Abstract
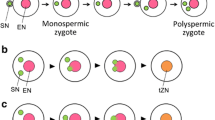
Barriers to polyspermy (fertilization of a female gamete by more than one sperm) are essential to successful reproduction in a wide range of organisms including mammals, echinoderms, fish, molluscs, and algae. In animals and fucoid algae, polyspermy results in early death of the zygote due to transmission of extra centrioles from the sperm and consequent disruptions to the mitotic spindle. Accordingly, a variety of mechanisms have evolved to prevent penetration of an egg by more than one sperm, or more than one sperm nucleus from fusing with an egg nucleus. The evolution of internal fertilization has also provided an opportunity to limit the number of sperm that gain access to each egg, as occurs in the mammalian female reproductive tract. Polyspermy and polyspermy barriers in plants have received much less attention. Plants lack centrioles and therefore, polyspermy would not be expected to cause lethal aberrant spindle organization. However, we find evidence from cytological, genetic and in vitro fertilization studies for polyspermy barriers in plants. Angiosperms, like mammals, are internally fertilized, and exert a high level of control over the number of sperm that have access to each female gamete. In particular, regulation of pollen tube growth ensures that in general only two sperm enter each embryo sac, where one fertilizes the egg and the other the central cell. Despite this 1:1 ratio of sperm to gametes within the embryo sac, angiosperms still require a mechanism to ensure that each female gamete is fertilized by one and only one sperm. Here, we present evidence suggesting that a polyspermy block on the egg may be part of the mechanism that promotes faithful double fertilization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In animal reproduction, thousands of sperm or more may compete to fertilize a single egg (Schuel 1984). A polyspermy block on the egg, preventing fusion of more than one sperm nucleus with the egg nucleus, is one strategy to avoid multiple fertilizations, which would otherwise lead to death of the embryo due to transmission of extra centrioles from the sperm and consequent disruptions to the mitotic spindle. Similarly, the eggs of fucoid algae are exposed to numerous sperm, and these also possess polyspermy barriers (Santelices 2002). For both animals and photosynthetic organisms, the move to land has been accompanied by a transition from external fertilization, as employed by marine “broadcast spawners” (e.g., sea urchins, fucoid algae), to internal fertilization (as in mammals and angiosperms). Although internal fertilization in mammals allows the mother to severely limit the number of sperm that have access to her gametes, polyspermy blocks have nevertheless been retained (Wong and Wessel 2006). In this review, we will explore the evidence for such blocks in flowering plants.
A defining characteristic of angiosperms is double fertilization, in which each pollen tube delivers two sperm into the embryo sac to fertilize the egg (forming the zygote) and central cell (generating endosperm, which among other functions supplies nutrients to the developing or germinating embryo). Although pollen tube guidance and repulsion systems normally ensure that only one pollen tube penetrates each ovule (Weterings and Russell 2004; Dresselhaus 2006), occasionally more than one pollen tube gains entry, as occurs in heterofertilization in maize (Sprague 1929, 1932). Even if there could be a strict limitation of one pollen tube per embryo sac, the resulting 1:1 ratio of sperm to female gametes would not preclude the need for polyspermy blocks. Flowering plants have a particular problem not faced by any other taxa: since typically only two sperm enter each embryo sac, where both egg and central cell must be fertilized to produce a viable seed, it is essential not just to block multiple fertilization but also to use every sperm that has access to a female gamete. So far there is no evidence for mechanisms of active transport of sperm to gametes, or predestination of each sperm for a particular gamete that are under strict enough control to account for the observed success of double fertilization. Therefore, it is conceivable that polyspermy blocks could form part of a mechanism for ensuring not only that female gametes avoid multiple fertilizations, but also that each one receives a sperm.
Polyspermy blocks are widespread among eukaryotes with different breeding strategies
In animals, multiple fertilization is usually embryo lethal (Gilbert 2006). An immediate cause of death is the formation of multipolar or supernumerary mitotic spindles in the zygote due to transmission of extra centrioles from the sperm, resulting in aberrant divisions (Schuel 1984; Navara et al. 1994). Thus there is selective pressure to avoid polyspermy, and accordingly barriers to multiple fertilization are found in a wide range of taxa. Some animals (e.g., birds and reptiles) employ “physiological polyspermy”, in which multiple sperm fuse with the egg but only one of these merges its nucleus with the egg pronucleus; but in most species, modifications of the fertilized egg prevent further sperm entry (Wong and Wessel 2006). These fall into two main categories: (1) “fast”, “electrical”, or “membrane” blocks, involving depolarization or hyperpolarization of the egg plasma membrane within several seconds of sperm entry, and (2) “slow” or “egg coat” blocks, involving physical changes to the egg extracellular matrix (e.g., the zona pellucida in mammals or vitelline envelope in amphibians, molluscs, and crustaceans) that prevent sperm binding (Gardner and Evans 2006; Wong and Wessel 2006).
Polyspermy barriers have also been well studied in the fucoid algae Fucus and Pelvetia, which like marine invertebrates reproduce by broadcast spawning (Brawley 1987, 1991, 1992; Pearson and Brawley 1996; Serrão et al. 1999; Santelices 2002). Three types of polyspermy block were identified in fucoids: a fast, transient sodium-dependent electrical block; a slow, permanent block involving the formation of a cell wall; and an intermediate block causing sperm to detach from the egg soon after fertilization, which may involve the destruction of enzymatic receptors for sperm on the egg plasma membrane. Fucoid zygotes resulting from multiple fertilization are not viable. As in animals, Fucus and Pelvetia sperm contribute centrioles to the zygote, and multipolar spindles followed by abnormal cytokinesis have been observed in polyspermic zygotes of F. distichus (Nagasato et al. 1999).
The occurrence of polyspermy in groups that clearly employ polyspermy blocks, including fucoids (Santelices 2002), sea urchins (Levitan et al. 2007), and mammals (Gardner and Evans 2006), indicates that the barriers are not completely effective. High sperm:egg ratios increase the chances of fertilization but also cause polyspermy, as multiple sperm are more likely to collide with the egg before there is time to raise a block (Tomaiuolo et al. 2007). Although sperm and egg concentrations are among the parameters used in modelling of reproductive strategies (e.g., Styan 1998), there is little published data on the ratios encountered in nature. Marine broadcast spawners produce vast quantities of gametes; for example approximately 1011 sperm can be collected from a single male of the sea urchin Arbacia, and 4 × 106 eggs from a female (Schuel 1984). The numbers of gametes spawned synchronously by male–female pairs of the bucktooth parrot fish Sparisoma radians have been counted, with males releasing a median of 8.94 × 106 sperm per pair spawn, and female partners releasing several hundreds to thousands of eggs (Marconato and Shapiro 1996). However, the number of sperm actually encountered by an egg may depend on many factors other than how many gametes are released by each parent. For example, the spawned gametes of marine organisms are quickly diluted in seawater, especially in turbulent conditions (Santelices 2002). Sperm:egg ratios measured near the time of fertilization in Fucus vesiculosus were found to range from only 10:1 to 70:1 (Berndt et al. 2002).
In animals with internal fertilization there are multiple opportunities to regulate the effective sperm:egg ratio. In humans, millions of sperm are ejaculated, but only a few reach the site of fertilization in the fallopian tube (Hunter 1996; Bedford 2004; Eisenbach and Giojalas 2006; Suarez and Pacey 2006). Most sperm are lost from the female reproductive tract (estimated at more than 99%, Suarez and Pacey 2006) and the retained sperm are stored in a lower segment of the fallopian tube until the time of ovulation, when hormones induce the release of a few. One reviewer commented that the sequestration of sperm “illustrates the biological fallacy of media images in which many spermatozoa are shown ‘competing’ for an unfertilised egg in vitro” (Bedford 2004). In addition, only about 10% of human sperm undergo capacitation, a series of changes in the plasma membrane required for penetration of the egg. Nevertheless, humans and other mammals employ polyspermy blocks (reviewed by Gardner and Evans 2006). Although there is no fast electrical block, some form of slower-acting membrane block is suggested by the observation of supernumerary sperm between the zona pellucida and the plasma membrane of fertilized monospermic eggs. The egg coat block in mammals involves exocytosis of cortical granules, causing the zona pellucida to lose its ability to bind sperm. Most mammalian species studied appear to use both forms of block.
Sperm availability is also severely limited in Drosophila melanogaster (Bloch Qazi et al. 2003). Of the 4,000 sperm transferred to the female, approximately 1,000 are stored in two types of organ (the seminal receptacle and the spermatheceae), and 40% of these may be used to fertilize eggs up to 2 weeks after storage. The storage reservoirs emit a few sperm as each egg passes down the oviduct, and this controlled release of sperm may be one reason why polyspermy rates are below 1%. Sperm–egg contact is further limited by the extremely restricted access to the egg plasma membrane: sperm entry is only possible through the micropyle, a single small opening in the chorion.
Figure 1 illustrates the spectrum of effective sperm:egg ratios in animals and algae as well as plants, which will be discussed below. Taxa with low ratios are characterised by internal fertilization and concomitant maternal control over accessibility of female gametes to sperm. However, even internally fertilizing animals maintain polyspermy blocks. Presumably this is because the consequences of multiple fertilizations are devastating, but a 1:1 ratio of sperm to eggs cannot be faithfully guaranteed.
The ratio of sperm to eggs as encountered near the site of fertilization varies with reproductive strategy. Organisms that release gametes into the ocean, such as sea urchins and fucoid algae, have relatively high sperm:egg ratios while in organisms with internal fertilization such as mammals and seed plants, few sperm have access to each egg. The lowest ratio of sperm to female gametes, 1:1, is found in flowering plants, where the two sperm delivered to an embryo sac must each fertilize a female gamete (egg or central cell). See text for discussion of fertilization in bryophytes, pteridophytes, Chlamydomonas, mouse, gymnosperms, and Drosophila. The correct relative positions of the organisms illustrated here are uncertain, as there is little data available on how many sperm are available to each egg in nature
Transition to internal fertilization in plant lineages and opportunities for polyspermy
In plants, as in animals, colonisation of the land involved a transition to internal fertilization and loss of dependence on water for reproduction. Nonseed plants such as bryophytes (e.g., mosses and liverworts) and pteridophytes (ferns) release sperm from the male gametophyte directly into a wet environment (Bell and Hemsley 2000). The flagellate sperm swim toward eggs, which are retained on the maternal gametophyte. In the extant seed plants, angiosperms and gymnosperms, sperm (or their developmental precursors) are dispersed within the pollen grain, which is a male gametophyte enclosed in a wall. Pollen does not require free water to move from male to female reproductive organs, but may be carried on the wind, by animals, or transferred by direct contact from the anther to the stigma of a self-fertilizing flower. Once on the stigma of an angiosperm, or the nucellus (or other receptive surface) of a gymnosperm, pollen germinates to form a pollen tube, an elongated cell of the male gametophyte, which grows through maternal tissue and transmits the sperm to the female gametophyte (also known as the embryo sac in angiosperms). Among seed plants only the sperm of cycads and Ginkgo biloba have retained flagella; in these groups the branched and long-lived pollen tubes are considered to be in transition towards a role in sperm delivery, with their primary role being nutrient uptake from female tissue for use by the developing sperm (Rudall and Bateman 2007).
What are the opportunities for polyspermy in plants? In taxa with naked motile sperm, the numbers of male gametes that can approach an egg will be determined by several factors including density of gametophytes, the numbers of gametes generated, the distance sperm can travel, and the structure of the female gametophyte. Bryophytes and pteridophytes may produce from a few to hundreds or thousands of spermatozoa in each antheridium (Bell and Hemsley 2000; Renzaglia and Garbary 2001). Seed plants may shed vast quantities of pollen—e.g., a single flower may produce thousands or millions of pollen grains (Cruden 2000)—but, as in internally fertilizing animals, the numbers of sperm reaching the female gametes are likely to be severely restricted. Only a fraction of pollen released is transmitted or adheres to the stigma of angiosperms; for example in Raphanus sativus (wild radish), flowers in the field had a mean pollen load of 112 grains on the stigma, and the pistils contained an average of 7.4 ovules (Marshall et al. 2007). Surveying literature on plant species with a range of breeding systems, Cruden (2000) concluded that a minimum of 4–6 pollen grains per ovule on a stigma are necessary for maximum seed set; this underlines the relatively small number of sperm that can effectively compete for each female gamete. Further control of sperm:gamete ratios is exerted at the level of the pollen tube, since it is this structure rather than naked sperm that migrates through the female reproductive organs. The pollen of gymnosperms and angiosperms contains two sperm, with only very few exceptions, the cycad Microcycas and the conifer Cupressus developing more than two sperm per grain (Rudall and Bateman 2007). Therefore, a mechanism for ensuring that only one pollen tube reaches a female gametophyte would in general limit entry to two sperm. Since the embryo sac of angiosperms contains both an egg and a central cell, which require fertilization, such an arrangement would ensure a strictly 1:1 ratio of sperm to female gametes. The situation in gymnosperms is more complex since there is no central cell, but occasionally there is fertilization of other cells of the female gametophyte than the egg, and in addition a female gametophyte may generate several archegonia, each containing an egg.
Evidence for polyspermy blocks in plants
There are hardly any direct investigations of polyspermy or polygamy blocks in plants in vivo (but see sections on heterofertilization and apomixis, below). In green algae, which unlike fucoid algae are in the plant lineage (Palmer et al. 2004), there is mixed evidence for polygamy blocks (prevention of fusion of more than two gametes). Chlamydomonas reinhardtii, a unicellular green soil alga, produces two types of gametes known as mt plus and mt minus. Gametes adhere in large clumps via agglutinin glycoproteins on their flagellar surface, and subsequently plus/minus pairs fuse (Goodenough et al. 2007). The agglutinins of newly formed zygotes lose their adhesive properties by an unknown mechanism, suggesting a polygamy block. However, Bryopsis plumosa (Chlorophyta) demonstrated polygamy levels in the laboratory of up to 25%, and was therefore considered to lack a barrier to multiple gamete fusion (Speransky et al. 2000). In Spirogyra and other members of the Zygnematales, an algal group closely related to land plants, the need for a polygamy block appears to be bypassed by the method of sexual reproduction, which involves conjugation of opposing cells in a pair of parallel filaments (Bell and Hemsley 2000).
A study of fertilization in the moss Funaria flavicans reported that antherozoids (the sperm of bryophytes) swim down the neck canal of the archegonium and into the venter containing the egg, and that “a large number approach the egg so that it has the appearance of being covered with very fine threads” (Beardsley 1931). After their entrance a mucilaginous plug appears in the canal, which appears to have been secreted by cells overlying the venter. The author proposes that this plug prevents the entry of any more sperm, noting that above the plug there are often many antherozoids tangled together. Although more than one antherozoid penetrates the egg, polyspermy is observed rarely, and only very soon after fertilization. The author concludes that the supernumerary sperm disintegrate, as they are never observed in the egg cytoplasm at later stages. These observations suggest that Funaria may employ something akin to a physiological polyspermy barrier. The occasional occurrence of polyspermy has been recorded in the fern Onoclea struthiopteris (discussed by Hoyt 1910). Potential barriers to multiple fertilizations were observed in another pteridophyte, Marsilea vestita (Myles 1978). The author noted that many spermatozoids are observed in the cavity above the egg, but their access to the egg plasma membrane is limited by a thick wall with an opening of only slightly greater diameter than a spermatozoid. Shortly after fertilization an extracellular layer is elevated above the upper surface of the zygote, between the plasma membrane and the thick wall, pushing the wall and degenerating spermatozoids against the jacket cells of the archegonium. The author proposed that the restricted access to the egg and the new extracellular layer may both prevent polyspermy. In the Polypodiales (pteridophytes), several spermatozoids commonly enter an open archegonium, but generally only one penetrates the egg, although others may be seen pressed against its surface (Bell and Hemsley 2000).
“Polyembryony” is a feature of gymnosperm reproduction but this is not the same phenomenon as polyspermy, as it results from fertilization of multiple eggs or other female cells in one ovule, or splitting of a fertilization product into several zygotes (Buchholz 1926; Berlyn 1962; Friedman and Carmichael 1996). It is also distinct from polyembryony in apomictic flowering plants, where multiple asexual embryos arise in an ovule (Nogler 1984). What is the fate of the second sperm in a gymnosperm pollen tube when it does not fertilize a second egg or other female cell? In Pinus species, both sperm enter the egg, but only one approaches the nucleus and fuses with it (Blackman 1898; McWilliam and Mergen 1958). The situation is similar in Picea (spruce) (Runions and Owens 1999) while in Taxus brevifolia (Pacific yew), the second sperm and pollen tube nucleus enter the vacuolate area of the archegonium and degenerate (Anderson and Owens 1999). In Picea it was also noted that a substance appearing to be a polysaccharide accumulates at the site of pollen tube entry into the egg, forming a plug, which could prevent penetration by other pollen tubes. These studies provide evidence for polyspermy barriers in gymnosperms.
A paper entitled “On polyspermy in the sunflower” (Vigfússon 1970) does not address polyspermy as described in the present review, but instead documents “polyspermy sensu lato”, “a discharge of more than one pollen grain into the embryo sac” (i.e., multiple pollen tube entry) rather than polyspermy sensu stricto, “the penetration of more than one sperm nucleus into the egg cell”. The consequences of discharging more than two sperm into the embryo sac are not discussed. Instances of “polyspermy” reported in other angiosperm species are tabulated (Vigfússon 1970, Table 4), but again this refers to cases where more than one pollen tube has entered an ovule (or rarely where a pollen tube contains more than two sperm), rather than entry of more than one sperm into the egg or central cell.
The prevailing view of fertilization control in flowering plants is that each ovule can receive only one pollen tube, and that each of the two sperm subsequently released into the embryo sac is actively transported to a female gamete. Figure 2 illustrates current understanding of pollen tube growth and sperm release in flowering plants. After penetrating the stigma, a pollen tube grows through the transmitting tract of the pistil into the ovary, where it emerges to extend over the surface of the funiculus (the stalk of the ovule) and into the micropyle, an opening in the ovule that allows access to the embryo sac. Long- and short-range attraction systems, along with repulsion between pollen tubes, are thought to ensure that one and only one pollen tube enters each embryo sac (Shimizu and Okada 2000; Weterings and Russell 2004; Dresselhaus 2006).
Models for pollen tube guidance to the embryo sac and sperm guidance within it. Left pollen germinates on the stigma and the pollen tubes penetrate the transmitting tissue of the pistil to grow toward the ovules. Middle pollen tubes are attracted to each embryo sac (ES) by a funiculus guidance signal (FS) and a micropylar guidance signal (MS), and also repel each other (male male repulsion, MMR). f, funiculus; m, micropyle. Right a single pollen tube enters one of the synergid cells flanking the egg and bursts. The sperm (S) are transported on actin tracks (black) to the egg (E) and central cell (CC). Left and middle adapted from Shimizu and Okada (2000)
However, the studies cited in Vigfússon (1970), and the evidence from heterofertilization in maize (discussed below), show that this system can fail, sometimes at a high frequency. Multiple pollen tube entry would then provide an opportunity for polyspermy. Would we expect this to be lethal in flowering plants? Angiosperms lack centrosomes (Lloyd and Chan 2006), and therefore, a polyspermic zygote or endosperm would not be expected to die for the same reason that an animal or algal zygote would fail. Polyploidy is also no barrier to plant development (Leitch and Bennett 1997). However, a disturbed ratio of maternal to paternal genomes is often lethal to endosperm, though not the embryo (Haig and Westoby 1991). Therefore, we would predict that multiple pollen tube entry would result in polyploid embryos if there is no polyspermy block on the egg, or seed abortion if there is no block on the central cell. Thus, heterofertilization experiments, though not designed to assay polyspermy, might provide clues to the existence of barriers to multiple fertilization.
Heterofertilization—an in vivo test for polyspermy?
Heterofertilization, first identified in maize, refers to fertilization of the egg and central cell by sperm from two pollen grains (Sprague 1929, 1932). This is graphically demonstrated in mixed pollinations where one pollen parent carries a colour marker—usually purple anthocyanin production—that is visible in both the embryo scutellum and the aleurone layer of the endosperm, and the other male parent is null for the marker. In Sprague’s experiments, pollination of a colourless seed parent with a mixture of the two pollen genotypes produced three easily identifiable types of kernel (Fig. 3a), the expected colourless embryo and endosperm, purple embryo and endosperm, and an unexpected “discordant” class, purple embryo and colourless endosperm. Kernels having a colourless embryo and purple endosperm were also produced, but these were difficult to observe directly, requiring confirmation by genetic analysis (Sprague 1932).
Heterofertilization in maize suggests there is a polyspermy block on the egg but not the central cell. a Illustration of the classic experiment by Sprague (1932) demonstrating that the egg and central cell of a single embryo sac could be fertilized by pollen from two different grains. A “colourless” seed parent with white endosperm (visible as a yellow kernel) and white embryo was pollinated with a mixture of pollen from another “colourless” plant, and a plant with purple endosperm and purple embryo. Approximately 2.5% of the kernels had purple endosperm and white embryo, or white endosperm and purple embryo, indicating mixed parentage. b Heterofertilization experiment by Kato (1997) using staggered mixed pollination could provide an assay for polyspermy. The first pollen parent was colourless and also treated with trifluralin to inhibit generative cell mitosis, so that most grains (approximately 90%) contained a single diploid male gamete rather than two haploid sperm. The second pollination, with normal purple pollen, occurred 1–4 days later (results are shown for 1 day). Three classes of plump kernel were obtained: two from homofertilization and one from heterofertilization (purple endosperm, white embryo). The ploidy of seedlings grown from these kernels shows whether a haploid or diploid male gamete fused with the egg. The combination of male gametes (haploid or diploid, white or purple) that could lead to each type of kernel is depicted below. Extra paternal genomes in the endosperm would produce shrivelled kernels (boxed). Paternal excess is generated if the central cell is fertilized by a diploid generative cell from the first pollination or two haploid sperm from the second (i.e., polyspermy). In the latter case the endosperm would not appear purple because it would die before expression of the colour marker. Paternal excess could explain why kernels with white endosperm and purple embryo were not recovered in this experiment, as they would most likely involve fertilization of the central cell by a white diploid generative cell, which would produce a shrivelled kernel. Photographs courtesy of Akio Kato
What causes heterofertilization and what is its implication for polyspermy? Experiments by Sprague (1932) indicated that heterofertilization is due to the fusion of the egg and central cell with sperm of different genotypes carried to the embryo sac in two different pollen tubes. There have been no reports since that challenge this idea. In support of this, Rhoades (1934) reported that 11% of maize embryo sacs contained more than one pollen tube. More recently Kato (2001) showed that doubly fertilized kernels are produced following pollination with grains containing only a single “sperm”, implying the cooperation of two pollen tubes. Heterofertilized kernels are not rare, with most maize strains giving a rate of about 1.25%. One exceptional genotype produced around 25% herterofertilized kernels (Sprague 1932). These figures represent an underestimate since fertilization of egg and central cell by sperm from two different pollen tubes but of the same genotype would evade detection. Presumably, therefore, around 2.5% of kernels in standard maize genotypes are produced by heterofertilization.
The implication of Sprague’s work is clear: fairly frequently, two pollen tubes enter the embryo sac and presumably deposit four sperm in the vicinity of the waiting egg and central cell. These supernumerary sperm therefore represent a potential challenge to the normal fertilization process, and an opportunity to probe for polyspermy barriers. Where both egg and central cells operate a polyspermy barrier, a normal seed will result. However, multiple fertilization of the egg, the central cell, or both, would result in embryos and/or endosperms with increased ploidy and altered parental genome ratios. These predicted outcomes provide a way to investigate whether more than two sperm participate in fertilization, since polyploid seedlings and aberrant parental genome ratios in the endosperm can be detected.
Does heterofertilization produce polyploid embryos? Sprague did not explicitly consider the possibility, and did not supply directly relevant data. However, genetic analysis of plants derived from heterofertilized kernels with white endosperm and purple embryos showed segregation ratios consistent with a heterozygous diploid constitution (Sprague 1929). This suggests that heterofertilization does not result in multiple fertilization of the egg, and in turn that the egg operates a polyspermy block.
Turning to central cell fertilization, the endosperm, but not the embryo, of maize is highly sensitive to parental genome ratio, with deviations from the normal two maternal:one paternal dose usually proving lethal (Kermicle 1971; Lin 1984). As early as 1935, Randolph had established that diploid–tetraploid intercrosses produced poorly developed kernels that rarely germinated. This experimental cross delivers the same paternal genome dose, albeit in a single diploid sperm, as would result from multiple fertilization of the central cell by two haploid sperm. Heterofertilization that resulted in multiple fertilization of the central cell should therefore cause seed abortion. Unfortunately, Sprague did not provide abortion data, precluding any estimate of the frequency of multiple central cell fertilization. Clearly though, the recovery of plump and viable herterofertilized seed, presumably therefore, with the normal 2m:1p endosperm ratio, indicates that single fertilization of the central cell did occur in some cases.
Kato (1997) extended investigations of heterofertilization with “staggered dual pollination” experiments involving a first pollination with mainly bicellular pollen containing a single diploid generative cell (generative cell division to form two sperm was prevented by the herbicide trifluralin), and a second pollination, 1–4 days later, with normal tricellular pollen (Fig. 3b). The tricellular pollen was genetically marked to produce purple anthocyanins in the embryo (scutellum) and endosperm (aleurone), as described earlier for heterofertilization, while the maternal parent and the first pollen parent were genetically colourless. In such experiments, the first paternal parent supplies a single gamete which, provided single fertilization is possible, will fuse with either the egg or the central cell; the second paternal parent then supplies genetically marked haploid sperm to fertilize whichever female gamete remains available. If the egg has been fertilized by the single diploid gamete, heterofertilization would allow production of endosperm and therefore, a viable seed. In this case, the kernel would have a (triploid) white embryo and purple endosperm. The experiment yielded four kinds of kernel: three of them plump—white embryo and white endosperm (which appeared as a yellow kernel due to the pericarp colour), purple embryo and purple endosperm, white embryo and purple endosperm—and one shrivelled (representing endosperm failure). In staggered pollinations where the second pollination occurred 1 day after the first, 10% of seeds had white embryo and endosperm (this corresponded to the percentage of white pollen that was tricellular, and accordingly nearly all seedlings grown from this seed class were diploid); 44% had purple embryo and endosperm, 6% had white embryo and purple endosperm, and 40% were shrivelled (Fig. 3b). The discordant (white embryo, purple endosperm) and shrivelled classes are the most interesting. Seedlings germinated from the discordant kernels were overwhelmingly triploid (approximately 96%), indicating that in most cases the “white” diploid generative cell had fertilized the egg, with the central cell subsequently fertilized by the “purple” sperm of the second pollen parent. This confirms that a viable seed can be produced even if the events of double fertilization are separated in time—i.e., simultaneous double fertilization is replaced with two single fertilizations.
Classical heterofertilization employing simultaneous application of purple and white marked pollen produces the two expected classes of discordant kernels, whilst staggered dual pollination produced only one, failing to generate any kernels with a purple embryo and white endosperm. Kato proposed that kernels with the latter genetic constitution are initiated but abort before formation of the scutellum and aleurone, rendering genotyping impossible—i.e., they reside within the shrivelled class of kernel. This proposal is reasonable since, as discussed earlier, fertilization of the central cell with a diploid paternal gamete is expected to result in a lethal parental genome dosage (2m:2p) in the resulting endosperm. The shrivelled class made up 40% of total kernels scored, but 88% of the kernels in which a white diploid generative cell was observed or inferred to participate in fertilization (i.e., visibly discordant or shrivelled). One interpretation of this figure is that where a white diploid generative cell fertilized one or other female gamete, it had a strong preference for the central cell. In contrast, Nowack et al. (2006) reported that single fertilization in Arabidopsis also shows a strong bias, but this time for the egg (discussed below). This species difference may be an artefact, and may point to an alternative explanation for the high frequency of seed abortion: the central cell does not exclude multiple sperm. Fertilization of the central cell by more than one haploid sperm would have the same lethal effect on the endosperm as fertilization by a single diploid gamete. Therefore, seeds where the egg was fertilized by a diploid generative cell in the first pollination, and the central cell by two sperm in the second, would die from lethal paternal excess and contribute to the class of large shrivelled seeds.
Is there any other evidence that the central cell of maize is susceptible to multiple fertilizations? The behaviour of tetraploid–diploid crosses provides a possible example. Such 4x × 2x crosses produced 1.5% plump kernels containing triploid embryos while the rest of the kernels aborted (Kato 2001). A 4m:2p endosperm ratio, like the 2m:2p ratio, is lethal in maize (Randolph 1935). Kato (2001) suggested that plump kernels in 4x × 2x crosses could arise from fertilization of the egg with a single haploid sperm from one pollen tube, and fertilization of the central cell with two haploid sperm from a second pollen tube, as previously proposed by Sarkar and Coe (1971) to explain the rare plump seeds they recovered in 4x × 2x crosses. However, it would be essential to karyotype the endosperms of such seeds to test this hypothesis.
The experiments highlighted above provide circumstantial evidence for multiple fertilization of the central cell in maize. Investigation of events in the embryo sac following heterofertilization, focusing on gamete fusion and the subsequent ploidy of endosperm as well as embryo, would shed much light on polyspermy barriers and also on the mechanisms of fertilization. In addition a more controlled substitute for heterofertilization would be use of mutants, such as tetraspore in Arabidopsis (Spielman et al. 1997), which regularly produces extra sperm in each pollen grain. Preliminary investigations using tes mutants have indicated that there is no polyspermy barrier on the central cell of Arabidopsis (Spielman et al. 2003).
Sperm transport in the embryo sac and polyspermy in vivo
There is much more known at present about the targeting of pollen tubes to the ovule than the transport of sperm within it. The pollen tube enters the embryo sac via one of the two synergids flanking the egg, where it bursts to release the sperm (Russell 1992). In Arabidopsis and in maize, the pollen tube contents are reported to move into the space between the egg and central cell plasma membranes (Mól et al. 1994; Faure et al. 2002) while in the orchid Phaius tankervilliae sperm are observed to migrate separately to the two female gametes (Ye et al. 2002). In species including maize, tobacco, Torenia fournieri (used for fertilization studies because its embryo sac partly protrudes from the micropyle), and P. tankervilliae, two actin “coronas” have been observed to form near the degenerated receptive synergid, disappearing soon after fertilization (Huang and Russell 1994; Huang and Sheridan 1998; Huang et al. 1999; Fu et al. 2000; Ye et al. 2002). One corona arcs from the degenerated synergid to the egg, and the other fills the space between egg and central cell. Support for the role of these structures in fertilization is provided by the maize mutant indeterminate gametophyte1, in which actin coronas are associated with fertilized eggs but not with unfertilized supernumerary eggs (Huang and Sheridan 1998). It is presumed that sperm migrate along the actin coronas using an actomyosin system. Myosin has been found associated with the pollen tube plasma membrane and not sperm membranes, but sperm injected into actively streaming internodal cells of the alga Nitella are able to move with actin bundles; therefore it was proposed that sperm acquire myosin on their surface after release from the pollen tube (Zhang et al. 1999; Zhang and Russell 1999).
How is it ensured that one sperm fuses with each female gamete? There are several possible mechanisms that are not necessarily mutually exclusive. There could be physical constraints or mechanisms in the embryo sac that allow only one sperm to be transported to each gamete. Each sperm could have different surface molecules allowing it to bind only to the egg or only to the central cell. There could be polyspermy blocks on the egg or central cell or both.
Most of the in vivo studies to address this question have focused on attempts to show preferential fertilization of a female gamete by each sperm in a pair. Ye et al. (2002) considered the fate of the two sperm in Phaius to be predetermined based on the observation that the sperm nearest the tip of the pollen tube always fuses with the central cell. Specific recognition could be a factor in preferential fertilization in species with dimorphic sperm (Wang et al. 2006). The sperm of Plumbago zeylanica are markedly different: each pair comprises a large sperm cell which is physically associated with the vegetative nucleus and contains numerous mitochondria but few plastids, and a small sperm with plastids but few mitochondria. Preferential fusion of the plastid-rich sperm with the egg was discovered by counting plastids in fertilized eggs and central cells (Russell 1985). This was interpreted as evidence for recognition between gametes. Preferential fertilization was also investigated in maize lines with supernumerary B chromosomes, which can undergo nondisjunction in the mitotic division that produces the sperm, so that one sperm carries two copies of the B chromosome while the other has none. Genetic analysis indicated the egg is fertilized about 65% of the time by the sperm carrying B chromosomes (Roman 1948). This is not a strong enough preference, however, to explain the targeting of one sperm to each female gamete that faithfully occurs in flowering plant reproduction. Faure et al. (2003) found a similar percentage of embryos expressing B chromosome-linked markers, but questioned whether this arose from preferential fusion. In addition, no preferential fertilization was found in vitro. Significantly, the two sperm from a single wild-type pollen grain could fertilize two different eggs in vitro, arguing against a system of gamete recognition (though this would have needed to survive the gamete isolation procedures). Instead the authors suggested that the choice of which sperm fuses with which female gamete in vivo may be influenced by factors such as differential positioning of sperm in the pollen tubes (though in general sperm travel in random order and can pass each other in the tube), non-random release into the embryo sac, or competition during adhesion of male and female gametes.
As discussed above, experiments with trifluralin-treated maize pollen containing a single male gamete could provide information about preferential fertilization, but as this question was not specifically investigated, the results are not complete enough to interpret. A similar experimental system is provided by the cdc2a mutant of Arabidopsis, which inhibits generative cell division so that the pollen grain contains only one haploid male gamete (Nowack et al. 2006). The mutant pollen can accomplish fertilization, and exclusive embryo expression of a marker normally active in both embryo and endosperm shows that the male gamete always fuses with the egg. The authors offered three explanations for this preferential fertilization: physical constraints in the embryo sac such as relative position or accessibility of the egg cell nucleus versus the central cell nucleus for arriving sperm; active signalling; or predetermined fertilization with either egg or central cell. It should be noted that the male gamete in cdc2a mutant pollen is larger than a sperm cell, and also more round than spindle-shaped, so it is conceivable there are physical constraints on its behaviour that would not apply to a normal sperm. Nevertheless, this work raises the intriguing possibility that either the first male gamete to leave the pollen tube (which in the case of the cdc2a mutant is the only gamete) is always transported to the egg, or else that both sperm initially compete for the egg but only one can enter. These models are challenged however, by the recent finding that msi1 mutants in Arabidopsis produce a small proportion of pollen grains each containing a single haploid male gamete expressing markers of sperm differentiation that appears to fertilize egg and central cell with equal frequency (Chen et al. 2008).
Observations of sperm in maize and Arabidopsis embryo sacs, and the disposition of the actin coronas in several species, suggest that at least in some species both sperm are transported to the space between the egg and central cell. In the absence of egg- and central cell-specific recognition systems, one or both female gametes might, therefore, require a polyspermy block to prevent fusion with both sperm. Attempts to induce multiple fertilizations of angiosperm eggs in vitro support the existence of a polyspermy block on this gamete. Faure et al. (1994) combined maize egg protoplasts with isolated sperm in a fusion medium containing CaCl2, moving gametes into contact with needles. When additional sperm were moved toward fertilized eggs, they could adhere but no fusion occurred, suggesting a polyspermy block. In a separate in vitro study using electrically mediated fusion, Kranz et al. (1995) observed release of cell wall components from the egg 30 s after fusion with a sperm. A fertilized egg could be fused with a second sperm up to 10 min after fertilization, but this happened more rarely between 20 and 30 min, and not at all after 35 min. It is possible that in vivo the cell wall barrier could provide an earlier polyspermy block than observed in vitro. Maize sperm have also been fused in vitro with central cells, but attempts to induce multiple fertilizations have not been reported (Faure et al. 1994; Kranz et al. 1998). However, Sun et al. (2000) reported a polyspermy block on both egg and central cell in tobacco: with a polyethylene-glycol mediated fusion system, additional sperm could adhere to fertilized eggs or central cells but could not fuse. However, tobacco may not be an ideal model for in vitro fertilization, as gametes do not fuse easily, and fused products do not continue development (Wang et al. 2006). It should also be noted that the female gametes used in all of the in vitro systems have had their cell walls removed (in vivo the egg and central cells are partly enclosed in walls; Russell 1993) and the fusion media may be very distant from the normal environment of the embryo sac—so it is difficult to assess how accurately any of these experiments reflect in vivo behaviour.
Apomixis—problems and solutions
Flowering plant species that reproduce by apomixis could provide a rich source of information about polyspermy barriers. In apomixis the embryo develops parthenogenetically, often from an unreduced egg, but in most apomictic species endosperm development still requires fertilization of the central cell, which is likewise usually unreduced (Nogler 1984). This immediately raises two problems: (1) how to ensure fertilization of the central cell but not the egg; and (2) how to cope with the disruption to parental genomic balance in the endosperm that would result from fusion of an unreduced central cell and a reduced sperm. Apomicts accordingly display a wide range of modifications to embryo sac morphology, fertilization, and tolerance to parental genomic imbalance (Nogler 1984; Savidan 2000), summarized in Fig. 4.
Embryo development in apomicts may begin before fertilization, with the embryo arising either from an egg or from another cell of the ovule. In the latter case, even if the egg is later fertilized it is out competed by the precocious asexual embryo. However, in many cases there is no precocious embryony, and therefore, a mechanism is required to prevent fertilization of the egg. In sexual embryo sacs, the egg cell wall is discontinuous (Russell 1993) while in some apomicts, a thickened egg cell wall has been observed e.g., in the grasses Dichanthium annulatum (Reddy and D’Cruz 1969) and Pennisetum ciliare (Vielle et al. 1995). This could prevent sperm entry, and has been interpreted as a precocious formation of the zygote wall. Another method of preventing egg fertilization, reminiscent of physiological polyspermy barriers, occurs in species where the nucleus of one sperm enters the egg cytoplasm but does not fuse with the egg nucleus, although the two nuclei may come into contact (“hemigamy” or “semigamy”) (Nogler 1984). The sperm nucleus may degenerate early, as in Atamosco (=Zephyranthes) texana (Pace 1913); or as described for the rainlily Cooperia pedunculata, it may divide several times and contribute to a small part of the embryo (Coe 1953). In still other species, both sperm are used to fertilize the central cell (see below), so there is no need to prevent sexual embryo formation.
The “Panicum” type of embryo sac, widespread in apomictic grasses, produces four nuclei instead of the more commonly found eight. The central cell nucleus is usually formed by fusion of two polar nuclei, but in “Panicum” type embryo sacs there is only one, unreduced polar nucleus: therefore fertilization with a single sperm generates a 2m:1p ratio in the endosperm (Brown and Emery 1958; Nogler 1984). In these species it may be important to prevent the second sperm from contributing to the endosperm, but to our knowledge there has been no investigation of a potential polyspermy block on the central cell in apomicts. Where the central cell contains two polar nuclei, it may be fertilized by both sperm, again generating a 2:1 ratio, as in Ranunculus auricomus (Goldilocks buttercup), Crataegus (hawthorn), and Mespilus (medlar) (Rutishauser 1954; Nogler 1984; Talent and Dickinson 2007). This would require absence of a polyspermy block on the central cell. Alternatively, the polar nuclei remain separate and one sperm fuses with each to form two primary endosperm nuclei with 2m:1p balance, as in D. annulatum (Reddy and D’Cruz 1969). Finally some species, such as Paspalum notatum (Quarín 1999), tolerate deviations from the 2m:1p balance; in these cases it may also be important to prevent the second sperm from entering the central cell.
Apomicts therefore demonstrate a range of variation on polyspermy and polyspermy blocks. The need to prevent fertilization of the egg in some cases appears to involve a precociously raised polyspermy block. The different mechanisms of central cell fertilization either require raising a polyspermy block if there had not been one in sexual ancestors, or dismantling one if there had.
Concluding perspectives
Polyspermy blocks are employed by a wide range of eukaryotes with very different breeding strategies. There has been little attention to this phenomenon in plants, but cytological observations suggest a physiological polyspermy barrier in some gymnosperms while genetic and in vitro fertilization experiments support the existence of a polyspermy block on the egg in angiosperms. The unique sexual reproduction strategy of flowering plants, double fertilization resulting in distinct products that are both required for successful seed development, could involve a novel purpose for a polyspermy block. While other organisms merely need to prevent fertilization of an egg by more than one sperm, flowering plants also need to promote the fertilization of one female gamete by every sperm that enters the embryo sac, except of course where there is multiple pollen tube entry as in heterofertilization. After release from the pollen tube, both sperm appear to arrive between the egg and central cell, and therefore could have access to either. There is at present no evidence that, in general, each sperm is only able to reach, bind, or penetrate a particular female gamete. In Arabidopsis, the strict preference of the single male gamete of cdc2a mutants for the egg (Nowack et al. 2006) raises the possibility that normally both sperm compete to fertilize this gamete. Initial transport of both sperm to the egg, combined with a polyspermy block, would serve the dual purpose of preventing multiple fertilization of the egg, and promoting sperm fusion with both egg and central cell.
References
Anderson ED, Owens JN (1999) Megagametophyte development, fertilization, and cytoplasmic inheritance in Taxus brevifolia. Int J Plant Sci 160:459–469
Beardsley ML (1931) The cytology of Funaria flavicans MichX. with special reference to fertilization. Ann Mo Bot Gard 18:509–540
Bedford JM (2004) Enigmas of mammalian gamete form and function. Biol Rev Camb Philos Soc 79:429–460
Bell PR, Hemsley AR (2000) Green plants: their origin and diversity, 2nd edn Cambridge University Press, UK
Berlyn GP (1962) Developmental patterns in pine polyembryony. Am J Bot 49:327–333
Berndt M.-L, Callow JA, Brawley SH (2002) Gamete concentrations and timing and success of fertilization in a rocky shore seaweed. Mar Ecol Prog Ser 226:273–285
Blackman VH (1898) On the cytological features of fertilization and related phenomena in Pinus silvestris L. Philos Trans R Soc Lond B Biol Sci 190:395–426
Bloch Qazi MC, Heifetz Y, Wolfner MF (2003) The developments between gametogenesis and fertilization: ovulation and female sperm storage in Drosophila melanogaster. Dev Biol 256:195–211
Brawley SH (1987) A sodium-dependent, fast block to polyspermy occurs in eggs of fucoid algae. Dev Biol 124:390–397
Brawley SH (1991) The fast block against polyspermy in fucoid algae is an electrical block. Dev Biol 144:94–106
Brawley SH (1992) Fertilization in natural populations of the dioecious brown alga Fucus ceranoides and the importance of the polyspermy block. Mar Biol 113:145–157
Brown WV, Emery WHP (1958) Apomixis in the Gramineae: Panicoideae. Am J Bot 45:253–263
Buchholz JT (1926) Origin of cleavage polyembryony in conifers. Bot Gaz 81:55–71
Chen Z, Hui JTL, Ingouff M, Sundaresan V, Berger F (2008) Chromatin assembly factor 1 regulates the cell cycle but not cell fate during male gametogenesis in Arabidopsis thaliana. Development 135:65–73
Coe GE (1953) Cytology of reproduction in Cooperia pedunculata. Am J Bot 40:335–343
Cruden RW (2000) Pollen grains: why so many? Plant Syst Evol 222:143–165
Dresselhaus T (2006) Cell–cell communication during double fertilization. Curr Opin Plant Biol 9:41–47
Eisenbach M, Giojalas LC (2006) Sperm guidance in mammals—an unpaved road to the egg. Nat Rev Mol Cell Biol 7:276–285
Faure JE, Digonnet C, Dumas C (1994) An in-vitro system for adhesion and fusion of maize gametes. Science 263:1598–1600
Faure JE, Rotman N, Fortune P, Dumas C (2002) Fertilization in Arabidopsis thaliana wild type: developmental stages and time course. Plant J 30:481–488
Faure JE, Rusche ML, Thomas A, Keim P, Dumas C, Mogensen HL, Rougier M, Chaboud A (2003) Double fertilization in maize: the two male gametes from a pollen grain have the ability to fuse with egg cells. Plant J 33:1051–1062
Friedman WE, Carmichael JS (1996) Double fertilization in gnetales: implications for understanding reproductive diversification among seed plants. Int J Plant Sci 157:S77–S94
Fu Y, Yuan M, Huang B-Q, Yang H-Y, Zee S-Y, O’Brien TP (2000) Changes in actin organization in the living egg apparatus of Torenia fournieri during fertilization. Sex Plant Reprod 12:315–322
Gardner AJ, Evans JP (2006) Mammalian membrane block to polyspermy: new insights into how mammalian eggs prevent fertilisation by multiple sperm. Reprod Fertil Dev 18:53–61
Gilbert SF (2006) Developmental Biology 8/e. Sinauer Associates, Sundlerland, MA, USA
Goodenough U, Lin H, Lee J-H (2007) Sex determination in Chlamydomonas. Semin Cell Dev Biol 18:350–361
Haig D, Westoby M (1991) Genomic imprinting in endosperm: its effect on seed development in crosses between species, and between different ploidies of the same species, and its implications for the evolution of apomixis. Philos Trans R Soc Lond B Biol Sci 333:1–13
Hoyt WD (1910) Physiological aspects of fertilization and hybridization in ferns. Bot Gaz 49:340–370
Huang BQ, Russell SD (1994) Fertilization in Nicotiana tabacum—cytoskeletal modifications in the embryo sac during synergid degerneration—a hypothesis for short-distance transport of sperm cells prior to gamete fusion. Planta 194:200–214
Huang BQ, Sheridan WF (1998) Actin coronas in normal and indeterminate gametophyte1 embryo sacs of maize. Sex Plant Reprod 11:257–264
Huang BQ, Fu Y, Zee SY, Hepler PK (1999) Three-dimensional organization and dynamic changes of the actin cytoskeleton in embryo sacs of Zea mays and Torenia fournieri. Protoplasma 209:105–119
Hunter RHF (1996) Ovarian control of very low sperm/egg ratios at the commencement of mammalian fertilisation to avoid polyspermy. Mol Reprod Dev 44:417–422
Kato A (1997) Induced single fertilization in maize. Sex Plant Reprod 10:96–100
Kato A (2001) Heterofertilization exhibited by trifluralin-induced bicellular pollen on diploid and tetraploid maize crosses. Genome 44:1114–1121
Kermicle JL (1971) Pleiotropic effects on seed development of the indeterminate gametophyte1 gene in maize. Am J Bot 58:1–7
Kranz E, von Wiegen P, Lörz H (1995) Early cytological events after induction of cell division in egg cells and zygote development following in vitro fertilization with angiosperm gametes. Plant J 8:9–23
Kranz E, von Wiegen P, Quader H, Lörz H (1998) Endosperm development after fusion of isolated, single maize sperm and central cells in vitro. Plant Cell 10:511–524
Leitch IJ, Bennett MD (1997) Polyploidy in angiosperms. Trends Plant Sci 2:470–476
Levitan DR, terHorst CP, Fogarty ND (2007). The risk of polyspermy in three congeneric sea urchins and its implications for gametic incompatibility and reproductive isolation. Evolution 61:2009–2016
Lin B-Y (1984) Ploidy barrier to endosperm production in maize. Genetics 107:103–115
Lloyd C, Chan J (2006) Not so divided: the common basis of plant and animal cell division. Nat Rev Mol Cell Biol 7:147–152
Marconato A, Shapiro DY (1996) Sperm allocation, sperm production and fertilization rates in the bucktooth parrotfish. Anim Behav 52:971–980
Marshall DL, Shaner MGM, Oliva J-P (2007) Effects of pollen load size on seed paternity in wild radish: the roles of pollen competition and mate choice. Evolution 61:1925–1937
McWilliam JR, Mergen F (1958) Cytology of fertilization in Pinus. Bot Gaz 199:246–249
Mól R, Matthys-Rochon E, Dumas C (1994) The kinetics of cytological events during double fertilization in Zea mays L. Plant J 5:197–206
Myles DG (1978) The fine structure of fertilization in the fern Marsilea vestita. J Cell Sci 30:265–281
Nagasato C, Motomura T, Ichimura T (1999) Influence of centriole behaviour on the first spindle formation in zygotes of the brown alga Fucus distichus (Fucales, Phaeophyceae). Dev Biol 208:200–209
Navara CS, First NL, Schatten G (1994) Microtubule organization in the cow during fertilization, polyspermy, parthenogenesis, and nuclear transfer: the role of the sperm aster. Dev Biol 162:29–40
Nogler GA (1984) Gametophytic apomixis. In: Johri BM (ed) Embryology of angiosperms. Springer, Berlin, pp 475–518
Nowack MK, Grini PE, Jakoby MJ, Lafos M, Koncz C, Schnittger A (2006) a positive signal from the fertilization of the egg cell sets off endosperm proliferation in angiosperm embryogenesis. Nat Genet 38:63–67
Pace L (1913) Apogamy in Atamosco. Bot Gaz 56:376–394
Palmer JD, Soltis DE, Chase MW (2004) The plant tree of life: an overview and some points of view. Am J Bot 91:1437–1445
Pearson GA, Brawley SH (1996) Reproductive ecology of Fucus distichus (Phaeophyceae): an intertidal alga with successful external fertilization. Mar Ecol Prog Ser 143:211–223
Quarín CL (1999) Effect of pollen source and pollen ploidy on endosperm formation and seed set in pseudogamous apomictic Paspalum notatum. Sex Plant Reprod 11:331–335
Randolph LF (1935) Cytogenetics of tetraploid maize. J Agric Res 50:591–605
Reddy PS, D’Cruz R (1969) Mechanism of apomixis in Dichanthium annulatum (Forssk) Stapf. Bot Gaz 130:71–79
Renzaglia KS, Garbary DJ (2001) Motile gametes of land plants: diversity, development, and evolution. CRC Crit Rev Plant Sci 20:107–213
Rhoades VH (1934) A study of fertilization in Zea mays. MS thesis, Cornell University, Ithaca, NY
Roman H (1948) Directed fertilization in maize. Proc Natl Acad Sci USA 34:36–42
Rudall PJ, Bateman RM (2007). Developmental bases for key innovations in the seed-plant microgametophyte. Trends Plant Sci 12:317–326
Runions CJ, Owens JN (1999) Sexual reproduction of interior spruce (Pinaceae). II. Fertilization to early embryo formation. Int J Plant Sci 160:641–652
Russell SD (1985) Preferential fertilization in Plumbago: ultrastructural evidence for gamete-level recognition in an angiosperm. Proc Natl Acad Sci USA 82:6129–6132
Russell SD (1992) Double fertilization. Int Rev Cytol 140:357–388
Russell SD (1993) The egg cell: development and role in fertilization and early embryogenesis. Plant Cell 5:1349–1359
Rutishauser A (1954) Die Entwicklungserregung des Endosperms bei pseudogamen Ranunculus-arten. Mitt. Naturforsch Ges Schaffhausen 25:1–45
Santelices B (2002) Recent advances in fertilization ecology of macroalgae. J Phycol 38:4–10
Sarkar KR, Coe EH (1971) Anomalous fertilization in diploid–tetraploid crosses in maize. Crop Sci 11:539–542
Savidan Y (2000) Apomixis: genetics and breeding. Plant Breed Rev 18:13–86
Schuel H (1984) The prevention of polyspermic fertilization in sea urchins. Biol Bull 167:271–309
Serrão EA, Brawley SH, Hedman J, Kautsky L, Samuelson G (1999) Reproductive success of Fucus vesiculosus (Phaeophyceae) in the Baltic Sea. J Phycol 35:254–269
Shimizu KK, Okada K (2000) Attractive and repulsive interactions in Arabidopsis pollen tube guidance. Development 127:4511–4518
Speransky SR, Brawley SH, Halteman WA (2000) Gamete release is increased by calm conditions in the coenocytic green alga Bryopsis (Chlorophyta). J Phycol 36:730–739
Spielman M, Preuss D, Li F-L, Browne WE, Scott RJ, Dickinson HG (1997) TETRASPORE is required for male meiotic cytokinesis in Arabidopsis thaliana. Development 124:2645–2657
Spielman M, Vinkenoog R, Scott RJ (2003) Genetic mechanisms of apomixis. Philos Trans R Soc Lond B Biol Sci 358:1095–1103
Sprague GF (1929) Hetero-fertilization in maize. Science 69:526–527
Sprague GF (1932) The nature and extent of hetero-fertilization in maize. Genetics 17:358–368
Styan CA (1998) Polyspermy, egg size, and the fertilization kinetics of free-spawning marine invertebrates. Am Nat 152:290–297
Suarez SS, Pacey AA (2006) Sperm transport in the female reproductive tract. Hum Reprod Update 12:23–37
Sun M-X, Moscatelli A, Yang H-Y, Cresti M (2000) In vitro double fertilization in Nicotiana tabacum (L.): polygamy compared with selected single pair somatic protoplast and chloroplast fusions. Sex Plant Reprod 13:113–117
Talent N, Dickinson TA (2007) Endosperm formation in aposporous Crataegus (Rosaceae, Spiracoideae, tribe Pyreae): parellels to Ranunculaceae and Poaceae. New Phytol 173:231–249
Tomaiuolo M, Hansen TF, Levitan DR (2007) A theoretical investigation of sympatric evolution of temporal reproductive isolation as illustrated by marine broadcast spawners. Evolution 61:2584–2595
Vielle J-Ph, Burson BL, Bashaw EC, Hussey MA (1995) Early fertilization events in the sexual and aposporous egg apparatus of Pennisetum ciliare (L.) Link. Plant J 8:309–316
Vigfússon E (1970). On polyspermy in the sunflower. Hereditas 64:1–52
Wang YY, Kuang A, Russell SD, Tian HQ (2006) In vitro fertilization as a tool for investigating sexual reproduction of angiosperms. Sex Plant Reprod 19:103–115
Weterings K, Russell SD (2004) Experimental analysis of the fertilization process. Plant Cell 16:S107–S118
Wong JL, Wessel GM (2006) Defending the zygote: search for the ancestral animal block to polyspermy. Curr Top Dev Biol 72:1–151
Ye XL, Yeung EC, Zee SY (2002) Sperm movement during double fertilization of a flowering plant, Phaius tankervilliae. Planta 215:60–66
Zhang ZJ, Russell SD (1999) Sperm cell surface characteristics of Plumbago zeylanica L. in relation to transport in the embryo sac. Planta 208:539–544
Zhang Z, Tian HQ, Russell SD (1999) Localization of myosin in sperm-cell-associated membranes of tobacco (Nicotiana tabacum L.). Protoplasma 208:123–128
Acknowledgments
We are grateful to Akio Kato (Faculty of Agriculture, Kyoto Prefectural University, Japan) for photographs of maize, to Fred Berger (Temasek LifeSciences Laboratory, Singapore) for sharing unpublished data, and to the BBSRC (UK) for funding MS.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Thomas Dresselhaus.
Rights and permissions
About this article
Cite this article
Spielman, M., Scott, R.J. Polyspermy barriers in plants: from preventing to promoting fertilization. Sex Plant Reprod 21, 53–65 (2008). https://doi.org/10.1007/s00497-007-0063-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00497-007-0063-7