Abstract
Clusia minor L. is a C3-CAM species in which Crassulacean acid Metabolism (CAM) is induced, among other factors, by water deficit. We propose that CAM induction by natural drought in C. minor shifts the sap flow pattern from daytime to a night-time one, and that the decreased osmotic potential due to increased malate content in droughted plants aids in the increase in nocturnal sap flow. In order to test these hypotheses, we followed for 2 years the seasonal changes in parameters of water relationships and sap flow velocity in one single, freestanding tree growing in Caracas. Leaf water and osmotic potential were measured psychrometrically, nocturnal proton accumulation by titration of aqueous leaf extracts and sap flow density with thermal dissipation probes. Leaf water, osmotic and turgor potential remained relatively high throughout the seasons. Nocturnal proton accumulation was nil under extreme drought or after frequent and heavy rains, and high after moderate rainfall. Estimated malate and citrate concentrations contributed up to 80 and 60%, respectively, of the value of osmotic potential. The shape of the daily courses of sap flow velocity varied seasonally, from mostly diurnal during the dry season to mostly nocturnal after a short dry spell during the rainy season, when nocturnal acid accumulation attained high values. There was a strong positive relationship between the proportion of the integrated sap flow courses corresponding to the night and dawn [H+] (r 2 = 0.88). Increased nocturnal sap flow in the CAM stage of the tree of C. minor may be explained by a lower osmotic potential due to an increased acid concentration, together with increased stomatal aperture, as suggested by increased nocturnal acid accumulation probably due to nocturnal CO2 fixation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The occurrence of Crassulacean acid Metabolism (CAM) in the genus Clusia (Clusiaceae) is well documented; Clusia is the only dicotyledonous tree genus known to possess CAM and C3-CAM species (Lüttge 2006).
Plants of C. minor are evergreen small freestanding trees, hemi-epiphytes or strangler holo-epiphytes; they live in a broad range of environments, from forests to savannas, and from the forest floor to tree branches (Lüttge 2006). In C. minor CO2 fixation is best characterised as C3-CAM; watered plants of C. minor show nil nocturnal acid accumulation (ΔH+), while a short period of drought is capable of inducing the appearance of nocturnal CO2 fixation with concomitantly high values of ΔH+ (Schmitt et al. 1988). CAM is extremely plastic in this species; in one single branch, leaves subjected to drier air performed CAM, whereas the opposite leaves, under humid air, did not (Schmitt et al. 1988). The shift between C3 photosynthesis and CAM is rapid and plastic under both field and controlled conditions (Borland et al. 1996; Haag-Kerwer et al. 1996; Schmitt et al. 1988; Mattos et al. 1999; Mattos and Lüttge 2001).
In plants of the C3-CAM hemi-epiphyte C. uvitana growing on a Ceiba tree, the seasonal variation in leaf water potential (ψ), leaf osmotic potential (ψ s) and turgor potential (ψ T) was small and little influenced by water availability or evaporative demand; ΔH+ was high under mild drought and nil after prolonged drought or rains (Zotz and Winter 1994).
It is currently accepted that CAM is an adaptation to dry environments that helps the plant attain higher water use efficiency than C3 or C4 plants (Winter 1985). CAM may not only serve as a water-saving adaptation but also as a water-collecting mechanism. In droughted plants of the CAM species Senecio medley-woodii, water uptake was correlated with an increase in morning osmotic pressure and malate content (Ruess and Eller 1985; Ruess et al. 1988); also, the accumulation of malate in the vacuole of Kalanchoe daigremontiana caused a reduction in ψ s, which may favour water absorption (Smith and Lüttge 1985).
Not only malate but also large amounts of citrate accumulate in C. minor and citrate accumulation has been suggested to act as a protection mechanism against light or water stress (Franco et al. 1992).
Daily courses of gas exchange in C. minor under moderate irradiance and low leaf-air water vapour gradient showed the four phases of CAM defined by Osmond (1978), with 24-h CO2 uptake and open stomata (Lee et al. 1989; Franco et al. 1990; Lüttge 2006). Changes in magnitude and daily pattern of sap flow velocity (V) reflect changes in transpiration, provided sap flow is driven by transpiration rather than replenishment of reservoirs. In trees of tropical C3 woody species, sap flow continued through the night and was accompanied by substantial stomatal conductance (Bucci et al. 2005). Therefore, we can expect to evidence the four phases of CAM in daily courses of V.
Since in plants of C. minor with CAM-induced stomatal aperture occurs mostly during all phases but III (Franco et al. 1990, 1991, 1992; Borland et al. 1996; Zotz et al. 1997; Mattos et al. 1999), sap flow must be faster during the night and the first morning hours than during the day. Nevertheless, up to 4% of daily carbon balance has been found in the field to occur during phase IV (Ball et al. 1991). In addition, acids accumulated during the night would cause a decrease in ψ s; therefore, the magnitude of the soil–plant–atmosphere water potential gradient in plants with CAM induced would favour water absorption during the night and on phase II. The CO2 uptake and acid content in C. minor has been shown to be high on phase II for as late into the daytime as 12 h.
We propose that CAM induction by natural drought in C. minor shifts the sap flow pattern from daytime to a nighttime one, and that the decreased ψ s due to increased organic acid content in droughted plants aids in the increase in nocturnal sap flow. In order to test these hypotheses, we followed for 2 years the seasonal changes in water relations and ΔH+ and for 1 year the changes in V in one single tree growing in the garden in Caracas.
Materials and methods
Study site and plant material
One freestanding individual of C. minor L., approximately 3-m-high with a 13-cm diameter at breast height, growing in the gardens of the Instituto de Biología Experimental (IBE) in Caracas (10°30′N–66°55′E) at an altitude of 1,000 m, was sampled from March 2003 until August 2004, covering two dry and two rainy seasons. Daily values of rainfall in Caracas were obtained from the Ciudad Universitaria weather station (Universidad Central de Venezuela, Caracas), at approximately 4 km from the IBE. The tree of C. minor was not watered throughout the period of study. Air RH, air temperature and total radiation (TR) were collected by a weather station mod. Vantage Pro (Davies Instruments, Hayward, CA, USA) at IBE. The crown of the tree of C. minor received an average of 20% of TR incident on the taller trees of Ficus obtusifolia and Hura crepitans that shaded the tree of C. minor; leaves of C. minor in the upper canopy received direct sunshine for a small portion of the day.
Water relations
Morning ψ was measured at 06.30 h placing one leaf disk (n = 6, different leaves every time) in each of six C52 chambers previously calibrated with NaCl solutions of known osmolality and connected to an HR33T psychrometer (Wescor Inc., Lincoln, UT, USA); measurements were made at 25 ± 1°C after a 30-min equilibration time. The ψ s was determined likewise in the same disks frozen in liquid nitrogen. Leaf water content (LWC) was determined in leaf disks (n = 6) collected between 06.30 and 07.00 h and between 17.00 and 18.00 h, weighed fresh and dried at 60°C for 72 h.
Nocturnal proton accumulation
Leaf disks to a total area of 2 cm2 (n = 6, different leaves) were collected at dawn (06.00 h) and dusk (18.30 h), weighed and placed in 10-ml plastic syringes maintained in liquid nitrogen until measurements were made. Syringes were placed in 50-ml plastic tubes and centrifuged for 5 min at 8,000 rpm in a centrifuge mod. Multex (MSE, Crawley, UK). The residue in the syringe was rinsed into the tube with distilled water to a final volume of approximately 20 ml; protons were titrated with 10 mM KOH to pH 7.0. The ΔH+ was calculated as the difference between dawn and dusk values.
Sap flow velocity
Sap flow velocity was measured during 2004 with Granier-type thermal dissipation probes (Granier, 1987) mod. TDP-30 (30-mm long) connected to a DL2e data logger (Dynamax Inc., Houston, TX, USA). Two holes 5-cm vertically apart were drilled using a portable drill into the trunk after removing the cortex. Once the probes were inserted in the holes a small piece of art eraser (Faber-Castell, Buenos Aires, Argentina) was used to ensure an adequate seal; probes were secured to the trunk with polystyrene foam half-spheres and duct tape (3M, Buenos Aires, Argentina) and covered with bubble plastic sheets and windshield aluminium foil protectors. On each date, five TDPs were inserted in the trunk at a height of 1.5 m above ground; records were taken for a minimum of 1 week. The output in mV from the probes was converted to °C (dT) by dividing the voltage by 0.04. From the empirical equation of Granier (1987) sap flow velocity was calculated as V = 104 × 0.0119 × K 1.231, where K = (dTm − dT)/dT, with dT the temperature difference between the downstream and the upstream thermocouples in the probe and dTm the maximum obtained, when sap flow is assumed to be nil. Voltage was adjusted to give an average dTm of approximately 8°C. One representative record per season is presented.
Transpiration rate
Transpiration rate (E) during 2004 was calculated as the difference in LWC (morning-afternoon) divided by 12 h.
Statistics
Values are mean ± one SE. Regressions were done using Sigmaplot 7.0 at P < 0.05.
Results
The seasonal changes during 2003–2004 in physiological parameters of the tree of C. minor are shown and compared to daily rainfall in Fig. 1. The ψ and ψ s remained relatively high throughout the seasons, maximum decreases amounting to 0.64 and 0.66 MPa, respectively (Fig. 1a, b). Turgor potential varied between 0.10 and 0.42 MPa and increased almost immediately with rainfall on certain dates, as in mid-April 2003, mid-May 2003 and mid-March 2004; LWC varied no more than 15% throughout the season (Fig. 1c). Dawn as well as dusk H+ content changed seasonally, dusk values responding rapidly to rainfall (Fig. 1d). The ΔH+ increased with moderate rainfall in April and June 2003, and March and July 2004, and decreased with either drought or heavy and/or frequent rains, as in August 2003 and the end of May to mid-June 2004 (Fig. 1e).
Seasonal changes during 2003 and 2004 in a tree of Clusia minor growing in the gardens in: a leaf water potential; b leaf osmotic potential; c turgor potential (closed circles) and leaf water content (open circles); d dawn (open circles) and dusk (closed circles) H+ content; e nocturnal acid accumulation. Values are mean ± one SE (n = 6). f daily rainfall
Noon atmospheric water-vapour saturation deficit (Δw) was twice as high during the dry season and the beginning of rains as during the rainy season (Fig. 2).
Nocturnal acid accumulation decreased with dusk H+ content (r 2 = 0.32). Low correlations were found between the cumulative rainfall for the previous seven or 15 days and ΔH+, ψ, ψ s or ψ T (r 2 < 0.2 in all cases, P < 0.05). Similarly, coefficients for the correlations between ψ, ψ s, ψ T and dawn H+ content, dusk H+ content or ΔH+ were all ≤0.12 (P < 0.05).
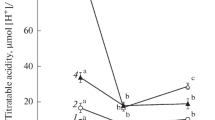
Malate and citrate concentrations were estimated from the dawn H+ content and the corresponding LWC, using the proportion of 1.3 malate:one citrate reported by Franco et al. (1990) for C. minor. The contribution to ψ s of malic and citric acid increased linearly with dawn H+ content (r 2 = 0.70) and explained up to 32 and 24%, respectively, of the variation in ψ s (Fig. 3).
Correlation between dawn H+ content in leaves of Clusia minor and the proportion of leaf osmotic potential contributed by a malate and b citrate. Acid concentration was calculated using the proportion 1.3 malate: one citrate reported by Franco et al. (1990). Values are individual data points measured in 2003–2004. The determination coefficient is r 2 = 0.48. Dotted lines, confidence intervals (P < 0.05)
In Fig. 4 are shown daily courses of V representative of all those recorded. Maximum V was lowest in May, followed by February and highest in July and March. The shape of the daily course varied seasonally, from mostly diurnal during the dry season to mostly nocturnal after a short dry spell during the rainy season, when nocturnal acid accumulation attained high values.
Changes during 2004 in the daily courses of sap flow velocity (dots) in a tree of Clusia minor growing in the garden and total radiation (open circles) on the indicated dates. Values are individual data points recorded at half-hourly intervals. The numbers inserted in each panel are values of nocturnal H+ accumulation (μmol cm−2, mean ± one SE, 6 ≤ n ≤ 12)
The relationship between the integrated nocturnal sap flow and ψ was poor (r 2 = 0.19), whereas a strong positive correlation was found between the proportion of total sap flow corresponding to the night and [H+] (Fig. 5).
Transpiration rate was solely diurnal until the beginning of the rainy season and solely nocturnal during the rainy season (Table 1).
Discussion
We have measured for the first time in C. minor the seasonal changes due to water availability in parameters of water relations together with xylem sap flow. Changes in ΔH+ were apparently governed by rainfall, although in an indirect manner. The shape of the daily course of sap flow changed with rainfall and obeyed changes in dawn H+ content.
Changes in ψ, ψ s and ψ T with changes in water supply were small, as previously shown in trees of C. uvitana growing in Barro Colorado Island, Panama throughout the dry and the rainy seasons, and between a drier and a wetter environment (Zotz and Winter 1993, 1994) . Similarly, in the present study ψ T reached values as low as 0.1 MPa only after prolonged dry periods. Such maintenance of leaf water status may owe, among other factors, to the succulent nature of leaves of CAM Clusia species. In C. minor, LWC was similar to values reported by Zotz et al. (1997) and varied less than 15% throughout the seasons; the leaf of C. minor has a hypodermis (Borland et al. 1996) that may contribute to the maintenance of leaf water and hence, of ψ T.
During the dry season/beginning of rains, ψ T increased rapidly in response to showers fallen the previous days, suggesting that water absorption was very efficient. Runoff from the crown and water absorption by the adventitious roots, which are abundant in this tree, may have rapidly improved leaf water status. This assumption is supported by the observation that hydraulic conductivity in C. uvitana was found to be higher in aerial roots than in stems (Zotz et al. 1994).
Values of ΔH+ were similar to those previously reported for C. minor at medium PPFD (Franco et al. 1992). The ΔH+ changed rapidly with rainfall, varying from high values during the dry season to a decrease at the end of the dry season (March-April), an increase with rains at the beginning of the rainy season, and a decrease after heavy rainfall during the rainy season. A similar trend of ΔH+ with rainfall was observed in C. uvitana, with large values occurring after periods of moderate rains and small values after either prolonged periods of drought or several months of rain (Zotz and Winter 1994).
In this study, when ΔH+ was nil under severe drought, both dawn and dusk [H+] were small but when it was nil after heavy rains, high values of both dawn and dusk [H+] content were measured, in concordance with reports in C. uvitana (Zotz and Winter 1993, 1994). The ΔH+ decreased with dusk [H+], as reported by Zotz and Winter (1993), but not in such good correlation as theirs, suggesting that decarboxylation was not always completed. The occurrence of high [H+] well into the morning and as late as 14.00 h during phase III due to delayed decarboxylation reported by Roberts et al. (1997, 1998), but not determined in the present work, may also help water absorption during this part of the photoperiod.
Assuming that part of the [H+] measured in the present study corresponds to citrate, high dawn and dusk [H+], together with low ΔH+ values may have resulted from a large citrate accumulation with little daily fluctuation, as suggested by Franco et al. (1992).
Maximum V, whether diurnal or nocturnal, was a fourth of the value in the range reported for 31 tree species, mostly tropical and all C3, with stem diameters of 15 cm (Meinzer 2003); such difference between C. minor and these tropical species is to be expected, given the low values of E measured and calculated by us and of stomatal conductance reported for C. minor (Franco et al. 1990, 1991, 1992; Mattos et al. 1999, Mattos and Lüttge 2001).
The shape of the daily courses of V in C. minor differed from those for gas exchange previously reported (Roberts et al. 1997, 1998) in that an increase in V during phase II was found only in May at the end of the dry season. Nevertheless, V was always high in the part of the light period corresponding to phase IV, in agreement with high photosynthetic rates during this phase (Roberts et al. 1997, 1998). During phases II to III a significant proportion of sap flow may have been due to refilling of reservoirs, rather than transpiration, whereas sap flow during phase IV was most probably due to stomatal opening. Such lag between sap flow and transpiration was found in hemi-epiphytic trees of C. minor and C. uvitana (Zotz et al. 1997). The observation that in July, with high ΔH+ values and presumably high rate of CO2 fixation on phase II, nocturnal sap flow peaked at around midnight to decrease towards dawn supports the hypothesis that a large proportion of this flow was due to transpiration.
Daily patterns of V changed seasonally, in concordance with E. In savanna trees, nighttime transpiration amounting up to 28% of daytime values has been reported (Bucci et al. 2005). Daily courses of E could aid in clarifying whether changes in V obeyed transpiration, rather than refilling, if it were not for the observation that in the same seedling of C. minor under controlled conditions one leaf may be performing CAM while the opposite may be performing C3 CO2 fixation (Franco et al. 1992). This situation would be exaggerated in the tree crown, where measurements of leaf E in individual leaves may not reflect whole crown transpiration. Stomatal conductance may be strongly decoupled from canopy transpiration due to a large boundary layer, making comparisons between leaf transpiration and whole-tree transpiration incorrect (Wullschleger et al. 1998).
The daily pattern of V changed in a consistent way with ΔH+, shifting from a mainly diurnal pattern during phase III of CAM with low ΔH+ to a mainly nocturnal one during phase I with high ΔH+. Similarly, in the hemi-epiphyte C. uvitana, 36% of daily sap flow, measured by heat dissipation probes in aerial roots, occurred during the night (Zotz et al. 1997) and, as drought progressed, CO2 fixation shifted from mostly diurnal to mostly nocturnal (Winter et al. 1992).
Maximum V was relatively independent of TR, except for the dry season in February, when V was half the maximum recorded, and the beginning of rains in May, when it was less than a third. In the first case, the tree had received no water during the previous 2 months and it may have been under severe stress aided by a high Δw, thus decreasing its leaf conductance and sap flow. An increase in Δw has been shown to reduce leaf gas exchange in leaves of C. minor (Schmitt et al. 1988). In the second case, the appearance in May of new leaves, with probably lower gas-exchange rate, as shown in young leaves of the same species (Borland et al. 1996), may have reduced whole crown transpiration despite a lower Δw.
The relative contribution of nocturnal sap flow to total daily sap flow was linearly related to [H+], in a manner consistent with results in S. medley-woodii, in which nocturnal water uptake was related to malate accumulation (Ruess et al. 1988). A nocturnal decrease of up to 2.2 MPa in leaf sap ψ s calculated from data of ΔH+ and free sugar contents in C. minor (Lüttge 2007) suggests that water absorption is favoured by CAM during phase II. Daily courses of ψ s, acid concentration and V should help elucidate the role of acids on osmotic adjustment and sap flow through the daily cycle.
The relationship between nocturnal sap flow and [H+] suggests nocturnal CO2 fixation and stomatal opening. This, together with changes in ΔH+, allows us to conclude that carbon fixation mode in leaves of C. minor shifted from CAM idling with very low ΔH+ after a prolonged drought to CAM with high values of ΔH+ under moderate rainfall, to C3 fixation after heavy and/or frequent rains with low or nil values of ΔH+, as found previously under field as well as greenhouse conditions (Lüttge 2006). The operation of CAM apparently favoured water absorption through nocturnal stomatal opening, lowering of ψ s and increase in the soil–plant–atmosphere ψ gradient.
References
Ball E, Hann J, Kluge M, Lee HSJ, Lüttge U, Orthen B, Popp M, Schmitt A, Ting IP (1991) Ecophysiological comportment of the tropical CAM-tree Clusia in the field. II. Modes of photosynthesis in trees and seedlings. New Phytol 117:473–481
Borland AM, Griffiths H, Maxwell C, Fordham MC, Broadmeadow MSJ (1996) CAM induction in Clusia minor L. during the transition from wet to dry season in Trinidad: the role of organic acid speciation and decarboxylation. Plant Cell Environ 19:655–664
Bucci SJ, Goldstein G, Meinzer FC, Franco AC, Campanello P, Scholtz FG (2005) Mechanisms contributing to seasonal homeostasis of minimum leaf water potential and predawn disequilibrium between soil and plant water potential in Neotropical savanna trees. Trees 19:296–304
Franco AC, Ball E, Lüttge U (1990) Patterns of gas exchange and organic acid oscillation in tropical trees of the genus Clusia. Oecologia 85:108–114
Franco AC, Ball E, Lüttge U (1991) The influence of nitrogen, light and water stress on CO2 exchange and organic acid accumulation in the tropical C3-CAM tree, Clusia minor. J Exp Bot 42:597–603
Franco AC, Ball E, Lüttge U (1992) Differential effects of drought and light levels on accumulation of citric and malic acids during CAM in Clusia. Plant Cell Environ 15:821–829
Granier A (1987) Evaluation of transpiration in a Douglas-fir stand by means of sap flux measurements. Tree Physiol 3:309–320
Haag-Kerwer A, Grams TEE, Olivares E, Ball E, Arndt S, Popp M, Medina E, Lüttge U (1996) Comparative measurements of gas-exchange, acid accumulation and chlorophyll a fluorescence of different species of Clusia showing C3 photosynthesis, or crassulacean acid metabolism, at the same field site in Venezuela. New Phytol 134:215–226
Lee HSJ, Schmitt AK, Lüttge U (1989) The response of the C3-CAM tree Clusia rosea to light and water stress. II. Internal CO2 concentration and water use efficiency. J Exp Bot 40:171–179
Lüttge U (2006) Photosynthetic flexibility and ecophysiological plasticity: questions and lessons from Clusia, the only CAM tree, in the neotropics. New Phytol 171:7–25
Lüttge U (2007) Clusia. A woody neotropical genus of remarkable plasticity and diversity. Springer, Heidelberg
Mattos EA, Lüttge U (2001) Chlorophyll fluorescence and organic acid oscillations during transition from CAM to C3-photosynthesis in Clusia minor L. (Clusiaceae). Ann Bot 88:457–463
Mattos EA, Herzog B, Lüttge U (1999) Chlorophyll fluorescence during CAM-phases in Clusia minor L. under drought stress. J Exp Bot 50:253–261
Meinzer FC (2003) Functional convergence in plant responses to the environment. Oecologia 134:1–11
Osmond CB (1978) Crassulacean acid metabolism: a curiosity in context. Annu Rev Plant Physiol 29:379–414
Roberts A, Borland AM, Griffiths H (1997) Discrimination processes and shifts in carboxylation during the phases of crassulacean acid metabolism. Plant Physiol 113:1283–1292
Roberts A, Borland AM, Maxwell K, Griffiths H (1998) Ecophysiology of the C3-CAM intermediate Clusia minor L. in Trinidad: seasonal and short-term photosynthetic characteristics of sun and shade leaves. J Exp Bot 49:1563–1573
Ruess BR, Eller BM (1985) The correlation between crassulacean acid metabolism and water uptake in Senecio medley-woodii. Planta 166:57–66
Ruess BR, Ferrari S, Eller BM (1988) Water economy and photosynthesis of the CAM plant Senecio medley-woodii during increasing drought. Plant Cell Environ 11:583–589
Schmitt AK, Lee HSJ, Lüttge U (1988) The response of the C3-CAM tree, Clusia rosea, to light and water stress. I. Gas exchange characteristics. J Exp Bot 39:1581–1590
Smith JAC, Lüttge U (1985) Day-night changes in leaf water relations associated with the rhythm of crassulacean acid metabolism in Kalanchoë daigremontiana. Planta 163:272–282
Winter K (1985) Crassulacean acid metabolism. In: Barber J, Baker NR (eds) Photosynthetic mechanisms and the environment. Elsevier, Amsterdam, pp 329–387
Winter K, Zotz G, Baur B, Dietz KJ (1992) Light and dark CO2 fixation in Clusia uvitana and the effects of plant water status and CO2 availability. Oecologia 91:47–51
Wullschleger SD, Meinzer FC, Vertessy RA (1998) A review of whole-plant water use studies in trees. Tree Physiol 18:499–512
Zotz G, Winter K (1993) Short-term regulations of crassulacean acid metabolism activity in a tropical hemiepiphyte, Clusia uvitana. Plant Physiol 102:835–841
Zotz G, Winter K (1994) A one-year study on carbon, water and nutrient relationships in a tropical C3-CAM hemi-epiphyte, Clusia uvitana Pittier. New Phytol 127:45–60
Zotz G, Tyree MT, Cochard H (1994) Hydraulic architecture, water relations and vulnerability to cavitation of Clusia uvitana Pittier: a C3-CAM tropical hemiepiphyte. New Phytol 127:287–295
Zotz G, Patiño S, Tyree MT (1997) Water relations and hydraulic architecture of woody hemiepiphytes. J Exp Bot 48:1825–1833
Acknowledgments
This work was partly funded by FONACIT grant S1-200000023. Rainfall data from the Ciudad Universitaria weather station were provided by Judith Hernández, Ingeniería Hidrometeorológica, Facultad de Ingeniería, Universidad Central de Venezuela. Total radiation, relative humidity and air temperature data were provided by the Arboretum Experimental Station, Instituto de Biología Experimental.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by U. Lüttge.
Rights and permissions
About this article
Cite this article
Herrera, A., Ballestrini, C. & Tezara, W. Nocturnal sap flow in the C3-CAM species, Clusia minor . Trees 22, 491–497 (2008). https://doi.org/10.1007/s00468-008-0209-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-008-0209-8