Abstract
Key message
Multivariate morphometric analyses were used for the first time to examine sexual dimorphism and shape variability of needles in natural populations of relict species Taxus baccata L.
Abstract
Needle morphology and intraspecific variability of Taxus baccata L. were examined for the first time in Serbian natural populations (in the central Balkans). A geometric morphometric approach was used to assess size and shape variation of needles and to address questions of sexual dimorphism and phenotypic plasticity. Population-specific sexual size dimorphism was found. In the Tara population, females had larger needles than males and this was in accordance with earlier reports on T. baccata dimorphism. We found it unusual that in the other two populations, Kopaonik and Malinik, sexual size dimorphism was constrained and the sexes had needles of equal size. Multivariate analysis of needle shape revealed that the Tara population differed from the other two populations and was characterized by straighter, more symmetric needles. The absence of sexual size dimorphism and the less symmetrical shape of needles could be signs of a deviation from optimal conditions for yew in the Kopaonik and Malinik populations as regards mean annual temperature, precipitation, humidity and soil quality. There is also an indication that sexual size dimorphism in needles arises from a modification in female morphology and that female yews carry more information about climate. However, the conclusions presented in this study have certain limitations because of the small number of individuals in the Tara population (only 3 female and 4 male plants) that represent the quasi-total of adult yews at this site.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Taxus baccata L. is Tertiary relict and dioecious gymnosperm species. In Serbia, T. baccata is listed as a rare and endangered species (Službeni glasnik RS, br. 5/2010). Its natural populations are small and defragmented, usually located on steep, calcareous slopes of river canyons or on high mountains (Vidaković 1982; Obratov-Petković et al. 2002).
Dioecy is extremely rare for conifers. Only 6–9% of higher plants (Sakai and Weller 1999) and 15% of tree species (Coder 2008) are dioecious. Among conifers, dioecy evolved within only a few genera, including Taxus (Kersten et al. 2017). A change of sex in individual yew trees is possible but rare, and the percentage of bisexual trees (bearing branches of different sex) in populations is around 1–2% (Thomas and Polwart 2003; Hageneder 2013).
Sexual dimorphism in plants is associated with the contrasting strategies of the sexes, particularly in growth and reproductive expenditure (Barrett and Hough 2013). Males and females often have unequal resource requirements and investments in reproduction (Cipollini and Whigham 1994; Obeso 2002), and therefore sexual dimorphism may be an evolutionary response to allocation disparity between the sexes (Kohorn 1995).
Differences between the sexes in reproductive features have been thoroughly studied for most dioecious plants, but the sexes may also differ from one another in many ways with regard to vegetative traits. For example, there were reports on differences in plant size, internode length, leaf size, leaf shape, growth rate and physiological traits (Lloyd and Webb 1977; Kohorn 1995; Midgley 2010; Chen et al. 2015). Differences between the sexes of T. baccata are typical for dioecious plants; males are taller and have a larger diameter (Cedro and Iszkuło 2011). However, females are characterized by longer needles, greater leaf area and stomata density (Iszkuło et al. 2009).
Landmark-based geometric morphometrics provides a powerful technique in quantitative biology for the study of shape variation in a wide range of disciplines (Adams et al. 2013). In plant sciences, a geometric morphometric approach is more commonly used to study interspecific variations in shape and it is extremely useful when dealing with hybridogenous species (Jensen et al. 1993, 2002; Viscosi et al. 2009; Vít et al. 2012; Vander Mijnsbrugge 2015). However, multivariate morphometric analyses can also be applied to assess intraspecific variations in shape related to sexual dimorphism, fluctuating asymmetry and variation along geographic and ecological transects (Loy 1996).
We decided to take advantage of these useful quantitative techniques to analyze needle size and shape variations in populations of T. baccata and to address the questions of sexual dimorphism and intraspecific variability. We focused on the following hypotheses:
-
1.
Taxus baccata sexes differ in needle size and shape, and
-
2.
Patterns of dimorphism vary between populations.
To the best of the authors’ knowledge, this is the first examination of the morphological characteristics of needles and intraspecific variability of naturally growing T. baccata in Serbia.
Materials and methods
Studied populations and plant material
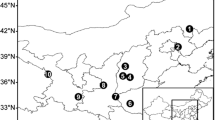
Plant material was collected from three natural populations of T. baccata from geographically differentiated areas in Serbia (central, eastern and western). The geographic and climatic characteristics of the studied populations are presented in Table 1.
Twigs with needles were sampled in autumn, and in accordance with Cornelissen et al. (2003) from well-irradiated, south-facing, middle parts of the crowns of 47 trees (Table 1). From every tree, ten needles were scanned with the abaxial surface up, using an Epson Perfection V370 scanner with a resolution of 600 dpi. We chose to analyze fully developed 2-year-old needles. Also, only needles from the right side of the twigs were sampled to avoid redundancy, because twigs are bilaterally symmetric (Fig. 1a).
Leaf measurements and statistical analysis
ImageJ (Version 1.47n) was used to measure needle length (NL), needle width (NW) and needle area (NA) on scanned samples. The number of stomata rows (SR) on one half of the abaxial needle side and stomata density (SD) were observed with light microscopy (Leica Galen III) at 10× magnitude and filmed with a Topica TP-5001 camera. SR was measured with two replications and SD with three replications on ten needles per tree. In addition, two relative indices were examined: needle length to needle width ratio (NL/NW) and number of stomata rows to needle width ratio (SR/NW).
For each population, hierarchical ANOVA was used to test if there is more variation in leaves of different trees than leaves from the same tree. If this was the case, ten measurements per tree for needle traits were averaged and used in further analyses. This is an important step in morphometric analyses of plants, because leaves of the same tree are not independent observations and represent to a certain degree pseudo replicates (Viscosi and Cardini 2011).
Procrustes ANOVA and pairwise tests were performed to assess population-specific differences between sexes in linear measures NL and NW.
The general linear model (GLM) with Population, Sex and their interaction as factors, and post hoc Bonferroni pairwise comparisons were performed to test for differences between populations and sexes in NA, NL/NW, SR, SR/NW and SD.
Principal component analysis (PCA) and hierarchical agglomerative cluster analysis were used on previously obtained and literature data to assess patterns of similarity among the yew populations. The values of five morphological characteristics (NL, NW, NA, NL/NW ratio and SD) were standardized prior to analysis. GPS coordinates of the yew populations were used as additional variables in PCA.
Statistical analysis of leaf measurements was carried out in Minitab 17 Statistical Software (2010) and the geomorph package in RStudio (Adams and Otárola-Castillo 2013).
Geometric morphometrics and statistical analysis
Scanned images were used to record 3 landmarks and 22 semi-landmarks for every specimen (Fig. 1b). Taxus baccata needles are asymmetric at the base (Di Sapio et al. 1997), so we decided to place two landmarks to define petiole and blade intersection: the first represented the intersection of the petiole and left needle margin and the second represented the intersection of the petiole and right needle margin. The third landmark defined the apex of the needle blade. To describe the outline, we drew the “upper curve” with 12 semi-landmarks along the left needle margin and the “lower curve” with ten semi-landmarks along the right needle margin. Digitalization was carried out using tpsUtil (Rohlf 2016a) and tpsDig (Rohlf 2016b) to obtain the x and y coordinates of the landmarks. During scanning, a ruler (10 mm) was placed beside the specimens and the scale factor was set in tpsDig.
To assess the variations in size and shape of T. baccata needles Procrustes-based geometric morphometry was used in RStudio (Version 1.0.136), and the geomorph package (Adams and Otárola-Castillo 2013). First, we imported the .tps file with the coordinates of landmarks and semi-landmarks of 470 specimens in R and carried out Generalized Procrustes analysis. During this procedure, all specimens were first scaled to unit centroid size and then translated and rotated using a least square algorithm to minimize the distances between the corresponding landmarks of all configurations (Gower 1975; Rohlf and Slice 1990). Because variation associated with differences in location, orientation and scale is removed by this procedure, any differences in the coordinates of corresponding landmarks between configurations must be the result of differences in shape between those configurations (Webster and Sheets 2010). Aligned specimens were then projected into a linear tangent space and their coordinates were used in “geomorph” as shape variables for further multivariate statistical analyses.
Size
During Procrustes superimposition, centroid size (CS) was computed from the coordinates of landmarks for each specimen. CS is a geometric measure of size, calculated as the square root of the summed squared distances of each landmark from the centroid of landmark configuration (Zelditch et al. 2004). In the present study, CS was highly correlated with NL (R 2 = 0.77, P < 0.001) and NA (R 2 = 0.74, P < 0.001), and therefore represented an adequate measure of size.
For each population, a hierarchical statistical design was used to test if there is more variation in the leaves of different trees than in those of the same tree. If this was the case, the mean CS for each tree was calculated and used for further analyses.
To test for population-specific differences in needle size between sexes, Procrustes ANOVA with a randomized residual permutation procedure was performed (Collyer et al. 2015).
Shape
To analyze and visualize variations in needle shape between populations and sexes, PCA was performed on a set of Procrustes-aligned specimens.
To assess population-specific differences in needle shape between sexes, hierarchical Procrustes ANOVA with the factors Population and Sex as the main effects and factor Tree as a random effect was carried out. The differences in shape between specimens were quantified as Procrustes distances.
A multivariate analysis of covariance (MANCOVA) was performed to assess shape covariation with size (allometry), with shape variables as dependent variables, Population as factor and CS as covariate. The aim was to test differences in shape after removing size-related variation (Klingenberg 2016). The homogeneity of allometric slopes between populations was tested with the following model:
Assessment of sex-specific response to bioclimatic factors
The relationship between morphological characteristics of needles and bioclimatic factors of habitat was assessed separately for female and male yews using linear regression analyses. From the WorldClim set of global climate layers, 19 bioclimatic variables were extracted with spatial resolution of 30 arc s for the period 1950–2000 (Hijmans et al. 2005) using DIVA-GIS 7.5. software (Hijmans et al. 2012).
Linear regression analysis was performed in Small STATA 12 Statistical Software (2011).
Results
Differences in needle size
According to morphometric analysis, the mean values of NL, NW and NA were 22.14 mm, 2.35 mm and 47.18 mm2, respectively, and ranges were 14.11–40.21 mm, 1.67–3.20 mm and 25.74–98.04 mm2, respectively, which is consistent with the reports of Cope (1998), Thomas and Polwart (2003) and Hageneder (2013). Populations in Serbia on average had 8.5 SR, which is in accordance with those previously reported by Strobel and Hess (1996) and Spjut (2007). The mean SD was 135.16 mm2 and ranged from 75 to 225 stomata per mm2.
Hierarchical ANOVA was performed separately for each population with factor Leaf nested in factor Tree to assess variation in leaf measurements and CS. Analysis revealed that there were many more variations between trees than within trees for NL, NW, NL/NW, NA and CS (see column % explained, ESM_1), so we gained enough evidence to average ten values per tree for these traits for further analysis. For SR, SR/NW and SD, a full dataset with nested structure was used in GLM (with Population, Sex as fixed effects and Tree, Leaf as random effects).
Procrustes ANOVA was performed on averaged CS and showed that populations of T. baccata differed in sexual size dimorphism (the Population*Sex interaction was marginally statistically significant: R 2 = 0.090, Z = 2.112, P = 0.05). Pairwise comparisons of groups showed that the difference in needle size between sexes was only significant in the Tara population (Table 2). Females from Tara had significantly larger CS when compared to males from the same population (Z = 2.210, P < 0.05) and compared to females from Kopaonik (Z = 1.648, P < 0.05) (Tables 2, 3).
Procrustes ANOVA and pairwise tests were also carried out on the linear measures NL and NW. Population*Sex interaction was significant for NL (R 2 = 0.108, Z = 2.63, P = 0.02).
GLM with Population, Sex and their interaction as factors was used to assess differences in NA, NL/NW, SR, SR/NW and SD. Mean values and homogenous groups are presented in Table 3.
Literature reports on the morphological characteristics of English yew needles from the UK, Poland, Ukraine, Mediterranean and Macaronesia (for details, see captures of Figs. 2, 3) were collected and systematized (ESM_3) together with results of this study. PCA revealed differentiation of yew populations from Europe and the Mediterranean (Fig. 2). The first and second principal components explained 90.72% of variability in the data. SD was negatively linked to PC1 and NW was positively linked to PC2 (Table 4). Cluster analysis showed that populations from Serbia were most similar to populations from Poland and Ukraine and the most divergent were populations from the Mediterranean and Macaronesia (Fig. 3).
Spatial diversity analyses of T. baccata populations were also carried out based on literature records of morphological characteristics (ESM_3) using DIVA-GIS 7.5 (Hijmans et al. 2012). Spatial diversity maps of yew populations are represented in ESM_4.
Differences in needle shape
PCA was performed to assess shape variation among specimens. The first two dimensions of tangent space explained 80.95% of total shape variation. There was no clear differentiation between populations (Fig. 4) or sexes (data not shown), except that all specimens from the Tara population were distributed along the positive part of PC1 and mainly separated from specimens from Kopaonik (Fig. 4). Deformation grids displayed the shape of specimens at the ends of the range variability along PC1 (which accounted for 66.29% of shape variation). From the shape coordinates of the extreme ends of PC1 and PC2 (ESM_5), it could be concluded that the most variable (semi)landmarks were: 1, 2, 3, 15, 16 and 25, which described shape of needle base and apex. According to results of PCA, specimens from Tara were characterized by straighter, more symmetric needles compared to specimens from Kopaonik and Malinik.
The hierarchical Procrustes ANOVA revealed statistically significant variation in needle shape only between populations (Z = 3.666, P < 0.01) (Table 5). In this model, geographical origin (population) explained 6% of variation in needle shape (R 2 = 0.059). This was not a meaningful biological variation, and considering the significant differences in needle size, we wanted to repeat shape comparisons in further analysis after controlling the effect of size to shape. Significant differences in needle shape between sexes were not recorded; therefore, we continued the analysis of allometric shape changes with pooled sexes.
The MANCOVA and homogeneity of a slopes test revealed that the populations displayed a common pattern of allometry—the shape-to-size relationship was the same across populations (Z = 1.486, P = 0.11, Fig. 5). In all populations, negative allometry was observed: as a needle increases in size, it becomes proportionally slenderer (Fig. 6).
Pairwise comparisons of mean shapes, after controlling the effect of allometry, showed that populations significantly differed in needle shape (R 2 = 0.171, Z = 3.622, P = 0.001). The Tara population was characterized by less curved needles compared to the Kopaonik and Malinik populations (Table 6; Fig. 7).
Relationship between morphological characteristics of needles and bioclimatic factors
The regression analysis revealed that for female yews, CS, NL, NW, NA, NL/NW and SR were statistically significantly correlated with a majority of bioclimatic variables (Table 7). For male yews, statistically significant correlations with bioclimatic variables were found only for NW and SR (Table 8). The linear relationship between SD or SR/NW and bioclimatic factors were not determined for both sexes.
The highest percentage of variability in morphological characteristics was explained by seven bioclimatic variables related to temperature: BIO1 (Annual Mean Temperature), BIO5 (Max Temperature of Warmest Month), BIO6 (Min Temperature of Coldest Month), BIO8 (Mean Temperature of Wettest Quarter), BIO9 (Mean Temperature of Driest Quarter), BIO10 (Mean Temperature of Warmest Quarter), BIO11 (Mean Temperature of Coldest Quarter) and 2 bioclimatic variables related to precipitation: BIO16 (Precipitation of Wettest Quarter) and BIO18 (Precipitation of Warmest Quarter) for female yews (Table 7).
Discussion and conclusion
Sexual size dimorphism of needles and sex-specific response to the environment in yew populations
Sexual size dimorphism of T. baccata needles was recorded only in the Tara population. Larger CS and NL for females in the Tara population than for males from the same population and females from Kopaonik indicated that size dimorphism may have arisen from a modification in female morphology. Iszkuło et al. (2009) found that females had greater NL and NA compared to males in all studied populations in Poland and Ukraine. Taking this into account, one could consider needle morphology in the Kopaonik and Malinik populations unusual, because females and males tend to have needles of equal size.
Comparison of our results with literature data showed that yew populations from Europe and the Mediterranean could be classified into four groups with regard to needle morphology. Defined groups are similar to those previously described by Vessella et al. (2013). The English yew colonized Europe from the east and populations in Europe could be divided into two gene pools—Eastern and Western (Mayol et al. 2015). Geographically, populations in Serbia belong to an Eastern gene pool (although they were not sampled in the study of Mayol et al. 2015), which is characterized by a greater genetic diversity (closer to an ancestral one). According to our findings, populations in Serbia were most similar to those in Poland and Ukraine, indicating that they are indeed part of an Eastern gene pool.
Considering needle morphology, sexual size dimorphism was found in some, but not in all yew populations from the Eastern gene pool. Mayol et al. (2015) reported that both geography and climate have played a significant role in shaping the genetic structure of T. baccata, pointing to a major effect of temperature on genetic divergence. However, it is not clear whether sexual size dimorphism in yew populations represents a short-term response to the environment or adaptation to ecologically differentiated areas.
The yew favors climates with mild winters, cool summers and sufficient rainfall. Yews require a minimum average precipitation of 500–1000 mm annually, but higher amounts (up to 2000 mm) are also beneficial. Particularly important is the rainfall in March to May, when the present year’s leaf buds open, and in July and August, when the leaf buds for the next year are formed. Considering humidity of habitat, T. baccata is submesophyte (it prefers mesophytic habitats, but can occur in xerophytic phytocenoses as well). Yews grow on different soil types, but do not tolerate stagnant water and high acidity. The ecological barrier limiting the distribution of yews in the east is the severe continental climate with cold winters and dry, hot summers (Kojić et al. 1997; Thomas and Polwart 2003; Hageneder 2013). Iszkuło et al. (2009) stated that female yews demand higher water resources than males and that they should be treated as more “oceanic”, especially near the edge of the continental range of the species.
In Serbian yew populations, a sex-specific response to bioclimatic factors of habitat was found. Considering the morphological characteristics of the needles, females had much more pronounced response to climatic factors, especially to MAT.
Lower MAT, MAP and MAH on Mts. Kopaonik and Malinik could be a constraint to sexual size dimorphism of T. baccata needles. Kopaonik and Malinik populations in the central and eastern parts of Serbia are characterized by a continental mountain climate (type 4.2 sensu Stevanović and Stevanović 1995) with an annual precipitation between 850 and 1400 mm. The Tara population in the western part of Serbia is influenced by a humid mountain climate of alpine type (type 4.1 sensu Stevanović and Stevanović 1995) with higher precipitation. Considering MAT, MAP and MAH, the climate on Mt. Tara is more favorable for female yews.
Other abiotic factors that should be discussed in relation to sexual size dimorphism of needles are light conditions and soil quality. Light variations could affect specific leaf area and leaf area (Gratani 2014). It is important to note that yew is shade-tolerant and, generally, light-induced plasticity is lower in shade-tolerant species than in light-demanding species (Longuetaud et al. 2013; Gratani 2014). The Tara population (the only one in Serbia where sexual size dimorphism was recorded) is yew-dominated and fully exposed to sunlight. On the contrary, in the Kopaonik population yews grow beneath dense stands of fir and birch, and in the Malinik population, beneath dense stands of birch. However, it is less likely that shade constrained sexual size dimorphism. Mitchell (1998) studied the acclimation of Taxus brevifolia and T. baccata to sun and shade and found no sex-specific acclimation in the foliar physiology and morphology in either species. Moreover, sexual size dimorphism of needles was found in all studied populations in Poland and Ukraine, where yews grow in shade, as a second layer of canopy (Iszkuło et al. 2009; personal communication with Dr. Grzegorz Iszkuło).
In dioecious species, females are more negatively affected by poor soil quality than males (Marques et al. 2002; Barrett and Hough 2013). Although, yew stands are often located on steep slopes (>30°) with shallow dry rendzina on limestone, yew prefers deep, well-drained soils (Thomas and Polwart 2003). The steep terrain on Mts. Kopaonik and Malinik cause faster water drainage from the soil and this could have a negative effect on yew growth. Moreover, the Kopaonik population stands on slightly acidic soil (Obratov-Petković et al. 2002), and yew prefers neutral or alkaline soils (Kojić et al. 1997). The well-drained and humus-rich, calcareous soil on Mt Tara (Tomićević 2005) is mostly beneficial for yew. Soil quality could be another constraint to the sexual size dimorphism of needles in the Kopaonik and Malinik populations.
Differences in needle shape and intraspecific variation
Presented analyses of needle shape revealed that the Tara population differed from the other two populations. Specimens from Tara were characterized by straighter, more symmetric needles compared to specimens from Kopaonik and Malinik. Since most of the variation was explained by PC1 (associated with needle curvature), there is an indication that within species leaf shape variation was concentrated in a single dimension of the shape space. In this case, Viscosi (2015) suggests that eventual evolutionary changes were more likely to occur in that direction.
Similar, but not the same, allometric patterns were observed among populations. Allometric trajectories were parallel for all populations, but the Tara population had a higher intercept. Leaf allometry can reflect the adaptation of plant populations to different environments (Viscosi 2015). It is known for many plant species that leaf size decreases with increasing elevation and decreasing temperature (Peppe et al. 2011; Toivonen et al. 2014), causing allometric responses in leaf morphological traits (Read et al. 2014).
Although T. baccata grows within its ecological range in all the studied populations in Serbia, the most favorable conditions with regard to temperature, humidity and soil quality are met in the Tara population and this is reflected in its needle size and shape. On the contrary, the more curved and less symmetrical needles in the Kopaonik and Malinik populations could mirror a partial deviation from optimal conditions for this species. However, more information is needed about the adaptive response of the species to be able to discuss the complex relationship between environmental factors and the morphology of needles.
The conclusions presented here have certain limitations because only three female and four male trees from the Tara population were analyzed. These seven trees represent a quasi-total of adult yew trees (not counting the young plants). In the past, yew was widespread and numerous at this site, as evidenced by the name of the local hill “Tisovo brdo” (meaning “yew hill”). In the previous century, anthropogenic factors (illegal cutting and cattle grazing) led to the devastation of the yew population at this site, and the analyzed trees could be considered rare survivals. However, because of favorable climatic conditions, good soil quality and natural regeneration at this site, it is possible to preserve this population with appropriate management.
Conclusion
Advanced quantitative analyses of biological size and shape allowed detection of subtle changes in needle morphology in T. baccata populations. Population-specific sexual size dimorphism and a sex-specific response to bioclimatic factors were found. Female yews carried more information about climate, especially mean annual temperature. There is an indication that sexual size dimorphism of T. baccata needles arises from a modification in female morphology and that it might be driven by environmental factors—temperature, humidity and soil quality. Nonetheless, further studies are needed on natural yew populations from a broader ecological range to determine the patterns of morphological responses to various abiotic conditions.
Author contribution statement
SM and BS designed the research. BS and VV did field research and collected plant material. SM and NB conducted microscopy and digital image analysis. SM performed statistical analysis and wrote manuscript. NB, MR and PZ provided critical feedback and contributed to manuscript revision.
References
Adams DC, Otárola-Castillo E (2013) Geomorph: an R package for the collection and analysis of geometric morphometric shape data. Methods Ecol Evol 4:393–399
Adams DC, Rohlf FJ, Slice DE (2013) A field comes of age: geometric morphometrics in the 21st century. Hystrix 24:7–14
Barrett SCH, Hough J (2013) Sexual dimorphism in flowering plants. J Exp Bot 64:67–82
Cedro A, Iszkuło G (2011) Do females differ from males of European yew (Taxus baccata L.) in dendrochronological analysis? Tree Ring Res 67:3–11
Chen J, Dong T, Duan B, Korpelainen H, Niinemets Ü, Li C (2015) Sexual competition and N supply interactively affect the dimorphism and competiveness of opposite sexes in Populus cathayana. Plant Cell Environ 38:1285–1298
Cipollini ML, Whigham DF (1994) Sexual dimorphism and cost of reproduction in the dioecious shrub Lindera benzoin (Lauraceae). Am J Bot 81:65–75
Coder KD (2008) Tree sex: gender & reproductive strategies. https://urbanforestrysouth.org/resources/library/citations/tree-sex-gender-reproductive-strategies. Accessed 2 Feb 2017
Collyer ML, Sekora DJ, Adams DC (2015) A method for analysis of phenotypic change for phenotypes described by high-dimensional data. Heredity 115:357–365
Cope EA (1998) Taxaceae: the genera and cultivated species. Bot Rev 64:291–322
Cornelissen JHC, Lavorel S, Garnier E, Díaz S, Buchmann N, Gurvich DE, Reich PB, ter Steege H, Morgan HD, van der Heijden MGA, Pausas JG, Poorter H (2003) Handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust J Bot 51:308–335
Dempsey D, Hook I (2000) Yew (Taxus) species—chemical and morphological variations. Pharm Biol 38:274–280
Di Sapio OA, Gattuso SJ, Gattuso MA (1997) Morphoanatomical characters of Taxus baccata bark and leaves. Fitoterapia 68:252–260
Gower JC (1975) Generalized Procrustes analysis. Psychometrika 40:33–51
Gratani L (2014) Plant phenotypic plasticity in response to environmental factors. Adv Bot 2014:208747-1–208747-17
Hageneder F (2013) Yew. Reaction Books, London
Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A (2005) Very high resolution interpolated climate surfaces for global land areas. Int J Climatol 25:1965–1978
Hijmans RJ, Guarino L, Mathur P (2012) DIVA-GIS Version 7.5, manual http://www.diva-gis.org/docs/DIVA-GIS_manual_7.pdf. Accessed 7 Mar 2016
Iszkuło G, Jasińska AK, Giertych MJ, Boratyński A (2009) Do secondary sexual dimorphism and female intolerance to drought influence the sex ratio and extinction risk of Taxus baccata? Plant Ecol 200:229–240
Jensen RJ, Hokanson SC, Isebrands JG, Hancock JF (1993) Morphometric variation in oaks of the Apostle Islands in Wisconsin: evidence of hybridization between Quercus rubra and Q. ellipsoidalis (Fagaceae). Am J Bot 80:1358–1366
Jensen RJ, Ciofani KM, Miramontes LC (2002) Lines, outlines, and landmarks: morphometric analyses of leaves of Acer rubrum, Acer saccharinum (Aceraceae) and their hybrid. Taxon 51:475–492
Kersten B, Pakull B, Fladung M (2017) Genomics of sex determination in dioecious trees and woody plants. Trees Struct Funct. doi:10.1007/s00468-017-1525-7
Klingenberg CP (2016) Size, shape, and form: concepts of allometry in geometric morphometrics. Dev Genes Evol 226:113–137
Kohorn LU (1995) Geographic variation in the occurrence and extent of sexual dimorphism in a dioecious shrub, Simmondsia chinensis. Oikos 74:137–145
Kojić M, Popović R, Karadžić B (1997) Vaskularne biljke Srbije kao indikatori staništa. Insititut za istraživanja u poljoprivredi “Srbija”, Institut za biološka istraživanja “Siniša Stanković”, Beograd
Lloyd DG, Webb CJ (1977) Secondary sex characters in plants. Bot Rev 43:177–216
Longuetaud F, Piboule A, Wernsdörfer H, Collet C (2013) Crown plasticity reduces inter-tree competition in a mixed broadleaved forest. Eur J For Res 132:621–634
Loy A (1996) An introduction to geometric morphometrics and intraspecific variation. In: Marcus LF, Corti M, Loy A, Naylor GJP, Slice DE (eds) Advances in morphometrics, part II. Springer, New York, pp 271–273
Marques AR, Fernandes GW, Reis IA, Assunção RM (2002) Distribution of adult male and female Baccharis concinna (Asteraceae) in the rupestrian fields of Serra do Cipó, Brazil. Plant Biol 4:94–103
Mayol M, Riba M, González-Martínez SC, Bagnoli F, de Beaulieu J-L, Berganzo E, Burgarella C, Dubreuil M, Krajmerová D, Paule L, Romšáková I, Vettori C, Vincenot L, Vendramin GG (2015) Adapting through glacial cycles: insights from a long-lived tree (Taxus baccata). New Phytol 208:973–986
Midgley JJ (2010) Causes of secondary sexual differences in plants—Evidence from extreme leaf dimorphism in Leucadendron (Proteaceae). S Afr J Bot 76:588–592
Minitab 17 Statistical Software (2010) Minitab Inc., State College, Pennsylvania, USA
Mitchell AK (1998) Acclimation of Pacific yew (Taxus brevifolia) foliage to sun and shade. Tree Physiol 18:749–757
Obeso JR (2002) The costs of reproduction in plants. New Phytol 155:321–348
Obratov-Petković D, Filipović M, Popović I, Đukić M (2002) Ekološka analiza zajednice tise na području NP Kopaonik. I Konferencija “S planinom u novi vek”, Kopaonik, pp 121–128
Peppe DJ, Royer DL, Cariglino B et al (2011) Sensitivity of leaf size and shape to climate: global patterns and paleoclimatic applications. New Phytol 190:724–739
Read QD, Moorhead LC, Swenson NG, Bailey JK, Sanders NJ (2014) Convergent effects of elevation on functional leaf traits within and among species. Funct Ecol 28:37–45
Rohlf J (2016a) tpsUtil v 1.70 (computer program). Stony Brook University, New York
Rohlf J (2016b) tpsDig2. v 2.26 (computer program). Stony Brook University, New York
Rohlf FJ, Slice DE (1990) Extensions of the Procrustes method for the optimal superimposition of landmarks. Syst Zool 39:40–59
Sakai AK, Weller SG (1999) Gender and sexual dimorphism in flowering plants: a review of terminology, biogeographic patterns, ecological correlates, and phylogenetic approaches. In: Geber MA, Dawson TE, Delph LF (eds) Gender and sexual dimorphism in flowering plants. Springer, Berlin, pp 1–31
Schirone B, Ferreira RC, Vessella F, Schirone A, Piredda R, Simeone MC (2010) Taxus baccata in the Azores: a relict form at risk of imminent extinction. Biodivers Conserv 19:1547–1565
Službeni glasnik RS, br. 5/2010: Pravilnik o proglašenju i zaštiti strogo zaštićenih i zaštićenih divljih vrsta biljaka, životinja i gljiva (Official Gazette of Republic of Serbia No. 5/2010: Regulation of proclamation and protection of strictly protected and protected wild species of plants, animals and fungi)
Smailagić J, Savović A, Marković D, Nešić D, Drakula B, Milenković M, Zdravković S (2013) Klimatske karakteristike Srbije, Republički hidrometeorološki zavod Srbije, Beograd (Climate characteristics of Republic of Serbia, Republic hydrometeorological service of Serbia)
Spjut RW (2007) A phytogeographical analysis of Taxus (Taxaceae) based on leaf anatomical characters. J Bot Res Inst Texas 1:291–332
STATA 12 (2011) StataCorp LP College Station. Texas, USA
Stevanović V, Stevanović B (1995) Osnovni klimatski, geološki i pedološki činioci biodiverziteta kopnenih ekosistema Jugoslavije. In: Stevanović V, Vasić V (eds.) Biodiverzitet Jugoslavije sa pregledom vrsta od međunarodnog značaja. Beograd: Ecolibri, Biološki fakultet, pp 75–95
Stojanović LJ, Jovanović B (1989) Ceno-ekološke i prirodne karakteristike sastojina bukve sa tisom na području Malinika u severoistočnoj Srbiji. Šumarstvo 2:3–18
Strobel GA, Hess WM (1996) A scanning electron microscopy study of Taxus leaves as related to taxonomy. Scanning Microsc 10:1111–1126
Thomas PA, Polwart A (2003) Taxus baccata L. J Ecol 91:489–524
Toivonen JM, Horna V, Kessler M, Ruokolainen K, Hertel D (2014) Interspecific variation in functional traits in relation to species climatic niche optima in Andean Polylepis (Rosaceae) tree species: evidence for climatic adaptations. Funct Plant Biol 41:301–312
Tomićević J (2005) Towards Participatory Management: Linking People, Resources and Management. A Socio-Economic Study of Tara National Park. Culterra, Schriftenreihe des Instituts für Landespflege der Albert-Ludwigs-Universität Freiburg
Vander Mijnsbrugge K (2015) Morphological dissection of leaf, bud and infructescence traits of the interfertile native A. glutinosa and non-native A. incana in Flanders (northern part of Belgium). Trees Struct Funct 29:1661–1672
Vessella F, Simeone MC, Fernandes FM, Schirone A, Gomes MP, Schirone B (2013) Morphological and molecular data from Madeira support the persistence of an ancient lineage of Taxus baccata L. in Macaronesia and call for immediate conservation actions. Caryologia 66:162–177
Vidaković M (1982) Četinjače—Morfologija i varijabilnost. Jugoslavenska akademija znanosti i umetnosti, Zagreb
Viscosi V (2015) Geometric morphometrics and leaf phenotypic plasticity: assessing fluctuating asymmetry and allometry in European white oaks (Quercus). Bot J Linn Soc 179:335–348
Viscosi V, Cardini A (2011) Leaf morphology, taxonomy and geometric morphometrics: a simplified protocol for beginners. PLoS One 6:e25630
Viscosi V, Fortini P, Slice DE, Loy A, Blasi C (2009) Geometric morphometric analyses of leaf variation in four oak species of the subgenus Quercus (Fagaceae). Plant Biosyst 143:575–587
Vít P, Lepší M, Lepší P (2012) There is no diploid apomict among Czech Sorbus species: a biosystematic revision of S. eximia and discovery of S. barrandienica. Preslia 84:71–96
Webster M, Sheets HD (2010) A practical introduction to landmark-based geometric morphometrics. In: Alroy J, Hunt G (eds) Quantitative methods in paleobiology, vol 16. The Paleontological Society Papers, Colorado, pp 163–188
Zarek M (2007) Variability of morphological features of needles, shoots and seeds of Taxus baccata L. in nature reservations of Southern Poland. Electron J Pol Agric Univ 10:05
Zelditch ML, Swiderski DL, Sheets HD, Fink WL (2004) Geometric morphometrics for biologists: a primer. Elsevier Academic Press, London
Acknowledgements
This research was supported by a Grant from the Ministry of Education, Science and Technological Development of the Republic of Serbia (Projects 173011 and 173029). We are very thankful to the reviewers who helped us to improve our manuscript with their useful suggestions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by J. Lin.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Stefanović, M., Nikolić, B., Matić, R. et al. Exploration of sexual dimorphism of Taxus baccata L. needles in natural populations. Trees 31, 1697–1710 (2017). https://doi.org/10.1007/s00468-017-1579-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-017-1579-6