Abstract
Key message
The olive pollen production showed a variation related to temperature and rainfall during dormancy period. A correlation was found between the number of olive tree inflorescences and airborne pollen counts.
Abstract
Analysis of pollen production in crops such as the olive is of particular agronomical value, in that it can help to predict final harvest outcomes. Since olive pollen is a major cause of allergy in the Mediterranean region, this analysis can also provide health-related benefits. The present study analyzed flower and pollen production in ‘Cornicabra’ olive cultivar, charting its correlation with airborne pollen counts, with phenological findings such as onset of pre-flowering period, and with environmental factors such as temperature and rainfall, elevation and northern vs. southern exposure of floral structures on the tree crown. Findings confirmed spatiotemporal variations for most of the parameters studied. Mean pollen grain production per flower of olive tree was 1.10 × 105 ± 0.25 × 105. Variations in the production of flowering branches, inflorescences, flowers and pollen reflected internal regulation processes linked to alternate bearing as well as year-on-year variations in weather conditions. Temperature and rainfall during dormancy were the weather-related variables most influencing flower, inflorescence and flowering-branch production. Early attainment of chilling requirements during dormancy was associated with increased production, coinciding with earlier budburst. A correlation was found between the number of inflorescences—and thus overall flower production—and airborne pollen counts. Production parameters also varied as a function of olive grove location, although that variability was not clearly attributable to the elevation. The correlation between production parameters and northern vs. southern exposure on the olive tree crown was significant only for flowering branches, whose production varied depending on their location on the tree crown.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The amount of pollen produced by a plant is governed by floral organ morphology and pollen dispersal strategies (Cruden 2000; Friedman and Barrett 2009). The olive (Olea europaea L.), as a largely wind-pollinated species, generates copious amounts of pollen to offset low pollination efficiency and thus ensure fertilization (Cruden 2000). Analysis of year-on-year variations in pollen and flower production is particularly valuable in the olive, given its tendency towards alternate bearing. Fruit production is mainly dependent on the vegetative growth of the previous growing season (Lavee 2007). This phenomenon is controlled by the balance between vegetative and reproductive cycles (Rallo and Suarez 1989) determined by the levels of hormones endogenous inhibitors and promoters of flowering, such as gibberellins, abscisic acid, and other substances (Baktir et al. 2004; Ulger et al. 2004).
However, the amount of pollen produced is not dictated by genetic and physiological factors alone (Subba and Reddi 1986); environmental factors also prompt considerable year-on-year variations in pollen production (Rogers 1993; Guerin and Sedgley 2007) as well as in the number of flowers per inflorescence and the number of inflorescences per tree (Rapoport 2008). Olive pollen production is influenced by weather conditions (Ribeiro et al. 2006), since each stage in the biological cycle is regulated by variables such as temperature, water availability and solar radiation (Connor and Fereres 2005), which affect both flowering events (Orlandi et al. 2002, 2009) and aspects of plant vegetative growth (Kozlowski and Pallardy 1997).
Geographical factors constitute another source of variability in olive pollen production (Damialis et al. 2011). Aspects such as the altitude and topographical orientation of olive groves affect a number of pollen production parameters including number of pollen grains per flower (Damialis et al. 2011; Aguilera and Ruiz 2012). Furthermore, some treatments applied in olive growing, as it happens to other species (Alcázar et al. 2004), may affect pollen production.
Knowledge of variations in pollen production is of agronomical value, as a means of predicting harvest outcomes for major crops such as the olive, since the amount of pollen released during pollination is a direct indicator of flowering intensity (Rosati et al. 2012) and thus of final fruit yield (Ribeiro et al. 2007). A number of authors have charted variations in final olive production and their correlation with flower and inflorescence production (Lavee et al. 1996, 1999).
Olive pollen, moreover, is responsible for 58 % of the allergies recorded in the Mediterranean region (D’Amato et al. 2007; Subiza et al. 2007). Pollen production estimates, and research into spatiotemporal variations in production, are therefore valuable when interpreting the findings of aerobiological studies carried out in the field of allergology (Hidalgo et al. 2003) and for predicting the potential severity of respiratory allergies.
Although airborne pollen counts have been widely studied (e.g., Díaz de la Guardia et al. 2003; Orlandi et al. 2009), few papers have addressed the issue of pollen production per tree (Tormo et al. 1996; Orlandi et al. 2003; Rovira and Tous 2005; Ferrara et al. 2007; Damialis et al. 2011; Aguilera and Ruiz 2012). Crop pollen and flower production varies even between varieties (Joppa et al. 1968; Ferrara et al. 2007), and little is known regarding production in the ‘Cornicabra’ variety.
The main aim of this study was to quantify the production of pollen and of the floral structures involved in pollen production (flowers, inflorescences and flowering branches) in ‘Cornicabra’ olives, charting correlations with airborne pollen counts, with phenological findings such as bud development after dormancy (pre-flowering), and with environmental factors such as temperature and rainfall, elevation gradient and northern vs. southern exposure of floral structures.
Materials and methods
Study area
The study was carried out in the province of Toledo (39°51′N, 4° 01′W), located in the Castilla-La Mancha region of central Spain. The most widely grown variety in this region is ‘Cornicabra’, which is the second largest Spanish variety in terms of surface area, covering over 270,000 Ha (Barranco et al. 2000). ‘Cornicabra’ is a late-flowering variety, coming into bloom between mid-May and early June (Rojo and Pérez-Badia 2014).
Fieldwork and phenological sampling
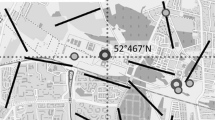
Sampling was conducted over a four-year period, from 2010 to 2013, at 7 sampling sites on 70 olive trees (10 trees per site) located across the whole elevation gradient (400–900 m a.s.l) in which olive groves are to be found in this part of central Spain (Fig. 1). The selected trees were situated in groves traditionally cultivated, all of them had similar size and crown shape and there was a separation of 10–12 m between them (Pastor et al. 2001). Geographical features of the sampling sites are shown in Table 1.
Phenological sampling was performed to determine the date of onset of the pre-flowering period at each sampling site, defined as the moment when 50 % of buds on the tree are swollen, i.e., phenophase 51 on the BBCH scale (Meier 2001).
Estimation of production parameters
Number of flowers, inflorescences and flowering branches per crown unit area
The number of flowers per inflorescence (flowers/inflorescence) was counted for all inflorescences on four flowering branches per tree (2 north-facing and 2 south-facing on the tree crown). The number of inflorescences on each of these flowering branches (inflorescences/branch) was also recorded. The number of flowering branches per square meter (branches/m 2) of crown was then calculated by examining 2 m2 of north-facing and 2 m2 of south-facing crown from randomly chosen areas of each tree.
Number of pollen grains per flower
The total number of pollen grains per flower (pollen/flower) was calculated following Dafni (1992) for three trees at each of the seven sampling sites. For each exposure (N/S) two flowers were collected immediately prior to anthesis (one flower per exposure in 2012 and 2013). Pollen from one anther of each flower was extracted in 1 ml of aqueous medium containing 70 % ethanol. Five replicates, each of 10 μl, were stained with fuchsine and pollen grains were counted under a light microscope (400×). The number of pollen grains per anther was calculated by extrapolation and multiplied by two (two anthers per flower) to obtain the total number of pollen grains per flower.
Airborne Olea pollen data
In tandem with field work and analysis of pollen production, airborne pollen counts were recorded using a Hirst-type volumetric spore trap (Hirst 1952) located in the city of Toledo, in accordance with Spanish Aerobiology Network (REA) protocols (Galán et al. 2007). The aerobiological sampling was carried out continuously during the period 2010–2013. The results obtained for each production parameter studied were compared with total daily airborne pollen counts over the Main Pollen Season (MPS) for each study year, calculated following Andersen (1991).
Weather data and statistical analysis
General Linear Models (GLM) were applied to test for potential variability in production parameters as a function of study year, elevation and N/S exposure of floral structures. Correlations between elevation and production parameters were also studied by linear regression for each study year.
Correlations between the various production parameters, and between those parameters and weather-related variables, were tested using either Pearson’s or Spearman’s correlation test, depending on data normality. For this purpose, data from four sampling sites (La Puebla de Montalbán, Toledo, Olías del Rey and Mascaraque) were used, because they were located close to weather stations.
Weather data were supplied by stations belonging to the Castilla La Mancha Air Control and Monitoring Network (http://pagina.jccm.es/medioambiente/rvca/calidadaire.htm) and the Integrated Irrigation Advice Network (SIAR, http://crea.uclm.es/siar/). The following weather-related variables were analyzed: maximum temperature (T max), minimum temperature (T min), mean temperature (T m), and accumulated rainfall (R). These variables were calculated for autumn (SON-1 September–October–November of the previous year) and winter (DJF December–January–February).
Annual airborne pollen counts over the pollen season were compared with production parameters and weather-related variables using Winsorized correlation tests, which are recommended when classical statistics cannot be applied (Wilcox 2012), in this case because data were available for only four sampling years.
Correlations between the date of onset of the pre-flowering phase and pollen production parameters (number of pollen grains, flowers, inflorescences and flowering branches) were analyzed by linear regression. The data used for the four studied years (2010–2013) were those obtained from the 7 sampling points (mean data in each point, 28 data in total).
All statistical analyses were carried out using the SPSS v. 19 software package, except for the Winsorized correlations, which were performed with R software (R Development Core Team 2008) and the WRS package (Wilcox 2012).
Results
Airborne pollen
The total airborne olive pollen count for the 4 years of the study (2010–2013) was 25,796 pollen grains, with an annual mean of 6190 ± 2877 pollen grains during the Main Pollen Season (MPS). The highest annual count was recorded in 2013 (9444 grains) and the lowest in 2012 (2537 grains), coinciding with a marked drop in winter rainfall that year (Fig. 2). Year-on-year variations in pollen counts seemed to coincide with fluctuations in accumulated rainfall over the previous autumn (Fig. 2).
A positive correlation (ρ w = 0.98) was observed between year-on-year variations in airborne olive pollen counts and number of inflorescences per flowering branch (Fig. 3), and thus, indirectly, with the flower production per tree.
Estimation of production parameters
Pollen production per flower
‘Cornicabra’ olive flowers contained a mean 110,105 ± 24,935 pollen grains (i.e., 55,052 ± 12,467 grains per anther). Grain counts varied significantly between years and sampling sites (Table 2). The highest pollen production per flower was recorded in 2013, when mean counts rose to 123,206 ± 20,420 grains (Fig. 3); the highest airborne pollen counts were also observed in that year. Mean pollen production counts for 2010 and 2011 were 114,863 ± 25,167 grains and 102,157 ± 19,719 grains, respectively. The lowest pollen production per flower was observed in 2012 (100,192 ± 27,705 grains).
There was no clear correlation between pollen/flower and elevation. A slight negative correlation was observed (R 2 = 0.53, although statistical significance was slightly greater than 0.05, Fig. 4) in 2010 and 2012, i.e., pollen production per flower was greater at lower elevations. This trend, however, was not recorded in other years.
Flower production per inflorescence
The mean number of flowers per inflorescence in ‘Cornicabra’ olives was 11.3 ± 2.4, though significant year-on-year variations were observed (Table 2). As Fig. 3 shows, the highest production rate was recorded in 2010 (12.2 ± 3.2 flowers), and the lowest in 2012 (9.7 ± 1.6 flowers). Flowers/inflorescence also varied as a function of sampling site location. In 2012, though not in other years, this variability was partly accounted for by elevation, with which a significant correlation was observed (R 2 = 0.66), i.e., flowers/inflorescence increased with elevation (Fig. 4).
Inflorescence production per branch
The largest number of inflorescences per branch was recorded in 2013 and 2011 (13.4 ± 3.2 and 11.8 ± 4.4 inflorescence, respectively). Production was slightly lower in 2010 (8.1 ± 3.8) and dropped sharply in 2012, to 4.2 ± 2.1 inflorescences/branch (Fig. 3). Significant variations were observed as a function of sampling site location in some years (year × elevation, Table 2). In 2012, a positive correlation was found between inflorescences/branch and elevation (R 2 = 0.66, Fig. 4).
Branch production per m2
The highest number of branches per square meter (Fig. 3) was recorded in 2013 (63.1 ± 15.4), followed by 2010 (54.5 ± 28.5) and 2011 (55.0 ± 21.0). However, numbers were significantly lower in 2012 (20.5 ± 16.8). Considerable inter-tree variability was also observed in 2012, with a high coefficient of variation of 82 %, implying major differences even between trees at the same location.
Number of branches/m 2 was the only parameter displaying significant variation as a function of N vs. S exposure (Table 2). Generally, more flowering branches were produced on the south-facing side of the tree (50.5 ± 31.6) than on the north-facing side (46.0 ± 28.7). This was true for all study years and for all sampling sites except Los Yébenes (Fig. 5).
Correlations between production parameters and both weather-related and phenological variables
Both flowers/inflorescence and inflorescences/branch correlated negatively with mean and maximum temperatures for the previous autumn (TmaxSON-1, TmSON-1) (Table 3). Moreover, a negative correlation was observed between flowers/inflorescence and maximum winter temperature (TmaxDJF). By contrast, a positive correlation was found between both minimum and mean winter temperature (TminDJF, TmDJF), and flowers/inflorescence, inflorescences/branch and branches/m 2 (Table 3).
A positive correlation was observed between cumulative rainfall over the previous autumn (RSON-1) and inflorescences/branch, and also between cumulative winter rainfall and flowers/inflorescence (Table 3).
A correlation was observed between all production parameters and the date of onset of pre-flowering, i.e., the date on which dormancy was broken. The correlation was negative for flowers/inflorescence, inflorescences/branch and branches/m 2 (Fig. 6). Production of inflorescences/branch accounted for 70 % of variance, while branches/m 2 and flowers/inflorescences accounted for 55 and 27 %, respectively, of total variance.
Linear regressions among the onset of preflowering period (DOY days of year) and the production parameters (pollen/flower, flowers/inflorescence, inflorescences/branch, branches/m 2). Number of cases = 28 (7 sites in 4 studied years). F F-Snedecor value. Significance levels: ns non-significative, *95, **99, ***99.9 %
A number of significant correlations were found between production parameters (Fig. 7), the most significant being between inflorescences/branch and branches/m 2 (Spearman correlation coefficient, 0.77). A correlation was also observed, albeit less significant, between these two parameters and both flowers/inflorescence (coefficients, 0.39 and 0.35, respectively) and pollen/flower (0.22 and 0.29) (Fig. 7).
Discussion
Mean pollen production in ‘Cornicabra’ olive flowers was 1.10 × 105 ± 0.25 × 105 grains (5.51 × 104 ± 1.25 × 104 grains per anther). Values are similar to those reported for the Spanish ‘Picual’ variety (Aguilera and Ruiz 2012), but differ from other varieties such as ‘Arbosana’ and ‘Arbequina’ (Ferrara et al. 2007), which are considerably higher (>9.00 × 104 grains per anther). Mean pollen production per anther in the olive was higher than that reported for other tree species, for example those belonging to the genera Juglans, Populus, Salix, Ulmus (Tormo et al. 1996), Morus (Mondal et al. 1998) and Quercus (Gómez-Casero et al. 2004). Mean values for pollen production per anther in other species of the Oleaceae family such as Fraxinus angustifolia Vahl are similar to those obtained here (Tormo et al. 1996), reflecting the similar flower morphology shared by members of the same taxonomic family (Andrés 2012; Vargas and Talavera 2012). On the other hand, mean number of flowers per inflorescence in ‘Cornicabra’ was 11, though the number varied between 8 and 14 depending on sampling site and year. Values in this range are also reported for Spanish varieties (Ferrara et al. 2007; Aguilera and Ruiz 2012).
Year-on-year variations in the production of pollen, flowers, inflorescences and flowering branches (Table 2) may be attributable in part to the alternate bearing characteristic of the olive’s biological cycle (Lavee 2007; Therios 2009), and also to year-on-year variations in weather conditions (Ribeiro et al. 2005). The findings of the present study seem to confirm the effect of alternate bearing on olive production: data pointed to a biannual pattern for inflorescences per flowering branch over the four study years (Fig. 3). This alternating pattern of flower and inflorescence production, also reported by Al-Shdiefat and Qrunfleh (2008) and by Aguilera and Ruiz (2012), is linked to the balanced synthesis of endogenous hormones which promote or inhibit flowering, and limit plant reproductive growth (Badr et al. 1970; Palese and Crocker 2002).
Annual airborne olive pollen counts displayed the same fluctuating pattern as inflorescences/branch, which influences number of flowers per tree (Fig. 3). As expected, years with lower pollen airborne pollen counts, i.e., 2010 and 2012 (“Off years”), were followed by years with higher counts, i.e. 2011 and 2013 (“On years”), a finding also reported by other authors (Ribeiro et al. 2005; García-Mozo et al. 2008). Differences were especially marked in 2012, which recorded the lowest annual airborne pollen counts of the study.
Positive correlations observed between flower, inflorescence and flowering-branch production (Fig. 7) may be due to the fact that floral induction is favored in “off years” (Fernández-Escobar et al. 1992; Lavee 2007). When this happens, therefore, there is also greater vegetative growth, and increased branch production (Castillo-Llanque and Rapoport 2011), providing a larger surface area for bud development the following year (Baktir et al. 2004; Rubio et al. 2007). This suggests that on trees with a larger number of flowering branches, those branches also produce a larger number of inflorescences.
With regard to the effects of weather-related factors, flower and inflorescence production was favored by a fall in September–November temperatures the previous year (Table 3), suggesting that low temperatures during dormancy favor the adequate development of olive reproductive organs (Hartmann and Porlingis 1957; Denney et al. 1985), by enabling buds initiated in mid-autumn to break dormancy (Rallo and Martín 1991). Increased production of flowers, inflorescences and flowering branches coincided with early budburst (pre-flowering) (Fig. 6), linked to an earlier attainment of chilling requirements (Orlandi et al. 2002). However, buds cannot break dormancy without a gradual increase in minimum temperatures over the months prior to pre-flowering, which enables the metabolic activation of the plant at the end of winter, prompting flower bud differentiation (Cesaraccio et al. 2004; Connor and Fereres 2005). This was evident here in the positive correlation observed between minimum and mean winter temperatures and flower, inflorescence and flowering-branch production (Table 3).
A positive correlation was also reported between flower and inflorescence production and rainfall over the previous autumn and winter (RSON-1, RDJF) (Table 3), when flower bud initiation and differentiation take place (Connor and Fereres 2005). This also coincides with increased competition for plant assimilates due to the development of floral organs (Rallo 1994). Moreover, severe water deficit inhibits olive reproductive growth in terms of productivity (Kozlowski and Pallardy 1997), considerably reducing the number of floral buds (Hartmann and Panestsos 1961).
All the parameters studied here displayed variations as a function of sampling site elevation (Table 2). In most cases, however, this variability could not be accounted for by a linear correlation with elevation (Fig. 4). Pollen grain production per flower recorded a mildly negative correlation with elevation in 2010 and 2012 (albeit with a significance level of p < 0.07, Fig. 4), but not in other years. Higher pollen production at lower elevations has been reported for the olive by Damialis et al. (2011), and also for other tree species (Moe 1998). However, Aguilera and Ruiz (2012) found the opposite: pollen production increased with elevation. A positive linear correlation was observed between elevation and flower and inflorescence production in 2012 (Fig. 4). Damialis et al. (2011) reported no significant variation in olive flower and inflorescence production as a function of elevation, although variation was observed in other species, including Cupressus sempervirens var. horizontalis. Further research is required into the effect of elevation on olive production parameters, and data are needed for more years to clarify these correlations.
With regard to the effect of N vs. S exposure on the olive tree crown, at most sampling sites flowering-branch production was greater in south-facing branches (Fig. 5). More populations need to be studied to confirm this effect. Lower production of floral buds in shadier areas of the tree has also been reported for other species (Flore 1981). The effect of exposure on flowering-branch production may be linked to variations in solar radiation between sunny and shady areas of the tree (Camargo et al. 2011). Solar radiation affects photosynthesis rates and nutrient distribution within the tree, which is particularly crucial in flowering processes (Privé et al. 1993). Similarly, Kozlowski and Pallardy (1997) report a reduction in floral bud numbers in shady areas of the tree due to reduced light intensity.
Conclusions
Findings pointed to marked spatiotemporal variability in all pollen production parameters studied (pollen grains, flowers, inflorescences and flowering branches), influenced by environmental and weather-related factors, which assume greater importance during floral organ development (initiation and differentiation). Temperature and rainfall during the dormancy period were the weather-related variables most influencing flower production.
Analysis of the influence of environmental conditions on olive pollen and flower production is rendered more complex by interactions with other processes involved in the olive biological cycle. Moreover, variations in pollen production may in part be linked to topographical factors such as the elevation gradient, although no clear correlation emerged here, with different trends depending on the year. Data for more years should be analyzed to determine the effect of elevation on pollen production. Confirming sensitivity to microclimatic variations, flowering-branch production varied as a function of small-scale geographical factors such as north vs. south exposure of branches on the olive tree crown.
The results obtained in this study are interesting from an agricultural point of view, since it can be used for crop forecasting models. They are also interesting from the point of view of allergies because they can be used for forecasting pollen production models that serve to alert the allergy sufferers about the increase of pollen concentrations.
References
Aguilera F, Ruiz L (2012) Microclimatic-induced fluctuations in the flower and pollen production rate of olive trees (Olea europaea L.). Grana 51(3):228–239
Alcázar P, Cariñanos P, De Castro C, Guerra F, Moreno C, Domínguez-Vilches E, Galán C (2004) Airborne plane-tree (Platanus hispanica) pollen distribution in the city of Córdoba, South-western Spain, and possible implications on pollen allergy. J Invest Allergol Clin Immunol 14(3):238–243
Al-Shdiefat SM, Qrunfleh MM (2008) Alternate bearing of the olive (Olea europaea L.) as related to endogenous hormonal content. Jordan J Agric Sci 4(1):12–25
Andersen T (1991) A model to predict the beginning of the pollen season. Grana 30:269–275
Andrés C (2012) Fraxinus L. In: Talavera S, Andrés C, Arista M, Fernández-Piedra MP, Gallego PL, Ortiz C, Romero C, Salgueiro FJ, Silvestre S, Quintanar A (eds) Flora ibérica, XI: Gentianaceae-Boraginaceae. Real Jardín Botánico, CSIC, Madrid, pp 143–151
Badr SA, Hartmann HT, Martin GC (1970) Endogenous gibberellins and inhibitors in relation to flower induction and inflorescence development in the olive. Plant Physiol 46:674–679
Baktir I, Ulger S, Kaynak L (2004) Relationship of seasonal changes in endogenous plant hormones and alternate bearing of olive trees. HortScience 39(5):987–990
Barranco D, Cimato A, Fiorino P, Rallo L, Touzani A, Castañeda C, Serafini F, Trujillo I (2000) Catálogo mundial de variedades de olivo. Consejo Oleícola Internacional, Madrid
Camargo MGG, Souza RM, Reys P, Morellato LPC (2011) Effects of environmental conditions associated to the cardinal orientation on the reproductive phenology of the cerrado savanna tree Xylopia aromatica (Annonaceae). An Acad Bras Cienc 83(3):1007–1019
Castillo-Llanque F, Rapoport HF (2011) Relationship between reproductive behavior and new shoot development in 5-year-old branches of olive trees (Olea europaea L.). Trees 25:823–832
Cesaraccio C, Spano D, Snyder RL, Duce P (2004) Chilling and forcing model to predict bud-burst of crop and forest species. Agric Forest Meteorol 126:1–13
Connor DJ, Fereres E (2005) The physiology of adaptation and yield expression in olive. Hortic Rev 31:155–229
Cruden RW (2000) Pollen grains: why so many? Plant Syst Evol 222:143–165
D’Amato G, Cecchi L, Bonini S, Nunes C, Annesi-Maesano I, Behrendt H, Liccardi G, Popov T, van Cauwenberge P (2007) Allergenic pollen and pollen allergy in Europe. Allergy 62:976–990
Dafni A (1992) Pollination ecology: a practical approach. New Oxford University Press, New York
Damialis A, Fotiou C, Halley JM, Vokou D (2011) Effects of environmental factors on pollen production in anemophilous woody species. Trees 25:253–264
Denney JO, McEachern GR, Griffiths JF (1985) Modeling the thermal adaptability of the olive (Olea europaea L.) in Texas. Agric Forest Meteorol 35:309–327
Díaz de la Guardia C, Alba F, Trigo MM, Galán C, Ruíz L, Sabariego S (2003) Aerobiological analysis of Olea europaea L. pollen in different localities of southern Spain. Forecasting models. Grana 42:234–243
Fernández-Escobar R, Benlloch M, Navarro C, Martin GC (1992) The time of floral induction in the olive. J Am Soc Hort Sci 117(2):304–307
Ferrara G, Camposeo S, Palasciano M, Angelo G (2007) Production of total and stainable pollen grains in Olea europaea L. Grana 46:85–90
Flore JA (1981) Influence of light interception on cherry production and orchard design. Ann Rpt Mich State Hort Soc 111:161–169
Friedman J, Barrett SCH (2009) Wind of change: new insights on the ecology and evolution of pollination and mating in wind-pollinated plants. Ann Bot 103:1515–1527
Galán C, Cariñanos P, Alcázar P, Domínguez-Vilches E (2007) Spanish Aerobiology Network (REA): management and quality manual. Servicio de Publicaciones de la Universidad de Córdoba, Córdoba
García-Mozo H, Pérez-Badia R, Galán C (2008) Aerobiological and meteorological factors’ influence on olive (Olea europaea L.) crop yield in Castilla-La Mancha (Central Spain). Aerobiologia 24:13–18
Gómez-Casero MT, Hidalgo PJ, García-Mozo H, Domínguez E, Galán C (2004) Pollen biology in four Mediterranean Quercus species. Grana 43:22–30
Guerin J, Sedgley M (2007) Cross-pollination in Olive Cultivars. Rural Industries Research and Development Corporation, Barton
Hartmann HT, Panestsos C (1961) Effect of soil moisture deficiency during floral development on fruitfulness in the olive. Proc Am Soc Hort Sci 78:209–217
Hartmann HT, Porlingis I (1957) Effect of different amounts of winter chilling on fruitfulness of several olive varieties. Bot Gaz 119(2):102–104
Hidalgo PJ, Galán C, Domínguez E (2003) Male phenology of three species of Cupressus: correlation with airborne pollen. Trees 17:336–344
Hirst J (1952) An automatic volumetric spore trap. Ann Appl Biol 36:257–265
Joppa LR, McNeal FH, Berg MA (1968) Pollen production and pollen shedding of hard red spring (Triticum aestivum L. em Thell.) and durum (T. durum Desf.) wheats. Crop Sci 8:487–490
Kozlowski TT, Pallardy SG (1997) Growth control in woody plants. Academic Press, California
Lavee S (2007) Biennial bearing in olive (Olea europaea). Ann Ser Hist Nat 17:101–112
Lavee S, Rallo L, Rapoport HF, Troncoso A (1996) The floral biology of the olive: effect of flower number, type and distribution on fruitset. Sci Hortic 66:149–158
Lavee S, Rallo L, Rapoport HF, Troncoso A (1999) The floral biology of the olive II. The effect of inflorescence load and distribution per shoot on fruit set and load. Sci Hortic 82:181–192
Meier U (2001) Growth stages of mono- and dicotyledonous plants. BBCH Monograph, 2nd edn. Federal Biological Research Centre for Agriculture and Forestry
Moe D (1998) Pollen production of Alnus incana at its south Norwegian altitudinal ecotone. Preliminary observations. Grana 37:35–39
Mondal AK, Mondal S, Mandal S (1998) Pollen production in some plant taxa with a supposed role in allergy in Eastern India. Aerobiologia 14:397–403
Orlandi F, Fornaciari M, Romano B (2002) The use of phenological data to calculate chilling units in Olea europaea L. in relation to the onset of reproduction. Int J Biometorol 46:2–8
Orlandi F, Ferranti F, Romano B, Fornaciari M (2003) Olive pollination: flowers and pollen of two cultivars of Olea europaea. N Z J Crop Hort Sci 31:159–168
Orlandi F, Sgromo C, Bonofiglio T, Ruga L, Romano B, Fornaciari M (2009) A comparison among olive flowering trends in different Mediterranean areas (south-central Italy) in relation to meteorological variations. Theor Appl Climatol 97:339–347
Palese AM, Crocker SJ (2002) Preliminary investigations of endogenous gibberellins in seeds of olive fruits. Acta Hort 586:525–528
Pastor M, Humanes J, Vega V, Castro J (2001) Diseño y manejo de plantaciones de olivar, 2ª edición. Consejería de Agricultura y Pesca, Sevilla
Privé JP, Sullivan JA, Proctor JTA, Allen OB (1993) Climate influences vegetative and reproductive components of primocane-fruiting red raspberry cultivars. J Am Soc Hort Sci 118(3):393–399
Rallo L (1994) Fructificación y producción en olivo. Agricultura 746:13–16
Rallo L, Martín GC (1991) The role of chilling in releasing olive floral buds from dormancy. J Am Soc Hort Sci 116(6):1058–1062
Rallo L, Suarez MP (1989) Seasonal distribution of dry matter within the olive fruit-bearing limb. Adv Hort Sci 3:55–59
Rapoport HF (2008) Botánica y morfología. In: Barranco D, Fernández-Escobar R, Rallo L (eds) El cultivo del olivo, 8ª edición. Junta de Andalucía y Ediciones Mundi-Prensa, Madrid, pp 37–62
R Development Core Team (2008) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, ISBN 3-900051-07-0. http://www.R-project.org
Ribeiro H, Cunha M, Abreu I (2005) Airborne pollen of Olea in five regions of Portugal. Ann Agric Environ Med 12:317–320
Ribeiro H, Santos L, Abreu I, Cunha M (2006) Influence of meteorological parameters on Olea flowering date and airborne pollen concentration in four regions of Portugal. Grana 45:115–121
Ribeiro H, Cunha M, Abreu I (2007) Improving early-season estimates of olive production using airborne pollen multi-sampling sites. Aerobiologia 23:71–78
Rogers CA (1993) Application of aeropalynological principles in palaeoecology. Rev Palaeobot Palynology 79:133–140
Rojo J, Pérez-Badia R (2014) Effects of topography and crown exposure on olive-tree phenology. Trees 28:449–459
Rosati A, Caporali S, Paoletti A (2012) Floral biology: implications for fruit characteristics and yield. In: Muzzalupo I (ed) Olive germplasm—the olive cultivation, table olive and olive oil industry in Italy. InTech Design Team, Rijeka, pp 71–80
Rovira M, Tous J (2005) Producción y viabilidad de polen. In: Rallo L (ed) Variedades de olivo en España. Ediciones Mundi-Prensa, Madrid, pp 295–299
Rubio G, Rallo L, Rapoport HF (2007) Crecimiento de brotes y yemas fructíferas de olivo. Actas de Horticultura n° 48:793–796
Subba C, Reddi NS (1986) Pollen production in some anemophilous angiosperms. Grana 25:55–61
Subiza FJ, Pola J, Feo F, Moral AJ (2007) Pólenes de interés en alergología en nuestro medio. In: Pélaez A, Dávila IJ (eds) Tratado de Alergología. Ergón, Madrid, Spain, pp 425–446
Therios IN (2009) Olives. Crop production science in horticulture n° 18. CAB International
Tormo R, Muñoz A, Silva I, Gallardo F (1996) Pollen production in anemophilous trees. Grana 35:38–46
Ulger S, Sonmez S, Karkacier M, Ertoy N, Akdesir O, Aksu M (2004) Determination of endogenous hormones, sugars and mineral nutrition levels during the induction, initiation and differentiation stage and their effects on flower formation in olive. Plant Growth Regul 42:89–95
Vargas P, Talavera S (2012) Olea L. In: Talavera S, Andrés C, Arista M, Fernández-Piedra MP, Gallego MJ, Ortiz PL, Romero C, Salgueiro FJ, Silvestre S, Quintanar A (eds) Flora ibérica, XI: Gentianaceae-Boraginaceae. Real Jardín Botánico, CSIC, Madrid, pp 135–139
Wilcox RR (2012) Introduction to robust estimation and hypothesis testing, 3rd edn. Academic Press, USA
Author contributions statement
JR field work and data collection, data analysis, experimental design and redaction; PS data analysis and redaction; RPB experimental design, data analysis, redaction and supervision.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Lin.
Rights and permissions
About this article
Cite this article
Rojo, J., Salido, P. & Pérez-Badia, R. Flower and pollen production in the ‘Cornicabra’ olive (Olea europaea L.) cultivar and the influence of environmental factors. Trees 29, 1235–1245 (2015). https://doi.org/10.1007/s00468-015-1203-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-015-1203-6