini T, de la Torre JR, Tessier-Lavigne M (1994) Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell 78:425–435
Kikuchi S, Kino H, Tanaka H, Tsujimoto H (2007) Pollen tube growth in cross combinations between Torenia fournieri and fourteen related species. Breed Sci 57:117–122
Kim S, Mollet J-C, Dong J, Zhang K, Park S-Y, Lord EM (2003) Chemocyanin, a small basic protein from the lily stigma, induces pollen tube chemotropism. PNAS 100:16125–16130
Lord EM, Russell SD (2002) The mechanisms of pollination and fertilization in plants. Annu Rev Cell Dev Biol 18:81–105
Lush WM (1999) Whither chemotropism and pollen tube guidance. Trends Plant Sci 4:413–418
Lush WM, Grieser F, Wolters-Arts M (1998) Directional guidance of Nicotiana alata pollen tubes in vitro and on the stigma. Plant Physiol 118:733–741
Maheshwari P (1950) An introduction to the embryology of angiosperms. McGraw-Hill, NY
Márton ML, Cordts S, Broadhvest J, Dresselhaus T (2005) Micropylar pollen tube guidance by EGG APPARATUS 1 of maize. Science 307:573–576
Márton ML, Dresselhaus T (2008) A comparison of early molecular fertilization mechanisms in animals and flowering plants. Sex Plant Reprod 21
Mascarenhas JP, Machlis L (1962a) The hormonal control of the directional growth of pollen tubes. Vitam Horm 20:347–372
Mascarenhas JP, Machlis L (1962b) Chemotropic response of Antirrhinum majus pollen to calcium. Nature 196:292–293
Mascarenhas JP, Machlis L (1964) Chemotropic response of pollen of Antirrhinum majus to calcium. Plant Physiol 39:70–77
Mori T, Kuroiwa H, Higashiyama T, Kuroiwa T (2006) GENERATIVE CELL SPECIFIC 1 is essential for angiosperm fertilization. Nat Cell Biol 8:64–71
Nowack MK, Grini PE, Jakoby MJ, Lafos M, Koncz C, Schnittger A (2006) A positive signal from the fertilization of the egg cell sets off endosperm proliferation in angiosperm embryogenesis. Nat Genet 38:63–67
Pagnussat GC, Yu H-j, Ngo QA, Rajani S, Mayalagu S, Johnson CS, Capron A, Xie L-F, Ye D, Sundaresan V (2004) Genetic and molecular identification of genes required for female gametophyte development and function in Arabidopsis. Development 132:603–614
Palanivelu R, Preuss D (2006) Distinct short-range ovule signals attract or repel Arabidopsis thaliana pollen tubes in vitro. BMC Plant Biol 6:7
Palanivelu R, Brass L, Edlund AF, Preuss D (2003) Pollen tube growth and guidance is regulated by POP2, an Arabidopsis gene that controls GABA levels. Cell 114:47–59
Prado AM, Porterfield DM, Feijó JA (2004) Nitric oxide is involved in growth regulation and re-orientation of pollen tubes. Development 131:2707–2714
Punwani JA, Drews GN (2008) Development and function of the synergid cell. Sex Plant Reprod 21
Ray S, Park SS, Ray A (1997) Pollen tube guidance by the female gametophyte. Development 124:2489–2498
Reger BJ, Chaubal R, Pressey R (1992) Chemotropic responses by pearl millet pollen tubes. Sex Plant Reprod 5:47–56
Rotman N, Rozier F, Boavida L, Dumas C, Berger F, Faure JE (2003) Female control of male gamete delivery during fertilization in Arabidopsis thaliana. Curr Biol 13:432–436
Rotman N, Durbarry A, Wardle A, Yang WC, Chaboud A, Faure JE, Berger F, Twell D (2005) A novel class of MYB factors controls sperm-cell formation in plants. Curr Biol 15:244–248
Sekimoto H (2005) Plant sex pheromones. Vitamins Hormones 72:457–478
Shimizu KK, Okada K (2000) Attractive and repulsive interactions between and male gametophytes in Arabidopsis pollen tube guidance. Development 127:4511–4518
Sogo A, Tobe H (2005) Intermittent pollen-tube growth in pistils of alders (Alnus). PNAS 102:8770–8775
Steffen JG, Kang I-H, Macfarlane J, Drews GN (2007) Identification of genes expressed in the Arabidopsis female gametophyte. Plant J 51:281–292
von Besser K, Frank AC, Johnson MA, Preuss D (2006) Arabidopsis HAP2 (GCS1) is a sperm-specific gene required for pollen tube guidance and fertilization. Development 133:4761–4769
Wolfe AD, dePamphilis CW (1998) The effect of relaxed functional constraints on the photosynthetic gene rbcL in photosynthetic and non-photosynthetic parasitic plants. Mol Biol Evol 15:1243–1258
Wolters-Arts M, Lush WM, Mariani C (1998) Lipids are required for directional pollen-tube growth. Nature 392:818–821
Wu HM, Wang H, Cheung AY (1995) A pollen tube growth stimulatory glycoprotein is deglycosylated by pollen tubes and displays a glycosylation gradient in the flower. Cell 82:395–403
Yang H, Kaur N, Kiriakopolos S, McCormick S (2006) EST generation and analyses towards identifying female gametophyte-specific genes in Zea mays L. Planta 224:1004–1014
Acknowledgments
Our work on pollen tube guidance is supported by the Ministry of Education, Culture, Sports, Science and Technology, Japan (grant-in-aid for scientific research on priority areas no. 18075004, grant-in-aid for Scientific Research (B) no. 19370017, and PRESTO to T.H.).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Thomas Dresselhaus.
Electronic supplementary material
Below is the link to the electronic supplementary material
Movie S1. See text and the legend of Fig.2 for details. This movie is 64 times faster than the normal speed. (MOV 25.1 mb)
Movie S2. See text and the legend of Fig.3 for details. This movie is 64 times faster than the normal speed. (MOV 17.8 mb)
Rights and permissions
About this article
Cite this article
Higashiyama, T., Hamamura, Y. Gametophytic pollen tube guidance. Sex Plant Reprod 21, 17–26 (2008). https://doi.org/10.1007/s00497-007-0064-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00497-007-0064-6