Abstract
Adult conifers are notoriously recalcitrant in vegetative propagation and micropropagation that would result in the regeneration of juvenile propagules through somatic embryogenesis (SE) has not been demonstrated to date. Because SE-derived material is more amenable in subsequent tissue culture experiments compared with seed-derived material, a multi-year study was conducted to investigate induction of SE from primordial shoot (PS) explants that were excised from shoot buds of somatic embryo-derived white spruce. The SE induction experiments were carried out first with greenhouse-grown and later with field-grown trees each year from 2002 (2-year-old) to 2010 (10-year-old). Of the four genotypes tested, 893-2 and 893-12 never responded, 893-1 responded up to year 4 and 893-6 consistently responded every year. In 2010, for the first time, three of the 17 893-6 clonal trees produced male strobili as well as SE from cultured PS explants. SE induction was associated with formation of a nodule on the surface of an elongated needle primordium or in callus. Early somatic embryos were detectable after about 3 weeks of culture. Of 11 genes whose expression profiles were followed during the PS cultures, CHAP3A, VP1, WOX2 and SAP2C were expressed exclusively in the early stages of SE, and could potentially be used as markers of embryogenecity. Mature somatic embryos and plants were produced from the explants of responding genotype. Implication of these results for future research on adult conifer recalcitrance in micropropagation is discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Somatic embryogenesis (SE) has revolutionized conifer tissue culture and has become a method not only for mass propagation, but also a stepping stone for the development of other biotechnologies leading to new products in forestry. Modern forest management relies on extensive breeding and reforestation programs to support the sustainability of forest productivity and conservation of natural forests. Thus, plantation forestry, with increased forest productivity, is likely to become the major source of wood products (Tzfira et al. 1998; Fenning et al. 2008). The advantage of vegetative propagation is that it allows large genetic gains to be achieved by capturing a large proportion of tree genetic diversity in a single selection cycle (Park 2002). Vegetative propagation of superior coniferous forest trees through SE has the potential to deliver a stable supply of superior seedlings for forest plantations.
Tree growth is characterized by more or less distinct phases that are recognized as embryonic phase, post-embryonic juvenile vegetative phase (free growth), the adult vegetative phase (the shoots grow from preformed buds), and the adult reproductive phase (the trees produce female and male strobili) (Poethig 1990; Greenwood 1995). In many conifer species, SE based on immature or mature seed embryos has been routinely used for the production of clonal trees, however, explants taken from adult trees either at the vegetative or reproductive phase of growth have proven to be recalcitrant (Bonga et al. 2010).
True rejuvenation of adult trees through SE, and production of somatic seedlings, could be compared with the recapitulation of the tree ontogeny, with the unprecedented benefit of allowing mass propagation. Indeed, such a technology could be applied to species and/or population conservation and restoration, as has been proposed for mature American chestnut trees that are resistant to blight fungus (Andrade and Merkle 2005).
Maturation in woody perennials is a developmental process associated with decreased growth rates, increased plagiotropism, changes in branching characteristics and foliar morphology, and the appearance of reproductive organs (Haffner et al. 1991; Greenwood 1995). These morphological changes are accompanied by numerous physiological and biochemical changes, such as reduced expression of the cab gene (Hutchison et al. 1990), differences in chlorophyll content and leaf xylem morphology, changes in phytohormone ratios, carbohydrates and carbon metabolism, polyamines and peroxidase activities, in addition to accumulation of phenolic compounds and anthocyanins (Bauer and Bauer 1980; Greenwood 1984; Haffner et al. 1991). Most notable is the inability of cuttings to root, and hence the inability to conduct vegetative propagation (Bonga 1985). Whether maturation is established at the level of individual cells of a shoot meristem or the entire meristem is not known (Hackett 1985). Nevertheless, the expression of maturation is displayed through changes in the activity of the apical meristems (Borchert 1976), and the reversal of these changes is particularly difficult in conifers (Greenwood 1995; Bonga et al. 2010). Attempts to achieve rejuvenation of adult trees through grafting or serial micrografting of buds from adult trees onto juvenile rootstock or through in vitro regeneration of adventitious shoots have not been entirely satisfactory and indicated that rejuvenation had not occurred (reviewed by Bonga and von Aderkas 1992). One notable exception was reported by Ewald and Kretzschmar (1996) who succeeded in rejuvenating old Larix decidua by first performing micrografting of its meristems onto aseptically grown seedlings in vitro followed by micropropagation of the grafted shoots.
In this study, we used clonal white spruce trees of four genotypes that were regenerated through SE induced from seed embryos. Our aim was to investigate whether SE could be induced from primordial shoot (PS) explants of these trees collected over several consecutive years (from 2002 to 2010). Clonal trees were grown in the field and their shoot buds were collected for in vitro culture each year at different dates and developmental stages. SE was initially induced from two genotypes, and later consistently from one genotype, which in 2010 was 10 years and produced male strobili. Potential markers of early SE were identified by expression profiling of 11 genes through absolute quantitative real-time PCR before and during the culture of PS explants.
Materials and methods
Production of somatic white spruce for field planting
Initiation and proliferation of SE
Somatic embryogenesis of white spruce (Picea glauca (Moench) Voss) was induced from embryos excised from stored seeds (lot # C9612893, CFS-LFC, Quebec, Canada) in July 2000. The medium formulation used for all stages of SE was according to Litvay et al. (1985) modified by Klimaszewska et al. (2001). The medium was supplemented with 2% (w/v) sucrose, 1 g l−1 Bacto™ casamino acids (Becton, Dickinson and Co., Sparks, MD) and 0.5 g l−1 l-glutamine (Sigma), the latter filter sterilized and added to sterile medium, both were adjusted to pH 5.8 before sterilization. This modified formulation depicted as MLV PGR free was solidified with 0.4% gellan gum (Phytagel™, Sigma). 2,4-dichlorophenoxyacetic acid (2,4-d) at 9.5 μM and 6-benzyladenine (BA) at 4.5 μM (depicted as MLV-S) were added for both initiation of SE and proliferation of embryonal mass (EM). EM pieces were subcultured onto fresh medium every 2 weeks. Physical culture conditions were darkness and approximately 24°C.
Maturation of somatic embryos
For 10 days before maturation, EM of four genotypes 893-1, 893-2, 893-6 and 893-12 was cultured on a filter paper disc (Whatman #2, 7 cm) placed on the surface of MLV-S in 9 × 1.5 cm Petri dish as described in Klimaszewska et al. (2001). For maturation, six samples of 100 mg fresh mass of EM were collected from filter papers and each suspended by vigorous shaking in 4 ml of MLV PGR-free liquid medium. The suspension was poured over a filter paper disc in a Büchner funnel and the liquid completely drained. Subsequently, filter paper with dispersed EM was put on MLV medium with 6% sucrose, 60 μM abscisic acid (racemic, Sigma) and solidified with 0.6% gellan gum (Klimaszewska et al. 2001). Cultures were kept in dim light (5 μmol m−2 s−1), 16-h photoperiod (Cool White, fluorescence tubes, 55 W, Sylvania F72T12) at approximately 24°C for 7–8 weeks.
Germination of somatic embryos
The mature somatic embryos were germinated in December 2000, in Petri dishes (9 × 1.5 cm) containing MLV plant growth regulator-free medium with 2% sucrose and 0.6% gellan gum according to Klimaszewska et al. (2001). The somatic seedlings were subcultured once after 6–8 weeks onto fresh medium (of the same composition), but in taller Petri dishes (9 × 2.0 cm) and cultured until transfer to a potting mix (see below). Hence, the ontogenic age of the somatic trees is calculated from December 2000.
Growth in a greenhouse
In March 2001, the somatic plantlets (approximately 50 clones per each of the four genotypes) were transferred from Petri dishes to peat:perlite:vermiculite (1:1:1 v/v) mix and grown in a mist chamber under a 16-h photoperiod (Lumalux High Pressure Sodium Lamps, 400 W, #LU400/ECO, Sylvania) at 20 μmol m−2 s−1, at 22–24°C day and 18°C night. The relative humidity was maintained at 85–90% for the first 4 days and then gradually lowered to the ambient level over the next 8 days. The plantlets were sprayed with a 0.46 g l−1 solution of 11-41-8 N-P-K (Plant Products Co. Ltd., Brampton, ON, Canada) daily. After 12 days, the plantlets were placed in the greenhouse at the same conditions as above except for a higher light intensity, 102 μmol m−2 s−1, and fertilized with a solution of 25% of N-P-K 20-20-20 and 75% of 20-8-20.
In November 2001, the photoperiod and day/night temperatures were gradually reduced over a period of 7 weeks to 8 h and 5°C to promote the first bud dormancy. In January 2002, the somatic trees were placed in a dark, cold chamber (2°C) for 6 weeks. Subsequently, the plants were returned to a greenhouse where the dormant buds flushed in March 2002 and developed new shoots. In March 2003, the somatic trees were transferred first to a nursery in Valcartier, QC, Canada and grown under natural conditions. In September 2003, clonal trees were planted in a plantation (Valcartier, QC, Canada) according to the configuration in Fig. 1. There were 20, 9, 19, and 10 clonal trees of 893-1, 893-2, 893-6, and 893-12 genotype, respectively. Every June, the trees were fertilized with 11.2 N, 8.1 P, 14.1 K and 2.9 Mg slow release fertilizer (SynAgri, Canada).
Shoot bud collection from somatic trees grown in a greenhouse in 2002 and 2003 (2–3 years)
Lateral buds were collected from entire trees. Attention was paid during shoot bud detachment from the branch to include a small amount of the underlying lignified tissue. Shoot buds were disinfected (see below) and used in the experiments immediately.
Shoot bud collection from somatic trees grown in plantation from 2004 to 2009 (4–9 years)
Lateral buds were removed from all trees but only from the top second and third whorl of branches and up to three-fourth of the length from the tip of a branch. The shoot buds from each genotype were placed in the 15-ml screw-top centrifuge tube and transported in a cooler with ice packs. Attention was paid during shoot bud detachment from the branch to include a small amount of the underlying lignified tissue. All collected shoot buds were used in experimentation the same day and/or the next day.
Shoot bud collection from somatic trees grown in plantation in 2010 (10 years)
In the 2010 experiments, each clonal tree of two genotypes (893-6 and 893-12) was marked with a letter. Shoot buds were collected like in the previous years except that they were separated into two groups: apical together with subapical and lateral. Subsequently, each group of shoot buds collected from each clonal tree was kept, disinfected and cultured separately. The shoot bud collection was done between 21 April and 1 May.
Surface disinfection of shoot buds and excision of primordial shoots (PS)
For surface disinfection, the buds had their few basal scales removed to facilitate disinfection, placed in 50 ml non-sterile centrifuge tubes (maximum 30 buds per tube) and then were washed two or three times in tap water with a small amount of the surfactant Tween 20 (T-20) by shaking. Each wash lasted for 2–3 min. After several brief rinses in tap water, the buds were disinfected in the same tubes with 70% ethanol (v/v) for 3 min immediately followed by a 12-min treatment with 10% (v/v) hydrogen peroxide with a small amount of T-20. During the treatment, the tubes were placed horizontally on an orbital shaker at 100 rpm or shaken. The disinfecting solution was discarded and the buds were rinsed two times in sterile distilled water, and a third time in 100 mg l−1 polyvinylpyrrolidone (PVP, Sigma) solution, then drained and placed in a Petri dish on a double layer filter paper disc soaked with PVP solution to protect the buds from drying and minimizing oxidation of polyphenols (commonly recognized as tissue browning). Under a stereomicroscope, the buds were cut lengthwise (Fig. 2b) and the two median sections of a PS were excised (Fig. 2c). Depending on the size of the PS, each section could be further cut lengthwise resulting in a maximum of four sections (explants) per PS. The explants were cultured on a semi-solid MLV-S medium (described above) that in the preliminary experiments on media survey (data not shown) proved to be suitable for SE induction from PS. Sections (two, three or four) of four PS in total were cultured per Petri dish (9 × 1.5 cm). The sections were placed with their cut surfaces in contact with the medium.
The cultures were frequently inspected for contamination and initiation of SE for up to 12 weeks. Bacterial and fungal contamination frequencies ranged from 0 to 50% depending on the genotype and experiment. Only those PS sections that were not contaminated after 12 weeks of culture were counted for the total and final number of PS cultured.
Plant regeneration from PS explants
Plants were regenerated using the same protocols as developed for seed-based SE in white spruce (see above).
Expression profiling of 11 genes during PS explant culture
In 2006, samples (80 mg fresh mass) of freshly collected shoot buds and PS explants collected randomly from three to four Petri dishes after 3 and 6 days of culture were immediately frozen in liquid nitrogen. Later, samples of specific tissue type, such as needles with nodules, callus with nodules, EM, and callus (NET, non-embryogenic tissue) were also collected. The samples were then stored at −80°C until used for RNA extraction with RNeasy Plant Mini Kit (Qiagen Sciences, Valencia, CA, USA). The extracts were treated with DNAse (Invitrogen). RNA was reverse transcribed using oligo dT and Superscript II (Invitrogen) at a concentration of 100 ng total RNA per microlitre, using the manufacturer’s recommended reaction conditions except that no RNase H treatment was performed. Following 10× dilution in 10 mM Tris to a final concentration of 10 ng total RNA per microlitre, aliquots of the reverse transcriptase reaction were stored at −20°C. Real-time qPCR was carried out according to Rutledge and Stewart (2008, 2010), using the primers presented in Table 1. These included primers for two reference genes, peroxisomal targeting signal receptor (PTSR) and hypothetical protein (HP) (Friedmann et al. 2007), to which data were normalized. All transcript quantities were expressed as the number of transcripts per 5 ng of total RNA.
Results
Induction of SE from PS explants of greenhouse and field-grown somatic trees collected in the spring (2002–2010)
Of the four genotypes, PS explants of 893-2 and 893-12 never responded, those of 893-1 responded up to age of 4 years and those of 893-6 consistently initiated SE, irrespective of the age of the somatic trees they were collected (Table 2). The number of responding PS explants varied among experiments, but SE lines were initiated from 893-6 trees every year. However, because the collected shoot buds, from individual clonal trees, were pooled together (until 2010), it was not known if all or only some clonal trees responded particularly in experiments where the SE frequency was low. Therefore, in 2010 (Fig. 2a), we cultured separately PS from each of the 17 (893-6) individual clonal trees of 19 available because 2 clones (B and R) were severely damaged by abundant snowfall during the winter of 2005. In addition, the buds were separated according to their position on a branch into apical together with subapical and lateral bud groups. Of the 17 clonal trees, 5 clones J, K, P, D, and E initiated SE from both groups of PS. Hence, the results were combined for presentation in Table 2. Upon close observation of the trees in the plantation, we found that among the responding clonal trees J, K, and P had also produced one to three male strobili from which shedding of mature pollen was visible, signalling initiation of phase change. The SE responding trees were scattered across the plantation and there was no discernible pattern in their specific locations that could indicate site (Fig. 1) or collection time influence (21 April to 1 May). Of the five clones, P showed the highest number of responding PS (82%) followed by D, J, K and E in descending order. Overall, the SE was produced by 32% of all cultured PS from responding clones. However, over 1,080 cultured PS collected from 17 clonal trees the SE induction was 7.5%.
Induction of SE from PS explants collected in the summer and fall (2009)
In 2009, in addition to experiments involving shoot buds collected in the spring (May), we tested PS explants of the 893-6 (responding) and 893-12 (non-responding) genotypes excised from buds collected in August, September and October. The goal of these experiments was to determine whether the early stages during formation of winter (dormant) buds would show more plasticity in their response in tissue culture and if certain genotypes favoured other specific primordial shoot developmental stages for SE initiation. In these experiments SE was initiated from 893-6 PS collected on each date, whereas 893-12 did not respond at any date (Table 2). These embryogenic lines were proliferated and mature somatic embryos were obtained using the standard white spruce protocol.
Origin of SE in PS explants
The PS response followed a sequence of events, each marked by morphological changes in the explants: after the first 5 days of culture, all explants displayed elongation of the green needle primordia (Fig. 2d), which continued for the next several days and was accompanied by thickening and a slight callusing of some needle bases. At the same time, a small amount of callus appeared at the basal part of the PS explant and along the cut surface of the PS stem. After 2 weeks, the amount of callus increased and some explants started to become brown, whereas others remained green or slightly chlorotic. On some needles, conspicuous and easily identifiable round, mostly translucent nodules were formed that were firmly attached to the needle (Figs. 3a, b, 4a). The nodules were also detected in the callus (Fig. 3c). A close examination of the nodules formed on the needles and in callus revealed that with time some of them started to form an elongation zone (composed of elongated cells) at the site of their attachments to the explant that resulted in the nodules that protruded above the needle or callus surface (Fig. 4b, c). It was possible to separate a few of these protruding nodules, with the attached tail of elongated cells (Fig. 3d).
a Nodule formed on a needle primordium. b Squashed and magnified nodule from photo (a). c Slightly elongated nodule formed in a callus produced by PS explant. d Microscopic view of a nodule subtended by elongated cells and early somatic embryos differentiated from cells of the elongated zone. n nodule, ez elongation zone, se somatic embryo. Bar 600 μm (a), 200 μm (b), 1 mm (c) or 360 μm
Time lapse photography of a PS explant of 893-6 genotype (10 years) after a 35, b 42 c 48 days, and d 2 months of culture showing stages of somatic embryogenesis induction from nodules formed on a needle primordium. b–d The same part of PS explant as indicated on a. Yellow arrowheads indicate the same nodule. Bar 1.4 mm (a), 0.7 mm (b), 0.9 mm (c, d)
Between weeks 2 and 3, on a few explants, the first early somatic embryos could be distinguished along with nodules at various stages of elongation. Microscopic observations of the nodules and their squashes revealed that the early somatic embryos were clearly derived from the cells of the nodule elongated zone (Fig. 3d), or alternatively from cells of a nodule through an intermediate stage of elongation. These early somatic embryos grew rapidly and could be easily distinguished from the callus by its characteristic phenotype identical to that derived from seed embryos (Figs. 4d, 5a, b). Upon subculture to a fresh medium, EM proliferated rapidly and a large amount of material could be obtained for plant production experiments.
a Primordial shoot cultures of 893-6 showing a mixture of nodules and early somatic embryos. b Microscopic phenotype of early somatic embryos. c Mature somatic embryos after 7 weeks of culture. d Somatic plants photographed in June 2010 (1.7 years), regenerated from PS from 2008 collections. Bar 2.5 mm (a), 170 μm (b), 4 mm (c) or 7 cm (d)
Production of plants
Somatic plants were regenerated from mature somatic embryos (Fig. 5c) that were produced by PS collected in 2008, and are being grown in the nursery. The plants displayed a juvenile, seedling-like morphology and growth pattern after their establishment in the greenhouse. During the next growing season, the buds flushed and initiated a normal, non-sexual, vegetative growth phase (Fig. 5d shows 1.7-year-old plants). The plants are being grown in the nursery under natural conditions.
Expression profiles of 11 genes in primordial shoots before and during tissue culture
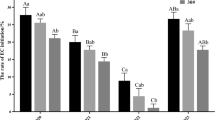
Culture of shoot explants resulted in production of callus only or callus and EM. We have chosen to follow expression profiles of 11 genes including transcription factors, which are known for their role in regulating the development of meristems and embryogenesis. Genes that were expressed in primordial shoots before culture were IAA2, SKN1, SKN2, SKN3, SKN4, SERK1, SAP2C and APL2 (Fig. 6). Of these, only three genes were expressed in callus, IAA2 and SKN3 were up-regulated 20- and 9.5-fold, respectively, whereas SERK1 was down-regulated fourfold compared with explants before culture. On the other hand, expression profiles for EM differed strikingly from that of callus because not only did it express IAA2, SKN1, SKN2, SKN3, SKN4 and SERK1, but also a different set of genes, namely CHAP3A, WOX2, SAP2C and VP1. The latter set of genes was not detectable in any other type of tissue or expressed at a much lower level, such as for SAP2C. When compared with non-cultured fresh shoots, the ones cultured for 3 and 6 days expressed IAA2 and SAP2C higher, whereas SKN1, SKN2, SKN3, SKN4, SERK1 and AP2-L2 were lower. Needles with nodules and callus with nodules, separated for the analysis from 3 to 4-week-old cultures, also expressed the transcription factors associated with EM (CHAP3A, WOX2, SAP2C and VP1) but at lower orders of magnitude. These results show that the expression of CHAP3A, WOX2, SAP2C and VP1 distinguishes EM from callus and other types of tissue present in the PS before and during culture.
Gene expression profiles in 893-6 PS before and during culture on SE induction medium. E Bud PS before culture, Bud 3d and Bud 6d PS cultured for 3 and 6 days, respectively, NwN needles with nodules, CwN callus with nodules, ET embryogenic tissue, NET non-embryogenic tissue (callus). Transcripts (copy number) are per 5 ng of total RNA
Discussion
Adult conifers are notorious for being recalcitrant in vegetative propagation and micropropagation, and true rejuvenation through organogenesis has not been achieved to date except for larch (Ewald and Kretzschmar 1996; reviewed by von Aderkas and Bonga 2000; Bonga et al. 2010). Potentially, SE is the most desirable micropropagation method for conifers owing to its efficiency and long-term cryogenic storage; however, to date the oldest tree that SE was reported for was a 3-year-old somatic Norway spruce (Harvengt et al. 2001). The potential benefits of propagating adult conifers through SE and the availability of a number of clonal somatic trees of white spruce prompted us to launch this multi-year study on induction of SE from PS explants excised from vegetative buds.
Repeated SE response from PS explants of somatic white spruce (2–10 years)
This multi-year study showed that not only did the shoot buds of 893-6 consistently respond during the last 8 years of growth when collected in the spring (coming out of dormancy), but also when collected in late summer and early fall (before becoming dormant). Therefore, no specific developmental stage of a bud was required for SE to occur as long as the entire primordial shoot was confined within the bud scales. Although we have not investigated to a great extent the SE potential of developed young needles from flushed buds, we did occasionally see SE occurring from the base of a needle (data not shown). Moreover, in 2010, clonal trees that produced male strobili also initiated SE from shoot explants, hence challenging the present concept that the onset of reproductive organs precludes any possibility of regenerating juvenile propagules of a conifer.
Regeneration potential of some plant species can be enhanced by the use of tissue culture-derived material, namely plants that were regenerated in vitro. In Norway spruce, 1-year old trees regenerated through SE showed a higher SE response in vitro when compared with plants of similar age, but of zygotic origin (Ruaud et al. 1992). The nature of this difference is not known, but one hypothesis could be that during the initial SE process, the capacity for SE becomes firmly fixed, and this fixation is primarily epigenetic. Gene expression is regulated by various factors, with DNA methylation being considered one of the chief regulators of the differential transcription (Finnegan et al. 1998). It has been also postulated that exposure to auxin raises methylation levels, and this in turn may stimulate cell division and differentiation leading to organ formation or SE (von Aderkas and Bonga 2000). Further research targeting methylation status of select key genes utilizing the identified white spruce genotypes could shed some light on the nature of differential response in vitro.
Genotype dependence for induction of SE from explants of woody perennials has been demonstrated in a number of other studies including angiosperms, such as Quercus robur (San-José et al. 2010), sweetgum (Merkle and Battle 2000), and Coffea canephora (Priyono et al. 2010). In the latter species, the authors compared a Congolese and Guinean group of plants and identified six quantitative trait loci (QTL) that were responsible for differential SE ability in leaf explant cultures. Conifers have not been a subject of extensive research for identifying QTLs correlated to regeneration capability, except for a few studies indicating that SE induction from seed embryos has a genetic background that is under a strong maternal effect (Park et al. 1993; MacKay et al. 2006).
To understand the molecular basis for the regenerative capability of the super-embryogenic line of Medicago truncatula, Imin et al. (2008) conducted a genome-wide transcriptional analysis during early tissue culture, which included its wild progenitor that is characterized by a low ability for SE. The study revealed essential differences between the two lines not only in many aspects of biochemical pathways but also in their response to auxin and cytokinin. These results may facilitate understanding of regulatory and metabolic networks involved in gaining totipotency and regeneration of somatic embryos.
SE proceeds from meristematic nodules
The first indication of SE induction in shoot explants of white spruce was the formation of a nodule, or multiple nodules either along needle primordium or embedded in callus formed on the cut surfaces. Some of these nodules either necrotized with time, or produced at the site of attachment to the explant, files of elongated cells that gave the nodule an early somatic embryo appearance. Hence, the pattern of SE induction is the same as that described for somatic seedlings of spruce species (Lelu and Bornman 1990; Mo and von Arnold 1991) and hybrid firs Salajová and Salaj (2001) where the nodule/somatic embryo formation was linked to meristematic cell centres created by periclinal and anticlinal cell divisions in the epidermal and subepidermal layers of cotyledons.
SE proliferation commenced usually from nodules adjacent to the medium, most likely because of direct access to nutrients and PGRs, whereas nodules formed on the upper surfaces of explants often dried out. SE initiation from nodules has been also described by von Aderkas et al. (2005) and Park et al. (2010) in Pinus strobus, P. banksiana and P. contorta, which were produced during culture of immature zygotic embryos. However, the nodules of P. strobus and P. banksiana could also directly develop into cotyledonary somatic embryos, although only those of P. strobus germinated. It is possible that because the pine nodules were formed in cultures of immature embryos, they might have been developmentally arrested somatic embryos, whereas in our study, the nodules of white spruce originated from cells of needle primordia or stem callus through formation of meristematic centres. Clearly, a detailed study comparing a “long cell tail” subtended nodule of white spruce with a similar sized and morphology somatic embryo is required to properly characterize the former and determine its potential to directly produce a plant. Equally intriguing are the signals that trigger activation of the elongation and anticlinal divisions of the nodule cells at the site attached to the explant, which results in a mimicry of early SE.
Gene expression profiles before and during the culture of PS explants
In an attempt to identify marker genes that could discriminate between callus, which is abundantly produced in PS culture of white spruce, and EM, which is produced in the first stage of SE, we followed the expression pattern of 11 genes before and during in vitro culture. The genes were chosen based on their sequence availability in conifer gene banks and a known role in regulating meristem and embryogenesis patterning. Ultimately, we would like to know which of these genes could signal the embryogenic nature of a culture even in the absence of any evident early somatic embryos, such as when embryogenic cells are mixed with predominantly callus cells. Our results showed that the transcription factors WOX2, CHAP3A, SAP2C and VP1 are potential markers of SE induction that allow EM to be distinguished from callus and other types of tissue present in cultured shoot buds. Our results corroborated those obtained for PaWOX2 and PcWOX2, which showed high expression in cultures with early stages of SE but were not detected in callus of Norway spruce (Palovaara and Hakman 2008) and lodgepole pine (Park et al. 2010). Similarly, CHAP3A (black spruce LEC1 homolog) transcripts were high in white spruce EM but not detected in callus. However, this is contrary to the findings in P. contorta (Park et al. 2010) and P. strobus (Klimaszewska, unpublished data) in which calli of these two pine species also expressed the LEC1 homolog, suggesting this gene may not be a reliable marker of SE induction in all conifer species.
Strong expression in somatic embryogenic lines of a Picea abies VP1 homolog described by Footitt et al. (2003) was confirmed in white spruce, although this gene was down-regulated during somatic embryo maturation. The same gene was further evaluated by Fischerova et al. (2008) in Norway spruce line that was characterized by a large number of early embryo structures and in a line that lacked any differentiated structure. High PaVP1 expression was found in the former line and no expression in the latter, consistent with our results with white spruce embryogenic line versus callus.
SAP2C, a spruce homolog of the Brassica napus Baby Boom that is expressed during microspore embryogenesis (Boutilier et al. 2002), appears to have a similar role in white spruce SE, as indicated by its high level of expression in EM in comparison with other tissue types. In contrast, AP2-L2 (Vahala et al. 2001) expression became progressively reduced in cultured tissue, with near undetectable expression in EM and callus. Similarly, SERK1 expression, which plays a key role in the acquisition of embryogenic competence (Schmidt et al. 1997) and which is highly expressed in embryogenic callus of grapevine (Schellenbaum et al. 2008) was over twofold lower in all tissues in culture, including EM, as compared with fresh shoot buds. When consistent with auxin induction in P. taeda (Goldfarb et al. 2003), IAA2 was found to be expressed most highly in cultured tissues, although there is no apparent difference between embryogenic and non-embryogenic tissues.
SKN1-4 which are spruce homologs of the KNOX I gene family (Guillet-Claude et al. 2004; Rutledge unpublished data), which play a central role in meristem development and maintenance in angiosperms (Hake et al. 2004; Hay and Tsiantis 2009) were found to have a diverse pattern of expression. While SKN1 and SKN2, which show similar expression patterns are most highly expressed in EM and nearly undetectable in callus, SKN3 is expressed most highly in callus with a 50-fold lower level expression in EM. In contrast, SKN4 expression was greatly reduced in all tissues in culture, with nearly undetectable levels in both EM and callus. Although functional differences between these SKN genes have yet to be demonstrated, these expression patterns suggest that the genes could be broadly divided into three function groups: SKN1/2, SKN3 and SKN4.
In conclusion, our results suggest that when working with non-domesticated conifers at the adult vegetative phase, it is necessary to screen/survey a number of genotypes in tissue culture to identify the ones with the ability for SE. While this type of experiment with adult conifers seemed to be futile in the past due to the universal inability to obtain SE from adult conifers, it is not clear if the lack of success was due to the recalcitrant genotype (most often only one or a few tested in a specific study) or to the unsuitable tissue culture medium/conditions. Now that we have identified a highly responsive somatic tree genotype, along with a suitable medium to support SE induction from PS of the adult vegetative phase and partial reproductive phase white spruce, continued experimentation throughout the life cycle of this genotype has become plausible. In particular, this provides a unique opportunity to assess the current hypothesis that the lack of SE potential in an adult, reproductive phase (mature) conifer is caused by biochemical and molecular modifications associated with phase change. For the first time in conifer tissue culture history, this provides the opportunity to resolve the assumed influence of phase change associated molecular events, from genotypic derived recalcitrance, the latter known to play a decisive role in the tissue culture response. Moreover, clonal somatic trees of various ages could also be utilized to study molecular changes that underpin phase change.
Abbreviations
- BA:
-
6-Benzyladenine
- 2,4-D:
-
2,4-Dichlorophenoxyacetic acid
- EM:
-
Embryonal mass
- HP:
-
Hypothetical protein
- LEC1:
-
LEAFY COTYLEDON1
- PS:
-
Primordial shoot
- PTSR:
-
Peroxisomal targeting signal receptor
- PVP:
-
Polyvinylpyrrolidone
- Qpcr:
-
Quantitative polymerase chain reaction
- SE:
-
Somatic embryogenesis
- SERK1:
-
Somatic embryogenesis receptor-like kinase 1
- WOX2:
-
WUSCHEL
References
Andrade GM, Merkle SA (2005) Enhancement of American chestnut somatic seedling production. Plant Cell Rep 24:326–334
Bauer H, Bauer U (1980) Photosynthesis in leaves of the juvenile and adult phase of ivy (Hedera helix). Physiol Plant 49:366–372
Bonga JM (1985) Vegetative propagation in relation to juvenility, maturity and rejuvenation. In: Bonga JM, Durzan DJ (eds) Tissue culture in forestry. Martinus Nijhoff/Dr W. Junk Publishers, Dordrecht, pp 387–412
Bonga JM, von Aderkas P (1992) In vitro culture of trees. Kluwer, Dordrecht
Bonga JM, Klimaszewska K, von Aderakas P (2010) Recalcitrance in clonal propagation, in particular of conifers. Plant Cell Tissue Organ Cult 100:241–254
Borchert R (1976) Differences in shoot growth patterns between juvenile and adult trees and their interpretation based on systems analysis of trees. Acta Hort 56:123–130
Boutilier K, Offringa R, Sharma VK et al (2002) Ectopic expression of BABY BOOM triggers a conversion from vegetative to embryonic growth. Plant Cell 14:1737–1749
Ewald D, Kretzschmar U (1996) The influence of micrografting in vitro on tissue culture behavior and vegetative propagation of old European larch trees. Plant Cell Tissue Organ Cult 44:249–252
Fenning TM, Walter C, Gartland KMA (2008) Forest biotech and climate change. Nat Biotechnol 26:615–617
Finnegan EJ, Genger RK, Peacock WJ, Dennis ES (1998) DNA methylation in plants. Annu Rev Plant Physiol Plant Mol Biol 49:223–247
Fischerova L, Fischer L, Vondrakova Z, Vagner M (2008) Expression of the gene encoding transcription factor PaVP1 differs in Picea abies embryogenic lines depending on their ability to develop somatic embryos. Plant Cell Rep 27:435–441
Footitt S, Ingouff M, Clapham D, von Arnold S (2003) Expression of viviparous 1 (Pavp1) and p34cdc2 protein kinase (cdc2Pa) genes during somatic embryogenesis in Norway spruce (Picea abies [L.] Karst). J Exp Bot 54:1711–1719
Friedmann M, Ralph SG, Aeschliman D, Zhuang J, Ritland K, Ellis BE, Bohlmann J, Douglas CJ (2007) Microarray gene expression profiling of developmental transitions in Sitka spruce (Picea sitchensis) apical shoots. J Exp Bot 58:593–614
Goldfarb B, Lanz-Garcia C, Lian Z, Whetten R (2003) Aux/IAA gene family is conserved in the gymnosperm, loblolly pine (Pinus taeda). Tree Physiol 23:1181–1192
Greenwood MS (1984) Phase change in loblolly pine: shoot developments as a function of age. Physiol Plant 61:518–522
Greenwood MS (1995) Juvenility and maturation in conifers: current concepts. Tree Physiol 15:433–438
Guillet-Claude C, Isabel N, Pelgas B, Bousquet J (2004) The evolutionary implications of knox-I gene duplications in conifers: correlated evidence from phylogeny, gene mapping, and analysis of functional divergence. Mol Biol Evol 21:2232–2245
Hackett WP (1985) Juvenility, maturation and rejuvenation in woody plants. Hort Rev 7:109–155
Haffner V, Enjalric F, Lardet L, Carron MP (1991) Maturation of woody plants: a review of metabolic and genomic aspects. Ann Sci For 48:615–630
Hake S, Smith HMS, Holtan H, Magnani E, Mele G, Ramirez J (2004) The role of KNOX genes in plant development. Anu Rev Cell Dev Biol 20:125–151
Harvengt L, Trontin JF, Reymond I, Canlet F, Pâques M (2001) Molecular evidence of true-to-type propagation of a 3-year-old Norway spruce through somatic embryogenesis. Planta 213:828–832
Hay A, Tsiantis M (2009) A KNOX family TALE. Curr Opin Plant Biol 12:593–598
Hutchison KW, Sherman CD, Weber J, Smith SS, Singer PB, Greenwood MS (1990) Maturation in larch: II. Effects of age on photosynthesis and gene expression in developing foliage. Plant Physiol 94:1308–1315
Imin N, Goffard N, Nizamidin M, Rolfe BG (2008) Genome-wide transcriptional analysis of super-embryogenic Medicago truncatula explant cultures. BMC Plant Biol 8:110. doi:10.1186/1471-2229-8-110
Klimaszewska K, Lachance D, Pelletier G, Lelu MA, Seguin A (2001) Regeneration of transgenic Picea glauca, P. mariana and P. abies after cocultivation of embryogenic tissue with Agrobacterium tumefaciens. In Vitro Cell Dev Biol Plant 37:748–755
Lelu M-A, Bornman CH (1990) Induction of somatic embryogenesis in excised cotyledons of Picea glauca and Picea mariana. Plant Physiol Biochem 28:785–791
Litvay JD, Verma DC, Johnson MA (1985) Influence of a loblolly pine (Pinus taeda L.) culture medium and its components on growth and somatic embryogenesis of the wild carrot (Daucus carota L.). Plant Cell Rep 4:325–328
MacKay JJ, Becwar MR, Park YS et al (2006) Genetic control of somatic embryogenesis initiation in loblolly pine and implications for breeding. Tree Genet Genomes 2:1–9
Merkle SA, Battle PJ (2000) Enhancement of embryogenic culture initiation from tissues of mature sweetgum trees. Plant Cell Rep 19:268–273
Mo LH, von Arnold S (1991) Origin of development of embryogenic cultures from seedlings of Norway spruce (Picea abies). J Plant Physiol 138:223–230
Palovaara J, Hakman I (2008) Conifer WOX-related homeodomain transcription factors, developmental consideration and expression dynamic of WOX2 during Picea abies somatic embryogenesis. Plant Mol Biol 66:533–549
Park YS (2002) Implementation of conifer somatic embryogenesis in clonal forestry: technical requirements and deployment considerations. Ann For Sci 59:651–656
Park YS, Pond SE, Bonga JM (1993) Initiation of somatic embryogenesis in white spruce (Picea glauca): genetic control, culture treatment effects, and implications for tree breeding. Theor Appl Genet 86:427–436
Park SY, Klimaszewska K, Park JY, Mansfield SD (2010) Lodgepole pine: first evidence of seed based somatic embryogenesis and the expression of embryogenesis marker genes in shoot bud cultures of adult trees. Tree Physiol 30:1469–1478
Poethig RS (1990) Phase change and the regulation of shoot morphogenesis in plants. Science 250:923–930
Priyono BF, Rigoreau M et al (2010) Somatic embryogenesis and vegetative cutting capacity are under distinct genetic control in Coffea canephora Pierre. Plant Cell Rep 29:343–357
Ruaud JN, Bercetche J, Pâques M (1992) First evidence of somatic embryogenesis from needles of 1-year-old Picea abies plants. Plant Cell Rep 11:563–566
Rutledge RG, Stewart D (2008) A kinetic-based sigmoidal model for the polymerase chain reaction and its application to high-capacity absolute quantitative real-time PCR. BMC Biotechnol 8:47
Rutledge RG, Stewart D (2010) Assessing the performance capabilities of LRE-based assays for absolute quantitative real-time PCR. PLoS ONE 5:e9731
Salajová T, Salaj J (2001) Somatic embryogenesis and plantlet regeneration from cotyledon explants isolated from emblings and seedlings of hybrid firs. J Plant Physiol 158:747–755
San-José MC, Corredoira E, Martínez MT, Vidal N, Valladares S, Mallón R, Vieitez AM (2010) Shoot apex explants for induction of somatic embryogenesis in mature Quercus robur L. trees. Plant Cell Rep 29:661–671
Schellenbaum P, Jacques A, Maillot P, Bertsch C et al (2008) Characterization of VvSERK1, VvSERK2, VvSERK3 and VvL1L genes and their expression during somatic embryogenesis of grapevine (Vitis vinifera L.). Plant Cell Rep 27:1799–1809
Schmidt EDL, Guzzo F, Toonen MAJ, De Vries SC (1997) A leucine-rich repeat containing receptor-like kinase marks somatic plant cells competent to form embryos. Development 124:2049–2062
Tzfira T, Zuker A, Altman A (1998) Forest-tree biotechnology: genetic transformation and its application to future forests. Trends Biotechnol 16:439–446
Vahala T, Oxelman B, von Arnold S (2001) Two APETALA2-like genes of Picea abies are differentially expressed during development. J Exp Bot 52:1111–1115
von Aderkas P, Bonga JM (2000) Influencing micropropagation and somatic embryogenesis in mature trees by manipulation phase change, stress and culture environment. Tree Physiol 20:921–928
von Aderkas P, Coulter A, White L, Wagner R, Robb J, Rise M, Temmel N, MacEacheron I, Park YS, Bonga J (2005) Somatic embryogenesis via nodules in Pinus strobus L. and Pinus banksiana Lamb.—Dead ends and new beginnings. Prop Ornamental Plants 5:3–13
Acknowledgments
We thank Dr. J. Bonga (Natural Resources Canada, Canadian Forest Service, Atlantic Forestry Centre) for his critical reading of the manuscript and valuable suggestions. Ms. P. Cheers (CFS-Laurentian Forestry Centre) is gratefully acknowledged for English editing. This work was financially supported by the Natural Resources Canada, Canadian Forest Service.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Klimaszewska, K., Overton, C., Stewart, D. et al. Initiation of somatic embryos and regeneration of plants from primordial shoots of 10-year-old somatic white spruce and expression profiles of 11 genes followed during the tissue culture process. Planta 233, 635–647 (2011). https://doi.org/10.1007/s00425-010-1325-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-010-1325-4