Abstract
Nowadays, a large number of health endpoints such as disease rates, treatment costs, and death, by air pollutants, have been a serious health problem for humans. One of the most hazardous air pollutants, which is highly dangerous for human health, is polycyclic aromatic hydrocarbons (PAHs). The existence of the emission of industries’ pollutants and seasonal variations are the primary agents affecting PAHs’ concentration. The purposes of this study were to calculate the cancer risk and measure PAHs’ exposure in the ambient air of Ahvaz, southwest of Iran, during 2017. Three distinct areas ((S1) industrial, (S2) high traffic, and (S3) residential) of Ahvaz metropolitan were selected. Omni sampler equipped with polytetrafluoroethylene (PTFE) filters were used for active sampling of PAHs. To detect the level of PAHs, gas chromatography with mass spectrometry (GC/MS) was used. Incremental lifetime cancer risk (ILCR) and lifetime average daily dose (LADD) were used to estimate the health risk caused by PAHs. The results showed that the residential and industrial areas had the lowest and highest level of PAHs. Moreover, the average levels of PAHs in industrial, high traffic, and residential areas were 8.44 ± 3.37, 7.11 ± 2.64, and 5.52 ± 1.63 ng m−3, respectively. Furthermore, ILCR in autumn and winter was higher than EPA standard, 0.06307 and 0.04718, respectively. In addition, ILCR in different areas was significantly higher than standard. Research findings imply that the levels of exposure to PAHs can increase ILCR and risk of health endpoint. The cancer risk attributed to PAHs should be further investigated from the perspective of the public health in metropolitans.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Air pollution consists of a mixture of particles, vapors, and gases (Geravandi et al. 2017; Goudarzi et al. 2017a). In recent years, anthropogenic air pollution, particularly development of industries (petroleum, gas, petrochemical), transportation, population growth, and dust storm (due to prolonged drought region) are the most critical factors threatening the humans and environment in Iran (Delfino et al. 2009; Dobaradaran et al. 2016; Elder et al. 2015; Fiala et al. 2001; Goudarzi et al. 2018; Hashemzadeh et al. 2017; Khaniabadi et al. 2017a; Neisi et al. 2016; Soleimani et al. 2013). Reducing its harmful effects and warnings to sensitive groups are one of the crucial government actions. Living in megacities with regard to breathing air pollutants can induce different health endpoints (Khaniabadi et al. 2017b). The consequences of air pollution on human health are a prominent issue addressed by several studies. Many studies conducted in cities across the USA, Europe, and Asia have reported the strong evidence regarding the deadly impact of air pollutants. Moreover, several recent studies have identified PAHs as a highly detrimental pollutant for human health (Chen et al. 2016; Goudarzi et al. 2017b; Liu et al. 2016). The main sources of emission PAHs are fossil-fuel combustion, petroleum refining, chemical manufacturing, coal, and dust storm (Alawi and Azeez 2017; Boonyatumanond et al. 2006; De Luca et al. 2005; Goudarzi et al. 2017b; Shen et al. 2014; Soclo et al. 2000; Wang et al. 2011). Some other studies state that the PAHs could increase ILCR in humans (Guerreiro et al. 2016; Hu et al. 2007; Kim et al. 2013; Pruneda-Álvarez et al. 2016; Tsai et al. 2001; Watanabe et al. 2009). Children are the most important group that can be influenced by PAHs’ exposure more than adults (Avagyan and Westerholm 2017; Chen and Liao 2006; Goudarzi et al. 2017b; Jerzynska et al. 2016; Oliveira et al. 2017; Perera et al. 2006). Allergic skin, thrombotic effects, decreased body weight, low IQ at age three, asthmatics, nausea, eye irritation, irritation, diarrhea, vomiting, impaired lung function, genotoxicity, and ILCR are the range of adverse health effects caused by PAHs’ exposure (Agency 2012; Alawi and Azeez 2016; Avagyan and Westerholm 2017; Balcıoğlu 2016; Boström et al. 2002; Chen and Liao 2006; Goudarzi et al. 2017b; Khaniabadi et al. 2017c; Kim et al. 2013; Kumar et al. 2016; Maragkidou et al. 2016; Tsai et al. 2001; Tseng et al. 2014). A series of studies have shown that smoking, inhalation, breathing exhaust fumes, and eating food contaminant are the primary factors transferring PAHs into the human body (Abdel-Shafy and Mansour 2016; Ali et al. 2016; Chetwittayachan et al. 2002; Hasanati et al. 2011; Hu et al. 2017; Kim et al. 2013; Oliveira et al. 2016; Rudel and Perovich 2009). Based on the report of an international organization, Ahvaz is one of the most polluted cities in the world. The pollution, of course, puts residents at risk of various health problems (Davar et al. 2014; Geravandi et al. 2015; Khaefi et al. 2016; Yari et al. 2016). In 2002, Boström et al. studied indicators, guidelines, and cancer risk assessment for PAHs (Boström et al. 2002). Furthermore, Chen et al. in 2006 assessed the exposure to environmental PAHs pollutants and its health risks for humans (Chen and Liao 2006). In a similar work, the quantification of carcinogenic risks related to the sources of particle-bound PAHs from Chengdu in China was studied by model-incremental lifetime cancer risk method (Liu et al. 2015). Moreover, Tseng et al. estimated the cancer risk attributed to incremental exposure to PAHs (Tseng et al. 2014).
Watanabe et al. in 2009, by epidemiologic data, compared ILCR computed for benzo[a]pyrene with lung cancer risk (Watanabe et al. 2009). Pankow et al. in 2007 evaluated risks of cancer for potentially reduced exposure product (Pankow et al. 2007). Alawi and Azeez in a wasteland in Iraq studied the effects of PAHs derived from Al-Ahdab oil field (Alawi and Azeez 2016). Kim et al. measured the level of airborne PAHs and its effects on human health (Kim et al. 2013). Furthermore, PAHs’ concentration in indoor dust samples in Kuwait and Jeddah was measured by Guirguis et al. (Ali et al. 2016).
In the previous study at animal scale lab, detrimental impacts of PM10 on many parameters including iNOS and eNOS mRNA expression levels, electrocardiogram parameters, blood pressure and oxidative stress were examined (Dianat et al. 2016a; Dianat et al. 2016b). The purpose of this study was to explore the association between cancer risk and PAHs originated from particulate in the ambient air of Ahvaz, southwest of Iran, during 2017.
Materials and methods
Methods
This cross-sectional study was carried out during 2017, in (S1) industrial, (S2) high traffic, and (S3) residential areas of Ahvaz. An active sampling system was used for measuring PAHs’ concentration within a specific period to cover autumn and winter.
Sampling
Samples were taken using Omni sampler (Bgi instruments USA Company; 231 model) equipped with polytetrafluoroethylene (PTFE) filters (8*10 in. Whatman, the USA) (Fig. 1). The flow rate of sampling was 5 l/s. Before sampling, the polytetrafluoroethylene was placed in an oven at 104 °C for 2 h.
Standard preparation and analysis
Air sampling was carried out at the same time of the day before and 24 h. After sampling, each of chosen filter loading samples was divided into four parts. One fourth of the exposed PTFE filter was cut into pieces and put in a Teflon container. The concentrated extract was cleaned up using a florisil column, according to the NIOSH 5515 method (Hassanvand et al. 2015). At the next stage, a mixture of nitric acid %5, distilled water, 5 mL methanol (ratio of 1–1 V%), and 5 mL dichloromethane (ratio of 1–1 V%) was added to it. The resultant solution was stored in a clean and sterile plastic bottle at 4 °C until further analyses. Finally, 1.5 mL resultant solution was picked up and thrown in Vaile for injection of GC-MS (7890N, AGILENT & MS 5975C, MODE). A fused silica capillary column (HP5-MS 30 m × 0.25 mm × 0.25 μm) was used for separation. The injected volume for PAHs was 3 and 2 μL/splitless, respectively. The temperature program of the injector was 230 °C. Helium was used as a carrier gas at 1–2 mL/min. The oven temperature was programmed from 80 °C (held for 2 min) and raised to 285 °C @ 7 °C/min (held for 4 min).
Method validation procedure
A linearity regression function for PAHs in the air samples was set up according to calibration measurement. The good linearity was observed in the detected range. The correlation coefficients (R2) were found to be 0.99, 0.98, 0.94, 0.97, 0.98, 0.98, 97, 0.96, 0.96, 0.95, 0.99, 0.97, 0.98, 0.99, 0.99, and 0.99 for naphthalene, acenaphthylene, acenaphthene, fluorene, phenanthrene, anthracene, fluoranthene, pyrene, benzo[a]anthracene, chrysene, benzo[b]fluoranthene, benzo[k]fluoranthene, benzo[a]pyrene, dibenz[a,h]anthracene, benzo[ghi]perylene, and indeno[1,2,3-cd]pyrene, respectively (Abdel-Shafy and Mansour 2016). In this study, the method of validation for analysis of urine samples was the limit of quantification (LOQ), determination of the limit of detection (LOD), precision, matrix effect, accuracy, recovery, and the calibration curve. The calibration curve showed a significant correlation with the linear regression profile (R2 = 0.99) (Abdel-Shafy and Mansour 2016). The average PAHs recovery efficiency ranged from 59 to 120%. In addition, the identification time of the 16 original combinations of PAHs ranged from 5.13 to 23.14 min. Moreover, the efficiency of PAHs recovery was extraction and analysis methods by determination of the standard deviation of 500, 1000, and 2000 μg/L spiked samples.
Description of study area
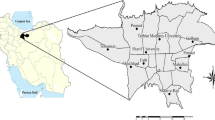
The experiment took place in three distinct areas of Ahvaz including industrial (31° 29′ N, 48° 72′ E), high traffic (31° 32′ N, 48° 69′ E), and residential areas (31° 42′ N, 48° 39′ E) (Geravandi et al. 2015; Goudarzi et al. 2015, 2016; Khaefi et al. 2017; Nashibi et al. 2017; Neisi et al. 2016). Locations of air samples are shown in Fig. 2.
Estimation of incremental lifetime cancer risk (ILCR) due to polycyclic aromatic hydrocarbons
PAHs enter the body by ingestion, inhalation, and dermal contact and induce health risks. To determine ILCR, lifetime average daily dose was calculated for PAH-exposed adults (Agency 2012; Kaur et al. 2013). LADD and ILCR were calculated based on following equation (Kaur et al. 2013; Liu et al. 2015; Shen et al. 2014):
where LADD is lifetime average daily dose (mg/kg/day); C is BaP exposure concentration (mg/m3); IR is inhalation rate (m3/day) (= 0.6 m3/h in this study); EF is exposure frequency (= 365 days/year in this study); ED is exposure duration (= 25 years for adults in this study); BW is body weight (kg) (= 50–70 kg in this study); AT is an averaging time (days) following US EPA (70 years or 25,550 days); CF is a conversion factor (10−3); ILCR is incremental lifetime cancer risk; and CSF is cancer slope factor (mg/kg/day)−1 (Kaur et al. 2013; Pankow et al. 2007; Shen et al. 2014; Tseng et al. 2014; Watanabe et al. 2009).
According to some studies and EPA report, the recommended amount of CSF for BaP by inhalation is 0.13 (Kaur et al. 2013).
Results
The characteristics of PAHs
This study assessed the PAHs’ exposure-associated cancer risk among individuals living in three distinct areas of Ahvaz, southwest of Iran, during 2017. The total of 16 PAHs (naphthalene, acenaphthylene, acenaphthene, fluorene, phenanthrene, anthracene, pyrene, fluoranthene, benzo(a)ant, chrysene, b(b)f, b(k)f, b(a)p, dbenzo[ah]anthracene, indeno[123 cd]pyrene, and benzo[ghi]perylene) in the outdoor air of Ahvaz are shown in Table 1.
Based on World Health Organization (WHO) report, the standard inhalation rates of benzo(a)pyrene and other PAHs for general population living in residential, high traffic, and industrial areas are 0.4, 0.7, and 1.5 ng m−3, respectively (Abdel-Shafy and Mansour 2016; Harrison et al. 2009; Lioy et al. 1988; Organization 2010). In this study, the lowest and highest levels of PAHs were observed in residential and industrial areas (Table 2). In addition, the average levels of PAHs in industrial, high traffic, and residential areas were 8.44 ± 3.37, 7.11 ± 2.64, and 5.52 ± 1.63 ng m−3, respectively (Table 2).
The concentration of 16 PAHs varied in a range of 1.84 to 14.76 ng m−3 (mean 7.02 ng m−3). In addition, B(k)F and anthracene had the lowest and highest average concentration (Table 2).
Incremental lifetime cancer risk
Carcinogenic and mutagenic effects of BaP, the best known of PAHs, have been already investigated (Nisbet and LaGoy 1992; Tseng et al. 2014). In a dose-response relationship of risk assessment, toxic equivalency factors (TEFs) are used for various pollutants to a given well-known toxic chemical (Nisbet and LaGoy 1992; Van den Berg et al. 1998). The relative carcinogenic effects of 16 typical PAHs have been estimated by TEFs. Cancer risk for a 70-year lifetime exposure is estimated through combining the unit risk of BaP, 8.7 × 10–5 (ng/m3)−1, and the corresponding PAHs in the target environments (Liu et al. 2015; Tseng et al. 2014).
Different amounts of BaPeq (TEQ) (ng/m3) (average amount in warm-cold seasons), ILCR, and LADD in adults were calculated based on the level of PAHs in industrial, high traffic, and residential areas, as summarized in Table 3. Incremental lifetime cancer risk is the first attempt that evaluated the potential cancer risk of human exposure to polycyclic aromatic hydrocarbons in the ambient air by using three different TEQ methods. Human activities and exposure to PAHs in urban region lay an important role in governing potential cancer risk of the human. In this study, the average values of ILCR of individuals living in three distinct areas of Ahvaz as estimated by three different TEQs were 6.7 and 8.9, for autumn and winter, respectively (Table 3).
Figures 3 and 4 present the level of BaP concentration in industrial, high traffic, and residential areas during warm-cold seasons. From these findings, poor air quality condition in Ahvaz is due to the higher BaP concentration compared to WHO guideline (1 ng/m3).
Discussion
This study explored the association between cancer risk and the PAHs’ concentration in the ambient air of Ahvaz, southwest of Iran, during 2017. As a result of production and emission of air pollutants, particularly PAHs, from industries and vehicles, health endpoints such cardiovascular disease, repository disease, and cancer risk are increasing among people living in industrial cities. Consequently, closer attention to decrease air pollutants through viable solutions is of the essence. All survey participants, living in three distinct areas of Ahvaz, were exposed to a higher level of PAHs compared to WHO air quality guidelines and region standard values (Abdel-Shafy and Mansour 2016; Harrison et al. 2009; Lioy et al. 1988; Neisi et al. 2016; Organization 2010; Tchounwou et al. 2012; Zhang et al. 2014). The high concentration of PAHs may occur when using them as a catalyst in oil, gas, and petrochemical industries. In addition, the petrogenic and pyrogenic sources of PAHs could be the reason for PAHs’ variations in ambient air of Ahvaz. The higher this ratio, the greater the contributions from combustion phenomena (oil, coal, gasoline, and gas oil) in the formation of these compounds. Balcıoğlu et al. studied the potential effects of PAHs in marine foods on human and suggested it as a risk for human health (Balcıoğlu 2016). Our study results, as well, identified PAHs as a substantial threat to human. Similar to our study, Rezaei et al. observed a higher level of exposure to PAHs during the cold season (Rezaei et al. 2015). It is worth mentioning that the temperature inversion occurs predominantly in autumn and winter and also demand sharply rises for heating fuels, crude oil, and natural gas in cold season, ultimately leading to higher refinery operation as well as higher level of PAHs’ concentration emission.
To evaluate the health risk of PAHs, BaP (BaPeq) method was used. Consequently, BaPeq (TEQ) was calculated by multiplying toxic equivalency factors (TEFs) in each PAH component. The total levels of BaP in winter and autumn were found to be 8.96 and 6.702 ng/m3, respectively. Moreover, BaP (about 76% of total BaP concentration) has the highest level of BaPeq (Table 3). The source and distribution of BaP are quite diverse. The assessment of ILCR of PAHs is mainly based on laboratory tests and epidemiologic work. ILCR and LADD for adult residents of Ahvaz in warm-cold seasons are given in Table 3. ILCR in autumn and winter, in our study, was 0.04718 and 0.06307, respectively. According to EPA guidelines, the acceptable ILCR is considered to be between 0.0001 and 0.00001 (Tseng et al. 2014; Watanabe et al. 2009). The results of this study disclosed the importance of ILCR and showed the higher ILCR than EPA guidelines among our study participants. In addition, results showed that people living in industrial and high traffic areas had higher ILCR compared to those living in a residential area; however, a comprehensive epidemiologic study is required to affirm our study results. The lower ILCR than EPA standard in China has been reported (Liu et al. 2015). Furthermore, a substantially higher ILCR (0.00435) than EPA standard has been reported for carbon black manufacturing workers exposed to PAHs (Tsai et al. 2001).
The comparison of the present study to the present existing studies and guidelines reveals that the city of Ahvaz due to its neighborhood and affiliation with the oil, gas, petrochemical, steel, and piping industries have a high concentration of air pollutants, particularly PAHs, which can increase ILCR as well as various health hazards among residents.
Limitations and strengths
We ran the study for two seasons just because of limited time for instruments. Observed trends may not be representative of a wider population because this study had a small sample size. Therefore, further studies should be implemented to investigate all areas of Ahvaz as well as other industrial cities in a longer period. Also, we can reduce citizenship exposure to hazardous contents of particulates by compressive enterprises in the field of industries and transportation patterns.
Conclusion
Health endpoints associated with exposure to noxious pollutants, particularly PAHs, are increasing among residents of large industrial cities, underlining the pressing need for governmental action to decrease the level of those health-threatening pollutants. Results showed that average amount of heavy metal was 7–8 times higher than the standard value. In addition, it appears reasonable to the high levels of PAHs observed in Ahvaz, Iran, were attributed to petroleum and gas industries, demographic, and climate characteristics of this city. The high concentration of PAHs in the air of large industrial cities primarily arises from industrialization, urbanization and climate change, and high consumption of fossil fuels.
Finally, careful monitoring, application of modern automobiles, developing the green area, and controlling the emission of PAHs will have important impacts on decreasing amount of these dangerous pollutants.
Change history
02 August 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00484-021-02171-4
References
Abdel-Shafy HI, Mansour MS (2016) A review on polycyclic aromatic hydrocarbons: source, environmental impact, effect on human health and remediation. Egypt J Pet 25:107–123
Agency UEP (2012) Risk assessment guidance for superfund, human health evaluation manual part A. Office of Emergency and Remedial Response, Washington, DC
Alawi MA, Azeez AL (2016) Study of polycyclic aromatic hydrocarbons (PAHs) in soil samples from Al-Ahdab oil field in Waset region. Iraq Toxin Rev 35:69–76
Alawi MA, Azeez AL (2017) Study of polychlorinated biphenyls (PCBs) in soil samples from Al-Ahdab oil field in Waset region/Iraq. Toxin Rev 36(2):109–115
Ali N, Ismail IMI, Khoder M, Shamy M, Alghamdi M, Costa M, Ali LN, Wang W, Eqani SAMAS (2016) Polycyclic aromatic hydrocarbons (PAHs) in indoor dust samples from cities of Jeddah and Kuwait: levels, sources and non-dietary human exposure. Sci Total Environ 573:1607–1614
Avagyan R, Westerholm R (2017) Target and suspect screening of OH-PAHs in air particulate using liquid chromatography-orbitrap high resolution mass spectrometry. Talanta 165:702–708
Balcıoğlu EB (2016) Potential effects of polycyclic aromatic hydrocarbons (PAHs) in marine foods on human health: a critical review. Toxin Rev 35:98–105
Boonyatumanond R, Wattayakorn G, Togo A, Takada H (2006) Distribution and origins of polycyclic aromatic hydrocarbons (PAHs) in riverine, estuarine, and marine sediments in Thailand. Mar Pollut Bull 52:942–956
Boström C-E, Gerde P, Hanberg A, Jernström B, Johansson C, Kyrklund T, Rannug A, Törnqvist M, Victorin K, Westerholm R (2002) Cancer risk assessment, indicators, and guidelines for polycyclic aromatic hydrocarbons in the ambient air. Environ Health Perspect 110:451–489
Chen S-C, Liao C-M (2006) Health risk assessment on human exposed to environmental polycyclic aromatic hydrocarbons pollution sources. Sci Total Environ 366:112–123
Chen Y, Zhang J, Ma Q, Sun C, Ha S, Zhang F (2016) Human health risk assessment and source diagnosis of polycyclic aromatic hydrocarbons (PAHs) in the corn and agricultural soils along main roadside in Changchun, China. Hum Ecol Risk Assess 22:706–720
Chetwittayachan T, Shimazaki D, Yamamoto K (2002) A comparison of temporal variation of particle-bound polycyclic aromatic hydrocarbons (pPAHs) concentration in different urban environments: Tokyo, Japan, and Bangkok, Thailand. Atmos Environ 36:2027–2037
Chuang JC, Mack GA, Kuhlman MR, Wilson NK (1991) Polycyclic aromatic hydrocarbons and their derivatives in indoor and outdoor air in an eight-home study. Atmos Environ Part B Urban Atmos 25:369–380
Davar H, Taghavirad SS, Mohammadi MJ (2014) The investigation of effects of silica on the environment and prevention of release of the silica particles with simulation of gas-solid flow in a gas cyclone. Res J Chem Environ 18:28–30
De Luca G et al (2005) Nature, distribution and origin of polycyclic aromatic hydrocarbons (PAHs) in the sediments of Olbia harbor (northern Sardinia, Italy). Mar Pollut Bull 50:1223–1232
Delfino RJ, Staimer N, Tjoa T, Gillen DL, Polidori A, Arhami M, Kleinman MT, Vaziri ND, Longhurst J, Sioutas C (2009) Air pollution exposures and circulating biomarkers of effect in a susceptible population: clues to potential causal component mixtures and mechanisms. Environ Health Perspect 117:1232–1238
Dianat M, Radmanesh E, Badavi M, Mard SA, Goudarzi G (2016a) Disturbance effects of PM 10 on iNOS and eNOS mRNA expression levels and antioxidant activity induced by ischemia–reperfusion injury in isolated rat heart: protective role of vanillic acid. Environ Sci Pollut Res 23(6)5154–5165
Dianat M, Radmanesh E, Badavi M, Goudarzi G, Mard SA (2016b) The effects of PM10 on electrocardiogram parameters, blood pressure and oxidative stress in healthy rats: the protective effects of vanillic acid. Environ Sci Pollut Res 23(19)19551–19560
Dobaradaran S et al (2016) Determination of cardiovascular and respiratory diseases caused by PM10 exposure in Bushehr, 2013. J Mazandaran Univ Med Sci 26:42–52
Elder A, Schwartz J, Oberdörster G (2015) Particulate air pollution and CNS health. Air pollution and health effects 269–288
Fiala Z, Vyskocil A, Krajak V, Viau C, Ettlerova E, Bukac J, Fialova D, Emminger S (2001) Environmental exposure of small children to polycyclic aromatic hydrocarbons. Int Arch Occup Environ Health 74:411–420
Geravandi S, Goudarzi GR, Vousoghi Niri M, Mohammadi M, Saeidimehr S, Geravandi S (2015) Estimation of the cardiovascular and respiratory mortality rate resulted from exposure to sulfur dioxide pollutant in Ahvaz. J Environ Stud 41:341–350
Geravandi S, Sicard P, Khaniabadi YO, de Marco A, Ghomeishi A, Goudarzi G, Mahboubi M, Yari AR, Dobaradaran S, Hassani G, Mohammadi MJ, Sadeghi S (2017) A comparative study of hospital admissions for respiratory diseases during normal and dusty days in Iran. Environ Sci Pollut Res 24:18152–18159
Goudarzi G et al (2015) Cardiovascular and respiratory mortality attributed to ground-level ozone in Ahvaz. Iran Environ Monit Assess 187:1–9
Goudarzi G, Geravandi S, Idani E, Hosseini SA, Baneshi MM, Yari AR, Vosoughi M, Dobaradaran S, Shirali S, Marzooni MB (2016) An evaluation of hospital admission respiratory disease attributed to sulfur dioxide ambient concentration in Ahvaz from 2011 through 2013. Environ Sci Pollut Res 23(21):22001–22007
Goudarzi G, Daryanoosh SM, Godini H, Hopke PK, Sicard P, de Marco A, Rad HD, Harbizadeh A, Jahedi F, Mohammadi MJ, Savari J, Sadeghi S, Kaabi Z, Omidi Khaniabadi Y (2017a) Health risk assessment of exposure to the middle-eastern dust storms in the Iranian megacity of Kermanshah. Public Health 148:109–116
Goudarzi G, Idani E, Alavi N, Salmanzadeh S, Babaei AA, Geravandi S, Mohammadi MJ, Mahboubi M, Moradi M (2017b) Association of polycyclic aromatic hydrocarbons of the outdoor air in Ahvaz, southwest Iran during warm-cold season. Toxin Rev 36(4):282–289
Goudarzi G et al (2018) Health risk assessment on human exposed to heavy metals in the ambient air PM 10 in Ahvaz, southwest Iran. Int J Biometeorol 1–9
Guerreiro C, Horálek J, de Leeuw F, Couvidat F (2016) Benzo (a) pyrene in Europe: ambient air concentrations, population exposure and health effects. Environ Pollut 214:657–667
Harrison R, Delgado-Saborit J, Baker S, Aquilina N, Meddings C, Harrad S, Matthews I, Vardoulakis S, Anderson H (2009) Measurement and modeling of exposure to selected air toxics for health effects studies and verification by biomarkers. Res Report (Health Effects Institute) 143:3–96 discussion 97–100
Hasanati M, Savari A, Nikpour Y, Ghanemi K (2011) Assessment of the sources of polycyclic aromatic hydrocarbons in Mousa inlet by molecular ratios. J Environ Studies 37:1–3
Hashemzadeh B et al (2017) Effects of PM 2.5 and NO 2 on the 8-isoprostane and lung function indices of FVC and FEV 1 in students of Ahvaz city, Iran Saudi. J Biol Sci https://doi.org/10.1016/j.sjbs.2016.11.008
Hassanvand MS et al (2015) Characterization of PAHs and metals in indoor/outdoor PM 10/PM 2.5/PM 1 in a retirement home and a school dormitory. Sci Total Environ 527:100–110
Hu Y, Bai Z, Zhang L, Wang X, Zhang L, Yu Q, Zhu T (2007) Health risk assessment for traffic policemen exposed to polycyclic aromatic hydrocarbons (PAHs) in Tianjin, China. Sci Total Environ 382:240–250
Hu R, Liu G, Zhang H, Xue H, Wang X (2017) Levels and sources of PAHs in air-borne PM2. 5 of Hefei City, China. Bull Environ Contam Toxicol 98(2):270–276
Jerzynska J, Podlecka D, Polanska K, Hanke W, Stelmach I, Stelmach W (2017) Prenatal and postnatal exposure to polycyclic aromatic hydrocarbons and allergy symptoms in city children. Allerg Immunol 45(1):18–24
Kaur S, Senthilkumar K, Verma V, Kumar B, Kumar S, Katnoria JK, Sharma C (2013) Preliminary analysis of polycyclic aromatic hydrocarbons in air particles (PM10) in Amritsar, India: sources, apportionment, and possible risk implications to humans. Arch Environ Contam Toxicol 65:382–395
Khaefi M et al (2016) An association between ambient pollutants and hospital admitted respiratory cases in Ahvaz, Iran. Fresenius Environ Bull 25:3955–3961
Khaefi M, Geravandi S, Hassani G, Yari AR, Soltani F, Dobaradaran S, Moogahi S, Mohammadi MJ, Mahboubi M, Alavi N, Farhadi M, Khaniabadi YO (2017) Association of particulate matter impact on prevalence of chronic obstructive pulmonary disease in Ahvaz, Southwest Iran during 2009–2013. Aerosol Air Qual Res 17:230–237
Khaniabadi YO, Daryanoosh SM, Amrane A, Polosa R, Hopke PK, Goudarzi G, Mohammadi MJ, Sicard P, Armin H (2017a) Impact of middle eastern dust storms on human health. Atmos Pollut Res 8:606–613
Khaniabadi YO, Daryanoosh SM, Hopke PK, Ferrante M, de Marco A, Sicard P, Oliveri Conti G, Goudarzi G, Basiri H, Mohammadi MJ, Keishams F (2017b) Acute myocardial infarction and COPD attributed to ambient SO2 in Iran. Environ Res 156:683–687
Khaniabadi YO, Fanelli R, de Marco A, Daryanoosh SM, Kloog I, Hopke PK, Conti GO, Ferrante M, Mohammadi MJ, Babaei AA, Basiri H, Goudarzi G (2017c) Hospital admissions in Iran for cardiovascular and respiratory diseases attributed to the middle eastern dust storms. Environ Sci Pollut Res 24:16860–16868
Kim K-H, Jahan SA, Kabir E, Brown RJ (2013) A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects. Environ Int 60:71–80
Kumar V, Kothiyal N, Saruchi, Vikas P, Sharma R (2016) Sources, distribution, and health effect of carcinogenic polycyclic aromatic hydrocarbons (PAHs)—current knowledge and future directions. J Chin Adv Mater Soc 4:302–321
Lioy PL, Waldman JM, Greenberg A, Harkov R, Pietarinen C (1988) The total human environmental exposure study (THEES) to benzo (a) pyrene: comparison of the inhalation and food pathways. Arch Environ Health 43:304–312
Liu G-R, Peng X, Wang RK, Tian YZ, Shi GL, Wu JH, Zhang P, Zhou LD, Feng YC (2015) A new receptor model-incremental lifetime cancer risk method to quantify the carcinogenic risks associated with sources of particle-bound polycyclic aromatic hydrocarbons from Chengdu in China. J Hazard Mater 283:462–468
Liu B, Xue Z, Zhu X, Jia C (2016) Long-term trends (1990–2014), health risks, and sources of atmospheric polycyclic aromatic hydrocarbons (PAHs) in the US. Environ Pollut 220(Part B): 1171–1179
Maragkidou A et al (2016) Occupational health risk assessment and exposure to floor dust PAHs inside an educational building. Sci Total Environ 579:1050–1056
Nashibi R, Afzalzadeh S, Mohammadi MJ, Yari AR, Yousefi F (2017) Epidemiology and treatment outcome of Mucormycosis in Khuzestan, southwest of Iran. Arch Clin Infect Dis 12:e37221
Neisi A, Goudarzi G, Akbar Babaei A, Vosoughi M, Hashemzadeh H, Naimabadi A, Mohammadi MJ, Hashemzadeh B (2016) Study of heavy metal levels in indoor dust and their health risk assessment in children of Ahvaz city, Iran. Toxin Rev 35:16–23
Nisbet IC, LaGoy PK (1992) Toxic equivalency factors (TEFs) for polycyclic aromatic hydrocarbons (PAHs). Regul Toxicol Pharmacol 16:290–300
Oliveira M et al (2016) Polycyclic aromatic hydrocarbons at fire stations: firefighters’ exposure monitoring and biomonitoring, and assessment of the contribution to total internal dose. J Hazard Mater 323:184–194
Oliveira M, Slezakova K, Delerue-Matos C, do Carmo Pereira M, Morais S (2017) Assessment of exposure to polycyclic aromatic hydrocarbons in preschool children: levels and impact of preschool indoor air on excretion of main urinary monohydroxyl metabolites. J Hazard Mater 322:357–369
Organization WH (2010) WHO guidelines for indoor air quality: selected pollutants. WHO 97892:89002134
Pankow JF, Watanabe KH, Toccalino PL, Luo W, Austin DF (2007) Calculated cancer risks for conventional and “potentially reduced exposure product” cigarettes. Cancer Epidemiol Biomarkers Prev 16:584–592
Perera FP et al (2006) Effect of prenatal exposure to airborne polycyclic aromatic hydrocarbons on neurodevelopment in the first 3 years of life among inner-city children. Environ Health Perspect 114(8):1287–1292
Pruneda-Álvarez LG, Pérez-Vázquez FJ, Ruíz-Vera T, Ochoa-Martínez ÁC, Orta-García ST, Jiménez-Avalos JA, Pérez-Maldonado IN (2016) Urinary 1-hydroxypyrene concentration as an exposure biomarker to polycyclic aromatic hydrocarbons (PAHs) in Mexican women from different hot spot scenarios and health risk assessment. Environ Sci Pollut Res 23:6816–6825
Rezaei F, Kakooei H, Ahmadkhaniha R, Azam K, Omidi L, Shahtaheri S (2015) Assessing effects of seasonal variation on occupational exposure of newsagent kiosks to polycyclic aromatic hydrocarbons found in the urban atmosphere of Tehran metropolitan Iranian. J Health Environ 8:203–216
Rudel RA, Perovich LJ (2009) Endocrine disrupting chemicals in indoor and outdoor air. Atmos Environ 43:170–181
Shen H et al (2014) Global lung cancer risk from PAH exposure highly depends on emission sources and individual susceptibility. Sci Rep 4:6561 https://doi.org/10.1038/srep06561
Soclo H, Garrigues P, Ewald M (2000) Origin of polycyclic aromatic hydrocarbons (PAHs) in coastal marine sediments: case studies in Cotonou (Benin) and Aquitaine (France) areas. Mar Pollut Bull 40:387–396
Soleimani Z, Goudarzi G, Naddafi K, Sadeghinejad B, Latifi SM, Parhizgari N, Alavi N, Babaei AA, Akhoond MR, Khaefi M, Rad HD, Mohammadi MJ, Shahsavani A (2013) Determination of culturable indoor airborne fungi during normal and dust event days in Ahvaz, Iran. Aerobiologia 29:279–290
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012) Heavy metal toxicity and the environment. In: Molecular, clinical and environmental toxicology. EXS 101:133–164
Tsai P-J, Shieh H-Y, Lee W-J, Lai S-O (2001) Health-risk assessment for workers exposed to polycyclic aromatic hydrocarbons (PAHs) in a carbon black manufacturing industry. Sci Total Environ 278:137–150
Tseng H-S, Liu S-P, Uang S-N, Yang L-R, Lee S-C, Liu Y-J, Chen D-R (2014) Cancer risk of incremental exposure to polycyclic aromatic hydrocarbons in electrocautery smoke for mastectomy personnel. World J Surg Oncol 12:31
Van den Berg M et al (1998) Toxic equivalency factors (TEFs) for PCBs, PCDDs, PCDFs for humans and wildlife. Environ Health Perspect 106:775–792
Wang W, Huang M-j, Kang Y, Wang H-s, Leung AO, Cheung KC, Wong MH (2011) Polycyclic aromatic hydrocarbons (PAHs) in urban surface dust of Guangzhou, China: status, sources and human health risk assessment. Sci Total Environ 409:4519–4527
Watanabe KH, Djordjevic MV, Stellman SD, Toccalino PL, Austin DF, Pankow JF (2009) Incremental lifetime cancer risks computed for benzo [a] pyrene and two tobacco-specific N-nitrosamines in mainstream cigarette smoke compared with lung cancer risks derived from epidemiologic data. Regul Toxicol Pharmacol 55:123–133
Yari AR, Goudarzi G, Geravandi S, Dobaradaran S, Yousefi F, Idani E, Jamshidi F, Shirali S, Khishdost M, Mohammadi MJ (2016) Study of ground-level ozone and its health risk assessment in residents in Ahvaz City, Iran during 2013. Toxin Rev 35:201–206
Zhang W-L, Du Y, Zhai M-M, Shang Q (2014) Cadmium exposure and its health effects: a 19-year follow-up study of a polluted area in China. Sci Total Environ 470:224–228
Funding
This work was part of a funded Ph.D. thesis of Mohammad Javad Mohammadi, a student at Ahvaz Jundishapur University of Medical Sciences (AJUMS), and the financial support of this study (U-95094) was provided by AJUMS.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Goudarzi, G., Geravandi, S., Alavi, N. et al. Association between cancer risk and polycyclic aromatic hydrocarbons’ exposure in the ambient air of Ahvaz, southwest of Iran. Int J Biometeorol 62, 1461–1470 (2018). https://doi.org/10.1007/s00484-018-1543-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00484-018-1543-1