Abstract
Key message
Embryogenic cultures of eastern and Carolina hemlocks could be initiated, and somatic embryos and plantlets produced using standard conifer protocols and media. Embryogenic hemlock cultures were cryostored and recovered.
Abstract
Eastern hemlock (Tsuga canadenesis) and Carolina hemlock (Tsuga caroliniana) are threatened with extirpation from their native ranges in eastern North America by the introduction of the hemlock woolly adelgid (HWA; Adelges tsugae), an exotic insect pest that has already killed millions of hemlock trees. Efforts to conserve and restore these members of the Pinaceae could be greatly enhanced by the availability of an in vitro propagation system. We conducted experiments to initiate embryogenic cultures from eastern and Carolina hemlock zygotic embryos at different stages of development using three media supplemented with 2,4-dichlorophenoxyacetic acid (2,4-D) and 6-Benzylaminopurine (BA). Cone collection date, medium and source tree had significant effects on induction of embryogenic tissue from zygotic embryo explants of both species, which ranged as high as 52 % for eastern hemlock and 17 % for Carolina hemlock. Embryogenic hemlock cultures could be cryostored using a protocol employing sorbitol and DMSO, and recovered following several months of frozen storage. Transfer of embryogenic tissue from proliferation media containing 2, 4-D and BA to a Litvay medium with abscisic acid promoted the development of somatic embryos, which were stimulated to mature by slow drying under semi-permeable plastic film. Embryos moved to an imbibition-germination medium without plant growth regulators and incubated in the light elongated and subsequently germinated. A small number of germinated embryos survived transfer to ex vitro conditions and grew into somatic seedlings. The embryogenesis and cryostorage systems developed in the study are already being integrated with hemlock breeding efforts to develop clones with resistance or tolerance to HWA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Eastern hemlock (Tsuga canadensis) is a major component of the Northern Hardwood Forest climax community in North America. The tree is distributed in the eastern U.S. from New England south through the Appalachian Mountains into north Georgia and Alabama and is considered to be a foundation forest species (Ellison et al. 2005), that, among other roles in the ecosystem, helps maintain stable stream temperatures in Appalachian coves. Carolina hemlock (Tsuga caroliniana) is a relatively rare endemic forest species found in scattered populations, mainly on rocky outcrops and dry ridges in the southern Appalachians. Stands of both eastern hemlock and Carolina hemlock are currently under attack from the hemlock woolly adelgid (Adelges tsugae; HWA), a non-native insect now present throughout the northeastern and mid-Atlantic region of the U.S., but which is having particularly severe impact in the Southern Appalachians (Vose et al. 2013). HWA is known to kill trees in as little as 4 years (McClure et al. 2001) and infestation by the adelgid threatens to greatly reduce or even eliminate the two hemlock species from eastern forests in the coming decades (Ellison et al. 2005). The loss of eastern hemlock is likely to have severe ecological consequences on forest composition, nutrient cycles, hydrologic processes, aquatic life in mountain streams and wildlife (Potter et al. 2008). While Carolina hemlock may not be as critical to ecosystem health as eastern hemlock, its loss would also dilute the value of the Appalachian forest.
While biocontrol measures for hemlock woolly adelgid (HWA) are being tested (reviewed in Vose et al. 2013), the pest continues to threaten the survival of both eastern and Carolina hemlocks. There can be little doubt that the genetic diversity of these species is declining every year, as populations of the trees are devastated by the pest. A system for long-term preservation of eastern and Carolina hemlock germplasm would help ensure that the genetic diversity of these species can be maintained for restoration purposes. Work to generate germplasm banks via both seed storage and establishment of seedlings or rooted cuttings outside the range of the pest is underway (Jetton et al. 2013). However, both of these approaches have potential drawbacks. The viability of seeds of eastern and Carolina hemlocks in cold storage may be limited. Eastern hemlock seeds could be stored under refrigeration for 2 to 4 years, but retention of viability varied considerably among seed lots (Olson et al. 1959). Plantings of hemlocks outside their natural ranges may expose them to new pests, pathogens or other stresses that they do not face in their natural range, leading to loss of the populations or sub-optimal growth and reproduction.
One alternative or supplement to storing seeds or installation of plantings outside the range of the pest to conserve genetic diversity of both hemlock species is to generate in vitro cultures from range-wide collections of eastern and Carolina hemlock genotypes and place samples of the cultures in cryostorage. Cryopreservation protocols have been successfully applied to embryogenic cultures of a number of coniferous species, including white spruce (Picea glauca; Kartha et al. 1988; DeVerno et al. 1999), black spruce (Picea mariana; Touchell et al. 2002), and radiata pine (Pinus radiata; Hargreaves and Smith 1992). Clone banks maintained as cryostored embryogenic cultures not only would be protected from the adelgid (and any other pests, pathogens or other threats that arise), but also could be held indefinitely. Here, we present the results of the first study to establish embryogenic cultures of eastern and Carolina hemlocks to regenerate somatic seedlings of these species and to demonstrate that embryogenic hemlock cultures can be successfully cryostored and recovered.
Materials and methods
Culture initiation
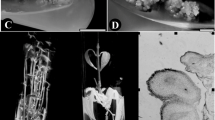
Cultures were initiated from eastern and Carolina hemlock seeds in two consecutive years (2006 and 2007). In a preliminary experiment in 2006, immature eastern and Carolina hemlock cones (Fig. 1a) were collected on three dates during the summer by USDA Forest Service (USFS) and Camcore (http://www.camcore.org/) cooperators. Eastern hemlock cones were collected by USFS cooperators from three North Carolina source trees on July 12. Carolina hemlock cones were collected by Camcore cooperators from three North Carolina source trees on July 20 and additional Carolina hemlock cones were collected by USFS cooperators from three different North Carolina source trees on August 7. Cones were shipped to us on cold packs via overnight courier and were refrigerated upon arrival. Prior to dissection to remove the seeds (Fig. 1b), cones were soaked in a 10 % solution of Roccal-D Plus (9.2 % didecyl dimethyl ammonium chloride, 13.8 % alkyl dimethyl benzyl ammonium chloride, 1 % bis-n-tributyltin oxide; Pfizer) for 1 min and rinsed with tap water. Seeds were surface-disinfested by a 3-s dip in 70 % ethanol and dissected to remove the megagametophytes with embryos. Depending on appearance of the seeds, we either cultured whole megagametophytes with associated embryos or dissected the embryos from the megagametophytes for culture without the megagametophyte. For the July 12 and July 20 collections, the seeds appeared to be somewhat shriveled (Fig. 1c), making it difficult to identify any embryos inside the megagametophytes, so whole megagametophytes with embryos were cultured (Fig. 1d, e). Seeds from August 7 collection appeared to be healthier and contained embryos that were almost fully mature, so they were dissected from the megagametophytes for culture (Fig. 1f). In all, approximately 320 megagametophyte and zygotic embryo explants were cultured. Explants were cultured in 100 × 20 mm plastic Petri dishes containing semi-solid culture initiation media of two types: (1) Merkle et al. (2005) pine induction medium (IM), which was supplemented with 13.57 µM 2,4-dichlorophenoxyacetic acid (2,4-D) and 2.22 µM 6-Benzylaminopurine (BA), and (2) EDM6 medium (Walter et al. 1998), which is Smith (1996) EDM supplemented with 4.52 µM 2,4-D and 2.22 µM BA. Both media contained 30 g/l sucrose and were gelled with 3 g/l Phytagel (Sigma). Cultures were incubated in the dark at 23 °C and checked for production of embryogenic tissue after 2 months in culture. In this study, embryogenic tissue was judged by morphology, which was semi-transparent with visible embryo heads (or embryos proper) and suspensors under a dissecting microscope (Tautorus et al. 1991). The embryogenic nature of the tissues was further confirmed by embryo maturation tests showing production of cotyledonary embryos from the tissue.
Eastern and Carolina hemlock cones, seeds, megagametophytes and zygotic embryos. a Immature eastern hemlock cone collected in July. Bar = 3 mm. b Eastern hemlock cone scale with two immature seeds. Bar = 3 mm. c. Eastern hemlock seed dissected from cone collected on July 12, 2006. Bar = 1 mm. d Dissected Eastern hemlock seed from cone collected on July 12, 2006, showing megagametophyte inside. Bar = 1 mm. e Eastern hemlock megagametophyte explant after 5 days in culture. Bar = 500 µm. f Almost mature Carolina hemlock zygotic embryo explant from cone collected on August 7, 2006. Bar = 1 mm
Based on the results of the preliminary culture initiation experiment, a larger experiment was conducted in 2007. Cones were collected from two eastern and two Carolina hemlock trees in Georgia on May 25, June 15, July 16 and August 10, except for the Carolina hemlock trees, which had already shed their seeds by August 10 and so were not collected on that date. Cones were collected by USFS cooperators from two eastern and two Carolina hemlock trees in North Carolina on June 25, July 10, July 23 and August 7. Shipping, storage, surface disinfestation and cone dissection protocols were the same as for the 2006 experiment. As in 2006, we either cultured whole megagametophytes with embryos or dissected the embryos from the megagametophytes, depending on appearance of the seeds. In 2007, over 2200 megagametophyte and zygotic embryo explants were cultured. Explants were cultured, six per plate, in 100 × 20 mm plastic Petri plates containing semi-solid culture initiation media of three types, the same two used in 2006, plus a third medium, DCR (Gupta and Durzan 1985), which was supplemented with the same levels of plant growth regulators as IM. Cultures were incubated in the dark at 23 °C. Explants were scored for production of embryogenic tissue that appeared to have embryogenic potential after 2 months in culture.
The 2007 initiation experiment was designed to test the effects of species (T. canadensis versus T. caroliniana), source tree location (Georgia or North Carolina), source tree genotype (four per species, nested within species), seed collection date, medium and any interactions between these variables, on induction of embryogenesis. At least four Petri plates, each with six seed explants, were tested for each source tree by collection date by medium combination. Following arcsin transformation of embryogenesis induction percentage data, treatment effects were tested by analysis of variance using PROC GLM and means were separated by Duncan’s multiple range test using the MEANS option of SAS (SAS Institute Inc. 2011).
Cryostorage and recovery
Embryogenic tissue from three Carolina hemlock and two eastern hemlock embryogenic culture lines initiated in 2007 was inoculated into 15 ml of liquid EDM6 in 50 ml flasks and grown in the dark on a rotary shaker at 100 rpm for 1 week. Cultures were pretreated by incubating in liquid EDM6 supplemented with 0.4 M sorbitol for 24 h prior to freezing. Then, they were inoculated into EDM6 with 0.4 M sorbitol and either 5 or 10 % dimethylsulfoxide (DMSO) as cryoprotectant. Aliquots of 1.8 ml of embryogenic suspension from each pretreatment were pipetted into pre-chilled (4 °C) 2 ml cryovials (Nalgene) and placed into a pre-chilled (4 °C) “Mr. Frosty” freezing container (Nalgene, Rochester, NY). Three cryovials were used for each line by treatment combination, for a total of 30 samples. The container was transferred to an ultra-low freezer (−70 °C) overnight. According to the manufacturer, the freezing container provides a sample cooling rate of approximately 1 °C per minute until −70 °C is reached. Samples were then placed into a cryobox and transferred to a Forma Cryomed II freezer, where they were held in liquid nitrogen at −196 °C for 7 months. Following their removal from liquid nitrogen, cryovials were transferred from the cryobox to a floating tray in a 37 °C water bath for 2 min to facilitate rapid thawing. Following thawing, embryogenic tissue was recovered by pouring vial contents through a 30-μm nylon mesh, over two layers of filter paper and paper towels. Then the mesh with embryogenic tissue was placed onto fresh EDM6 gelled with 0.8 % Phytagar (Life Technologies, Rockville, MD) in 100 mm plastic Petri plates and incubated in the dark at 24 °C. Nylon mesh with embryogenic tissue was transferred to fresh semisolid EDM6 at 1 h, 24 h, and 7 days, to facilitate dilution of residual DMSO. Embryogenic tissue was transferred directly to semisolid EDM6 after 1 month and assessed for regrowth 2 months post-thaw.
Somatic embryo and somatic seedling production
Six embryogenic hemlock culture lines (three Carolina hemlock and three eastern hemlock) that had been initiated in 2007 and cryo-stored were recovered from cryostorage as described above and re-grown on plates of semisolid EDM6 for 3 months, with transfer to fresh medium every 3 weeks. For each of the six lines, approximately 2 g of embryogenic tissue was weighed out, suspended in 20 ml of liquid EDM6 in 50 ml Erlenmeyer flasks and grown for 1 week on a gyratory shaker at 100 rpm in the dark. Then 2 ml aliquots of the suspension were pipetted onto disks of Nitex nylon mesh (Sefar America, Depew, NY, 30 µm pore size) in a Büchner funnel and subjected to mild vacuum to remove excess liquid medium. Each nylon mesh disk with embryogenic tissue was placed on one of two semisolid media in 100 mm plastic Petri plates for somatic embryo development: (1) Smith (1996) EMM2 medium, which includes 57 µM (±) cis, trans-abscisic acid (ABA), 3 percent sucrose and 4.5 g/L Phytagel, or (2) a modified half-strength Litvay (Litvay et al. 1985) medium described in Kong and von Aderkas (2007) supplemented with 3 % maltose, 5 % PEG 4000 (Sigma) and 50 µM ABA and gelled with 5 g/L Phytagel. Approximately 100 mg embryogenic tissue was inoculated onto each of four plates for each genotype × treatment combination, for a total of 48 plates. Plates were incubated in the dark at 24 °C until formation of pre-cotyledonary stage somatic embryos was observed (between 30 and 60 days). Then, lids of all plates were removed and plates were covered with water vapor-permeable film (Fisher Scientific brand polyvinyl-chloride all-purpose laboratory wrap) for approximately 2 weeks of slow desiccation under the same conditions. Following desiccation, the numbers of mature embryos produced per mg of plated embryogenic tissue were counted and the effect of medium type on embryo production per plate was tested by analysis of variance using PROC GLM of SAS (SAS Institute Inc. 2011). Individual embryos were harvested and transferred to 100 mm plastic Petri plates containing semisolid imbibition/germination medium, which was modified Litvay medium with 1 percent sucrose, 1 g/L NH4NO3, 3 g/L activated charcoal (Sigma catalog #C-4386) and 3 g/L Phytagel. Plates were incubated in a lighted incubator under cool white fluorescent lights (100 μmol m−2 s−1) with 16 h of light/day. After embryos began to germinate, they were transferred, four per vessel, to GA7 vessels (Magenta Corp.) containing 100 ml of semisolid imbibition/germination medium to complete conversion under the same light and temperature conditions. Somatic seedlings were removed from in vitro conditions, potted in Hillson-type Roottrainers (Spencer-Lemaire) containing Fafard 3B potting mix and placed in a Plexiglas acclimatization chamber with cool white fluorescent lights (100 μmol m−2 s−1) with 16 h of light/day under high relative humidity, for hardening off.
Results
Culture initiation
Very low response numbers and confounding of collection date, species and source tree did not allow statistical analysis for the effects of these variables in embryogenesis induction for the 2006 culture initiation. Very few of the Eastern hemlock megagametophytes from the July 12 collection proliferated and only two cultured on IM produced embryogenic tissue (Fig. 2a). Similarly, few of the Carolina hemlock megagametophytes collected in July 20 produced embryogenic tissue on both media, while relatively more embryogenic tissue were induced from August 7 explants on both media (Fig. 2b).
Eastern and Carolina hemlock megagametophytes cultured in 2006. a Embryogenic tissue produced from megagametophyte/zygotic embryo from eastern hemlock green cone collected on July 12, 2006. Bar = 1 mm. b Carolina hemlock embryogenic tissue proliferating on IM 2 months following initiation. Bar = 1 mm
Because collection dates differed for the North Carolina source trees and Georgia source trees in 2007, data were analyzed separately for source trees from each state to determine the effect of collection date on embryogenesis induction. For trees from both states, analysis of variance results indicated that cone collection date had a significant effect (p = 0.02, F = 3.38, df = 3, for North Carolina trees and p < 0.0001, F = 106.38, df = 3, for Georgia trees) on the induction of embryogenesis. For North Carolina trees, there was no significant interaction between collection date and species, while this interaction was significant for Georgia trees, but this probably was due to the fact that the Carolina hemlocks in Georgia had very low induction on all dates, while the Georgia eastern hemlocks had very high induction (52 %) for one collection date and low or no induction for the others (Fig. 3b). For North Carolina eastern hemlocks, embryogenesis induction peaked at 8 % for the July 10 collection, but this was not statistically different from the June 25 (4 %) or July 23 (5 %) collections, while mature embryos explants collected on August 7 had 0 % induction (Fig. 3a). Similarly, there were no significant differences in embryogenesis induction among Carolina hemlock seeds collected in North Carolina on June 25 (13 %), July 10 (14 %) or July 23 (17 %), and mature embryo explants collected on August 7 had 0 % induction (Fig. 3a). The only collection date resulting in embryogenesis induction for Carolina hemlock seeds from the Georgia trees was June 15, and even for this collection, only 1 % of the explants produced embryogenic tissue (Fig. 3b). The Georgia Carolina hemlocks were from a very small surviving population in Tallulah Gorge State Park, so the low induction frequency for these seeds may be associated with low pollination/fertilization rates. Data from North Carolina and Georgia trees were combined to determine the effect of medium on embryogenesis induction. Culture medium had a significant (p = 0.0002, F = 8.76, df = 2) effect on embryogenesis induction for both eastern and Carolina hemlocks, with both EDM6 and IM giving higher induction than DCR, according to Duncan’s multiple range test results (Fig. 4).
Effect of cone collection date on embryogenesis induction (percent of explants that produced embryogenic tissue) from eastern hemlock and Carolina hemlock megagametophyte and zygotic embryo explants. Bars (percentage values) represent means calculated from 144 explants (two source trees × three media × four plates × six explants per plate). In each graph, bars for the same species that have the same letter are not significantly different according to Duncan’s test. a Embryogenesis induction from North Carolina trees. b Embryogenesis induction from Georgia trees
Effect of induction medium on embryogenesis induction (percent of explants that produced embryogenic tissue) from eastern and Carolina hemlock megagametophyte and zygotic embryo explants. Bars (percentage values) represent means calculated from 384 explants (four source trees × four collection dates × four plates × six explants per plate). Bars for the same species that have the same letter are not significantly different according to Duncan’s test
Cryostorage and recovery
Of the five hemlock lines subjected to cryostorage, material from all three cryovials of all three Carolina hemlock lines and one of the two eastern hemlock lines regrew strongly following 7 months of cryostorage, regardless of whether the cryoprotectant was 5 or 10 % DMSO (data not shown). Only one of the three cryovials of the other eastern hemlock regrew, and it exhibited slow regrowth. Regrowth of embryogenic tissue of both hemlock species could first be observed within 2 weeks following removal from liquid nitrogen, and within 7 weeks, embryogenic tissue proliferation resembled that of the original cultures prior to cryostorage (Fig. 5).
Somatic embryo and somatic seedling production
The first “pre-cotyledonary” somatic embryos (Fig. 6a) became visible on embryogenic tissue of some genotypes about 30 days following plating of the suspension cultures and more embryos continued to arise over the following month. Within 60 days, lids of all plates with visible pre-cotyledonary embryos were replaced with semi-permeable film to allow slow desiccation, and over the next 2–3 weeks, embryos continued to develop and mature, with elongating hypocotyls and emerging cotyledons (Fig. 6b, c). The numbers of embryos produced on each plate (i.e. per approximately 100 mg of plated suspension culture) showed a significant (p < 0.0001, F = 12.86, df = 1) difference between the two maturation media. Litvay medium was superior to EMM2, averaging about 66 embryos per 100 mg of plated tissue, compared to an average of less than one embryo per 100 mg of tissue on EMM2 for eastern hemlock and about 11 embryos per 100 mg of tissue for Carolina hemlock, which produced no embryos on EMM2 (Fig. 7). The interaction between genotype and medium was significant (p < 0.0001), but the interaction was due to the fact that all three of the eastern hemlock genotypes and one of the Carolina hemlock genotypes produced much higher numbers of somatic embryos on Litvay medium than on EMM2, while somatic embryo production for two of the Carolina hemlock genotypes was low or zero on both media. Even using Litvay medium, somatic embryo production varied widely among genotypes, ranging from 1.3 to 27 embryos per 100 mg of tissue among the three Carolina hemlock genotypes tested and from 27 to 125 embryos per 100 mg of tissue among the three eastern hemlock genotypes tested (data not shown). Embryos with emerging cotyledons transferred to plates of imbibition/germination medium and incubated in the light continued to elongate, hypocotyls and cotyledons greened, and radicles elongated into taproots (Fig. 6d–g). Germinated embryos of both eastern and Carolina hemlock transferred to GA7 vessels produced true leaves, although growth was slow (Fig. 6h). Over 90 % selected mature embryos could germinate, but only about 50 % of the germinants could convert into plantlets with both shoot and root. Somatic seedlings transferred to potting mix and moved to the hardening off chamber grew slowly. To date, only a few have survived hardening-off and these were moved to larger pots (Fig. 6i, j).
Eastern and Carolina hemlock somatic embryo and somatic seedling production. a Pre-cotyledonary eastern hemlock somatic embryo following 5 weeks on Litvay maturation medium. Bar = 1 mm. b Eastern hemlock somatic embryos with emerging cotyledons following 8 weeks on Litvay maturation medium. Bar = 1 mm. c Mature eastern hemlock somatic embryo with well-developed cotyledons and developing hypocotyl following 10 weeks on Litvay maturation medium. Bar = 1 mm. d Elongating eastern hemlock somatic embryo following 1 week on imbibition/germination medium in the dark. Bar = 1 mm. e Eastern hemlock somatic embryo following 1 week on imbibition/germination medium in the dark and 4 days in the light. Embryo is just beginning to green and radicle has begun elongation. Bar = 2 mm. f Germinating Carolina hemlock somatic embryo following 1 week on imbibition/germination medium in the dark and 4 weeks in the light, with first true needles emerging, and elongating hypocotyl and radicle. Bar = 4 mm. g Germinated Carolina hemlock somatic embryo with expanded true needles and taproot. Bar = 5 mm. h Carolina hemlock somatic seedlings on Litvay imbibition/germination medium in a GA7 vessel. i Carolina hemlock somatic seedlings following transfer to potting mix in a humidifying chamber. j Carolina hemlock somatic seedling following repotting and transfer to the greenhouse
Discussion
Our results with eastern and Carolina hemlock indicate that the same methods and media that has been used to initiate embryogenic cultures of other members of the Pinaceae and to produce somatic embryos and somatic seedlings from them are applicable to these two hemlock species. Three different basal media formulations (DCR, IM and EDM6) all were capable of inducing somatic embryogenesis from megagametophyte (with zygotic embryo) and excised zygotic embryo explants from immature seeds, although EDM, originally developed for radiata pine (Smith 1996), and IM, originally developed for southern U.S. pines (Merkle et al. 2005), were superior to DCR (Gupta and Durzan 1985), which was originally developed for Douglas-fir and sugar pine. It should be noted here that each of these media were tested using the plant growth regulator concentrations and other supplements that were used in the publications in which the media were described, so it was not only the basal media formulations that varied. There may be other conifer basal media formulations that would be superior for hemlock embryogenesis induction and subsequent embryogenic tissue proliferation, including the modified Litvay (Litvay et al. 1985; Kong and von Aderkas 2007) formulation that we tested for somatic embryo development and maturation, but did not test for induction. In a recent preliminary test, hemlock embryogenic tissue appeared healthier (i.e. less watery, with more well-defined suspensors and embryo heads) on a modified LV proliferation medium when compared with IM (L. Kong, unpublished data). Megagametophyte explants of both hemlock species from cones collected over a span of about 7 weeks during June and July 2007 were capable of undergoing embryogenesis induction, while more mature embryos, collected in early August, did not produce any embryogenic cultures. The length of this developmental “window” for embryogenesis is very similar to that reported for pine species (Becwar et al. 1990; Finer et al. 1989), but quite different from the relatively broad range of explants that have been used for spruce (Picea) embryogenesis induction, which includes not only immature and mature zygotic embryos, but parts of seedlings up to 30 days old (Mo and von Arnold 1991) and buds of somatic embryo-derived trees up to 10 years old (Klimaszewska et al. 2011). In this study, however, we did not attempt to determine the relationship between the actual stage(s) of zygotic embryo development and embryogenesis induction, as has been done with several other conifers, with the exception of confirming that no embryogenesis induction occurred with fully mature zygotic embryos from the final (early August) collections. It is also true that we cultured zygotic embryos at earlier stages in their megagametophytes, while later stages were dissected from the megagametophytes and cultured without them. Thus, embryo developmental stage and the presence or absence of the megagametophyte was confounded in our experiment. A more intensive study now underway with weekly collections of cones from local eastern hemlock trees as the zygotic embryos develop will allow us to document the relationship between stage(s) of zygotic embryo development (and the presence or absence of megagametophyte) and embryogenesis induction.
Some components of a system we used to produce somatic seedlings of southern pines (Merkle et al. 2005) worked well for hemlock somatic seedling production, including the use of semi-permeable film to allow a controlled rate desiccation of the somatic embryos to encourage maturation. We found that the modified half-strength Litvay medium (mLV, Kong and von Aderkas 2007) with the combination of supplements described in Kong and von Aderkas (2011) significantly improved mature embryo production compared to Smith (1996) EMM2 medium. The combination of using mLV as the basal medium, maltose as a major carbon source and polyethylene glycol (PEG) to increase medium osmolarity stimulated somatic embryo maturation in hemlock, just as it did in Douglas-fir (Kong and von Aderkas 2011), which is closely related to hemlocks. In this study, although the embryo germination rate was high, the conversion rate was relatively low. In previous studies, PEG enhanced somatic embryo maturation but reduced embryo conversion in spruces (Kong and Yeung 1995; Bozhkov and von Arnold 1998). The relatively low conversion rates and slow growth of somatic seedlings following transfer to potting mix are indications that we still need to make significant improvements in hemlock somatic embryo quality if the system is to be useful for propagation.
Standard osmotic pre-treatment and cryoprotectant treatments developed for other conifer embryogenic cultures appear to be adequate for successful cryostorage and recovery of embryogenic eastern and Carolina hemlock cultures. The ability to safely hold hemlock cultures for long periods using this approach has great potential utility for hemlock germplasm conservation. In addition, the combination of somatic embryogenesis and cryostorage with breeding programs can offer a very powerful approach for reforestation with HWA-resistant or HWA-tolerant genotypes. If native trees with apparent natural resistance or tolerance are identified, they can be crossed with other such trees and the seeds used to initiate embryogenic cultures. Once established, the embryogenic cultures can be held indefinitely in cryostorage while somatic seedlings derived from them are screened for HWA resistance or tolerance. Then, if screening results indicate that any of the clones are especially promising, the cultures from which they were derived can be thawed, regrown and scaled-up to make somatic seedlings for restoration purposes. Similarly, hybrid breeding between susceptible native hemlocks and resistant Asian species, such as Chinese hemlock (Tsuga chinensis), can be combined with somatic embryogenesis and cryostorage to produce clones of HWA-resistant hybrid trees. Thus, the multiplying power of somatic embryogenesis and the long-term storage potential offered by cryostorage make for a very powerful combination with regard to both conservation and propagation of these threatened forest trees.
Reference
Becwar MR, Nagmani R, Wann SR (1990) Initiation of embryogenic cultures and somatic embryo development in loblolly pine (Pinus taeda). Can J For Res 20:810–817
Bozhkov PV, von Arnold S (1998) Polyethylene glycol promotes maturation but inhibits further development of Picea abies somatic embryos. Physiol Plant 104:211–224
DeVerno LL, Park YS, Bonga JM, Barrett JD (1999) Somaclonal variation in cryopreserved embryogenic clones of white spruce [Picea glauca (Moench) Voss.]. Plant Cell Rep 18:948–953
Ellison AM, Bank MS, Clinton BD, Colburn EA, Elliott K, Ford CR, Foster DR, Kloeppel BD, Knoepp JD, Lovett GM, Mohan J, Orwig DA, Rodenhouse NL, Sobczak WV, Stinson KA, Stone JK, Swan CM, Thompson J, Von Holle B, Webster JR (2005) Loss of foundation species: consequences for the structure and dynamics of forested ecosystems. Front Ecol Environ 3:479–486
Finer JJ, Kriebel HB, Becwar MR (1989) Initiation of embryogenic tissue and suspension cultures of eastern white pine (Pinus strobus L.). Plant Cell Rep 8:203–206
Gupta PK, Durzan DJ (1985) Shoot multiplication from mature trees of Douglas-fir (Pseudotsuga menziesii) and sugar pine (Pinus lambertiana). Plant Cell Rep 4:177–179
Hargreaves C, Smith DR (1992) Cryopreservation of Pinus radiata embryogenic tissue. Comb Proc Intl Plant Prop Soc 42:327–333
Jetton RM, Whittier WA, Dvorak WS, Rhea JR (2013) Conserved ex situ genetic resources of eastern and Carolina hemlock: eastern North American conifers threatened by the hemlock woolly adelgid. Tree Plant Notes 56:59–71
Kartha KK, Fowke LC, Leung NL, Caswell KL, Hakman I (1988) Induction of somatic embryos and plantlets from cryopreserved cell cultures of white spruce (Picea glauca). Plant Physiol 132:529–539
Klimaszewska K, Overton C, Stewart D, Rutledge RG (2011) Initiation of somatic embryos and regeneration of plants from primordial shoots of 10-year-old somatic white spruce and expression profiles of 11 genes followed during the tissue culture process. Planta 233:635–647
Kong L, von Aderkas P (2007) Genotype effects on ABA consumption and somatic embryo maturation in interior spruce (Picea glauca x engelmanni). J Exp Bot 58:1525–1531
Kong L, von Aderkas P (2011) A novel cryopreservation method for conifer immature somatic embryos without cryoprotectant. Plant Cell Tissue Organ Cult 106:115–125
Kong L, Yeung EC (1995) Effects of silver nitrate and polyethylene glycol on white spruce (Picea glauca) somatic embryo development: enhancing cotyledonary embryo formation and endogenous ABA content. Physiol Plant 93:298–304
Litvay JD, Verma DC, Johnson MA (1985) Influence of a loblolly pine (Pinus taeda L.) culture medium and its components on growth and somatic embryogenesis of the wild carrot (Daucus carota L.). Plant Cell Rep 4:325–328
McClure MS, Salom SM, Shields KS (2001) Hemlock woolly adelgid. USDA Forest Service Forest Health Enterprise Technology Team Report FHTET-2001-03, Morgantown
Merkle SA, Montello PM, Xia X, Upchurch BL, Smith DR (2005) Light quality treatments enhance somatic seedling production in three southern pine species. Tree Physiol 26:187–194
Mo LH, von Arnold S (1991) Origin and development of embryogenic cultures from seedlings of Norway spruce (Picea abies). J Plant Physiol 138:223–230
Olson JS, Stearns FW, Nienstaedt H (1959) Eastern hemlock seeds and seedlings: response to photoperiod and temperature. Connecticut Agric Expt Stn Bulletin 620, New Haven
Potter KM, Dvorak WS, Crane BS, Hipkins VD, Jetton RM, Whittier WA, Rhea R (2008) Allozyme variation and recent evolutionary history of eastern hemlock (Tsuga canadensis) in the southeastern United States. New For 35:131–145
SAS Institute Inc (2011) SAS/STAT 9.3 User’s Guide, Cary, NC: SAS Institute Inc
Smith DR (1996) Growth Medium. US Patent No. 5,565,355
Tautorus TE, Fowke LC, Dunstan DI (1991) Somatic embryogenesis in conifers. Can J Bot 69:1873–1899
Touchell DH, Chiang VL, Tsai CJ (2002) Cryopreservation of embryogenic cultures of Picea mariana (black spruce) using vitrification. Plant Cell Rep 21:118–124
Vose JM, Wear DN, Mayfield AE III, Nelson CD (2013) Hemlock woolly adelgid in the southern Appalachians: control strategies, ecological impacts, and potential management responses. For Ecol Manag 291:209–219
Walter C, Grace LJ, Wagner A, White DWR, Walden AR, Donaldson SS, Hinton H, Gardner RC, Smith DR (1998) Stable transformation and regeneration of transgenic plants of Pinus radiata D. Don. Plant Cell Rep 17:460–468
Author contribution statement
SAM designed culture initiation experiments and helped design somatic embryo production experiments, conducted data analysis, took photos and wrote all drafts of the manuscript; PMM conducted culture initiation, somatic embryo production and cryopreservation experiments and took photos; HMR conducted culture initiation experiments; LK designed and conducted somatic embryo and somatic seedling production experiments and took photos. All authors approved the final draft of the manuscript.
Acknowledgments
The research reported here was supported by a grant from the USDA Forest Service—Forest Health Protection. The authors would like to thank the USDA Forest Service, the Georgia Department of Natural Resources, Blue Ridge Outdoor Education Center, Camcore, Rusty Rhea, Jim Compton, Chuck Gregory, Danny Tatum, Greg Yates, Bill Dvorak, Robert Jetton and Josh Rood for help with collecting hemlock material, Dale Smith for technical advice and Christine Holtz for help with statistical analysis.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by K. Klimaszewska.
Rights and permissions
About this article
Cite this article
Merkle, S.A., Montello, P.M., Reece, H.M. et al. Somatic embryogenesis and cryostorage of eastern hemlock and Carolina hemlock for conservation and restoration. Trees 28, 1767–1776 (2014). https://doi.org/10.1007/s00468-014-1084-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-014-1084-0