Abstract
Mimosa tenuiflora and Piptadenia stipulacea are commonly accepted as drought-tolerant species but little is known about their response to drought followed by rehydration. Therefore, the interplay between leaf water potential and osmotic adjustment on photosynthetic and growth parameters of these species was examined. A greenhouse study was conducted in a split-plot design with two water conditions in the main plots (control; drought followed by rehydration), and eight sampling times in the subplots (1, 4 and 7 days of drought, and 1, 3, 6, 12, and 17 days of rehydration). Plant water status and biochemical changes were assessed as well as leaf gas exchange and subsequent growth. Under drought stress, both species maintained a low leaf water potential throughout the day by accumulating compatible solutes, thus allowing a rapid and full recovery of water status when rehydrated. Although these plants minimized water loss by closing their stomata, neither showed stomatal limitations to photosynthesis. The inhibition of this process during drought was possibly related to mesophyll limitations as well as to a reversible downregulation of photosystems, along with adjustments of their stoichiometry. Water deficits also triggered morphological adaptations at the whole plant level, leading to reduced growth, mainly of the shoots in M. tenuiflora and the roots in P. stipulacea.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Drought is one of the main factors causing tree mortality and forest decline, thus altering the carbon balance of terrestrial ecosystems (Choat et al. 2018; Serra-Maluquer et al. 2018; Jiao et al. 2021). Therefore, further information on the ability of trees to survive low water availability is of paramount importance to predict changes in carbon cycling (Santiago et al. 2016; Pritzkow et al. 2020). Drought stress triggers physiological, biochemical and molecular alterations in plants (Ashraf and Harris 2013). Unfavourable water status induces turgor loss and consequently reduces cell growth (Fox et al. 2018). Furthermore, it usually promotes stomatal closure as well as non-stomatal limitations to photosynthesis, inhibiting carbon assimilation (Gomes et al. 2008; Duan et al. 2020; Antezana-Vera and Marenco 2021). Under field conditions, drought may also lead to several other abiotic stresses such as light, temperature and nutrient stress.
Similar to what is observed in other tropical dry forests, drought events are frequent in the Brazilian Caatinga (Sampaio 1995). The annual rainfall ranges from 250 to 750 mm and is distributed over three to four months, followed by a dry season that lasts the rest of the year (Silva et al. 2010; Barros et al. 2020). In addition to low water availability, Caatinga plants face high irradiance and temperatures, making water deficits a common phenomenon (Silva et al. 2010; Dombroski et al. 2014), especially because the potential evapotranspiration exceeds 1500 mm a−1 (Sampaio 1995). Nevertheless, water deficits will intensify in the following years, with impacts on flora characteristics (Frosi et al. 2016). In fact, Campos et al. (2020) reported an increase in tree mortality from 2009 to 2019, along with a decrease in biomass production of a Caatinga fragment.
Mimosa tenuiflora (Willd.) Poir. (Mimosaceae) and Piptadenia stipulacea (Benth.) Ducke (Mimosaceae) are woody species widely distributed in tropical dry forests from Mexico to Brazil. These pioneer species play a key role in the ecological succession of the Caatinga dry forest vegetation (Alves and Freire 2019; Barros et al. 2019). However, it is unclear how they cope with the extreme conditions of this semi-arid habitat, particularly low and erratic rainfall (Sampaio 1995). To thrive in such environment, species depend on the ability to withstand droughts and on the capacity to recover (Gallé et al. 2007; Xu et al. 2010). This is especially important when considering the occurrence of short but frequent drought events. Yet there is far more information available on drought stress than on stress recovery, although the latter may determine survival (Santiago et al. 2016; Choat et al. 2018).
Despite being regarded as drought-tolerant little is known about the responses of M. tenuiflora and P. stipulacea to drought followed by rehydration. Lima and Meiado (2018) assessed the effect of hydration and dehydration cycles on the germination of M. tenuiflora and concluded that seedlings benefited from a discontinuity in the imbibition process, showing increased shoot heights, stem diameters and total dry weight. Alves and Freire (2019) evaluated the physiological response of one-year-old seedlings to water deficits and rewatering and found that drought-induced changes in relative water content and gas exchange parameters were normalized within three days of rehydration. As for P. stipulacea, apart from germination tests, there are few studies on biomass production and allocation as affected by drought (Barros et al. 2019; Campos et al. 2020), with no information on biochemical and physiological responses of this species nor on its recovery from drought stress.
Drought-triggered tree mortality can result in vegetation shifts with unknown environmental consequences (Serra-Maluquer et al. 2018; Thammanu et al. 2021). There is a wide variation in the response of plants to this stress as well as in their recovery dynamics on rewatering (Yordanov et al. 2000; Taiz et al. 2015; Niinemets 2016). Understanding these intrinsic characteristics may be useful in the implementation of management practices to ensure the resilience of tropical dry forests under climate change conditions (Xu et al. 2010; Serra-Maluquer et al. 2018; Stan et al. 2021). To elucidate some of the mechanisms behind the drought tolerance of M. tenuiflora and P. stipulacea, the interplay between leaf water potential and osmotic adjustment on photosynthetic and growth parameters of these plants was studied by evaluating their responses to drought followed by rehydration.
Material and methods
Plant material and experimental conditions
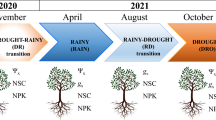
A greenhouse study was conducted in Mossoró, Brazil (5° 12′ 16″ S, 37° 19′ 29″ W) where M. tenuiflora and P. stipulacea were grown in polyethylene bags (1.9 L). A Thermo Recorder TR-72U (T&D Corporation, Matsumoto, Nagano, Japan) monitored air temperature and relative humidity, which had average values of 28.8 °C and 62.4%, respectively. Two independent experiments, one for each species, were performed in a split-plot design with two water conditions in the main plots (1 – control and 2 – drought followed by rehydration) and eight sampling dates in the subplots (1, 4 and 7 days of drought, and 1, 3, 6, 12, and 17 days of rehydration). Treatments were laid out in a randomized complete block design, replicated 10 times, with eight bags per experimental unit.
M. tenuiflora and P. stipulacea seeds were immersed in hot water at 100 °C for 4 min to break dormancy (Benedito et al. 2017, 2019), sown in a substrate of topsoil (Table S1) mixed with 200 mg P2O5 dm−3 and subjected to five top dressings (40, 50, 60, 70 and 80 days after sowing), totalling 500 mg N dm−3 and 250 mg K2O dm−3. Thinning was carried out 21 days after sowing to one seedling per bag. The plants were watered to field capacity in the early mornings and late afternoons for six months. Half were then subjected to the treatment shown in Fig. 1. While control plants were kept well-watered, drought was imposed by suspending irrigation for seven days, causing net photosynthesis of both species to be almost completely suppressed (Suresh et al. 2012; Freitas et al. 2018). Drought-stressed plants were then rehydrated until their photosynthetic activity returned to control levels (Gomes et al. 2008), which took 17 days for M. tenuiflora and 12 days for P. stipulacea.
Water potential (Ψw) measurements
To assess plant water status, fully expanded leaves from the middle-third (Schimpl et al. 2019) of one plant per treatment were used for measurements with a Scholander pressure chamber (PMS Instrument Company, Albany, OR, USA). Predawn and midday Ψw (leaf water potential) were recorded immediately after excision (Karimi et al. 2015), from 4 to 6 a.m. and from 11 am to 1 pm
Biochemical assay
On the seventh day of drought, the highest stress level, newly expanded leaves of plants from five replications were collected and stored at − 18 °C for subsequent analysis of proline, soluble sugars and amino acids and photosynthetic pigments (chlorophyll a and b). To ensure the accuracy of the results, data were collected in triplicate and expressed on a dry weight basis. Proline accumulation was determined after reaction with acid-ninhydrin solution (Bates et al. 1973). Soluble sugars and amino acids were estimated using the phenol–sulphuric acid method (DuBois et al. 1956) and the ninhydrin method (Yemm et al. 1955), respectively. Chlorophyll (Chl) a and b, and their ratio, were calculated from absorbance (A) values at 645 and 663 nm following acetone extraction (Lichtenthaler 1987), where: Chl a = 12.25 × A663 − 2.79 × A645 and Chl b = 21.5 × A645 − 5.1 × A663.
Leaf gas exchange
Gas exchange parameters were measured between 9 and 10 a.m. in one newly expanded leaf of each experimental unit using a LI-6400 portable photosynthesis system (LI-COR Biosciences, Lincoln, NE, USA). After setting light to 1200 µmol m−2 s−1, CO2 concentration to 400 µmol mol−1 and flow rate to 400 µmol s−1, the following parameters were simultaneously recorded: stomatal conductance, transpiration rate, net photosynthesis, intercellular CO2 concentration and carboxylation efficiency. Net photosynthesis was used to establish the length of the drought and rehydration periods, as proposed by Freitas et al. (2018), and it was also plotted against stomatal conductance and intercellular CO2 concentration data to assess their relationships.
Growth traits
Once considered recovered from drought (after rewatering M. tenuiflora for 17 days and P. stipulacea for 12 days), all plants were irrigated to field capacity for a further two months. This allowed for the evaluation of the effects of drought stress on subsequent biomass production and allocation within the plant. Leaves, stems and roots of two seedlings per treatment were harvested and placed in a forced-air drying oven at 65 ± 2 °C for three days. Based on their dry weights, the total dry weight and the shoot/root ratio were calculated, where: shoot = leaf + stem.
Statistical analysis
Data were analysed by Student’s t-test at the 5% level, comparing control and drought stress conditions at each sampling date using the Sisvar software, version 5.6 (Federal University of Lavras, Lavras, MG, Brazil). Regression analysis was also performed when assessing the relationships between photosynthetic parameters.
Results
Predawn and midday Ψw
Leaf measurements revealed that these pioneer species had a high ability to adjust leaf water potential (Ψw) to cope with decreasing soil water availability. In general, the recorded values were naturally lower at midday than at predawn (as observed for control plants), but suspending irrigation resulted in much greater reductions over time. After seven days of drought, the Ψw of M. tenuiflora ranged from − 5.0 MPa at predawn (Fig. 2a) to − 6.3 MPa at midday (Fig. 2b). Intriguingly, the former decreased even further to − 6.0 MPa on the first day following rehydration, suggesting a lingering response to drought. It was only on the sixth sampling date that leaf Ψw returned to control levels. At maximum stress, compared with well-watered plants, there were 5.9 and 2.8-fold decreases in predawn (Fig. 2c) and midday (Fig. 2d) Ψw of P. stipulacea, respectively. Nevertheless, this species rehydrated relatively rapidly, and water status was normalized three days after rewatering.
Biochemical changes
Suspending irrigation for seven days resulted in significant accumulation of compatible solutes by augmenting the levels of proline, soluble sugars and soluble amino acids in the seedlings. The highest differences between control and treated plants were in proline content, with 30.7 and 32.6-fold increases for M. tenuiflora and P. stipulacea, respectively (Fig. 3a). Unlike free proline, the accumulation of soluble sugars (Fig. 3b) and amino acids (Fig. 3c) was proportionally higher in the former species. After the drought treatment, chlorophyll a of M. tenuiflora went from 1.12 ± 0.20 to 2.15 ± 0.87 mg g−1 (P < 0.05) and there was also a significant increase of chlorophyll a/b ratio (Fig. 3d). The other photosynthetic pigments analysed did not differ statistically and their contents were as follows (mg g−1): M. tenuiflora – chlorophyll b of 0.62 ± 0.13; P. stipulacea – chlorophyll a of 2.33 ± 0.66 and chlorophyll b of 0.39 ± 0.07.
Photosynthetic responses
The response patterns of stomatal conductance and net photosynthesis to drought were similar, suggesting that photosynthetic activity was driven by changes in stomatal aperture and perhaps limited by a low CO2 uptake. After only four days without irrigation, the stomatal conductance of M. tenuiflora seedlings decreased by 90.1% relative to the controls (Fig. 4a), which also led to a 79.3% decrease in net photosynthesis (Fig. 4b). This last parameter was almost completely suppressed within seven days of drought, requiring a 17-day period to fully recover. The performance of P. stipulacea under stress was analogous to that of M. tenuiflora except that its photosynthetic activity was completely restored by the 12th day of rehydration (Fig. 4c, d). For both species, stomatal closure resulted in a decrease in transpiration rate (Fig. S1).
Stomatal conductance (a, c) and net photosynthesis (b, d) of M. tenuiflora and P. stipulacea over time as a function of drought and rehydration; arrows indicate the beginning of the recovery period; values are means ± SD (n = 10); asterisks denote significant differences from controls (**, P < 0.01; *, P < 0.05)
Even though by the third sampling date stomatal conductance was reduced to near zero, there were increases in intercellular CO2 concentration, indicating that CO2 was not a limiting factor for photosynthesis. Moreover, the fact that at that time net photosynthesis was suppressed, despite a great availability of CO2, resulted in extremely low carboxylation efficiency values. At the highest stress level, intercellular CO2 concentration of M. tenuiflora was 76.0% higher than that of control seedlings (Fig. 5a), and its carboxylation efficiency was normalized by the 17th day of rehydration (Fig. 5b). P. stipulacea showed essentially the same responses but the increase in intercellular CO2 concentration was of 105.7% with a significant difference shortly afterwards (Fig. 5c), and it took only 12 days to recover its carboxylation efficiency (Fig. 5d).
Intercellular CO2 concentration (a, c) and carboxylation efficiency (b, d) of M. tenuiflora and P. stipulacea over time as a function of drought and rehydration; arrows indicate the beginning of the recovery period; values are means ± SD (n = 10); asterisks denote significant differences from controls (**, P < 0.01; *, P < 0.05)
As indicated previously, drought-induced stomatal closure did not have a negative effect on intercellular CO2 concentration. Nevertheless, by plotting net photosynthesis against stomatal conductance and intercellular CO2 concentration data, it was confirmed that photosynthetic activity was indeed coupled with stomatal aperture but not limited by a low CO2 influx into the sub-stomatal chamber. For both M. tenuiflora and P. stipulacea, there was a high correlation between the first two parameters, while the third was weakly and negatively correlated with the first one (data not shown). Regression analysis showed a quadratic relationship between net photosynthesis and stomatal conductance, where the photosynthetic activity increased with stomatal opening (Fig. 6a, c). On the other hand, most of the net photosynthesis occurred between 150 and 300 µmol CO2 mol−1, with basically no carbon assimilation above this optimal range (Fig. 6b, d).
Biomass production and allocation
With regards to subsequent biomass production as compared to controls, treated plants invested considerably less in leaf and stem tissues and, in the case of P. stipulacea, also in root tissues. Consequently, the total dry weight of the two species was negatively affected by approximately 30%. Furthermore, exposure to a single drought period was enough to alter biomass allocation within the plant. Although the reductions in shoot and root dry weight (the latter not significant) of M. tenuiflora were relatively equivalent, they were quite different for P. stipulacea, where the shoot dry weight of previously stressed plants was 28.9% smaller than that of well-watered seedlings, whereas the root dry weight was affected by 45.0%. Therefore, there was an increase in the shoot/root ratio, apparently favouring shoot growth at the expense of root growth (Table 1).
Discussion
One of the first responses of plants to drought stress is a decrease in leaf water potential (Ψw) (Haider et al. 2018), which allows for a rapid recovery of cell turgor and photosynthetic activity upon rewatering (Niinemets 2016; Ruehr et al. 2019). M. tenuiflora and P. stipulacea coped with the increasing water deficit by considerably reducing their Ψw throughout the day. In fact, their predawn and midday Ψw after seven days of drought were much lower than those recorded for six Caatinga trees in the dry season (Dombroski et al. 2011), as well as for other tropical species under drought stress, such as Hevea brasiliensis Müll. Arg., − 1.85 MPa at predawn (Falqueto et al. 2017), Bertholletia excelsa Bonpl., − 4.7 MPa at midday (Schimpl et al. 2019), and Erythrina velutina Willd., − 0.31 MPa at predawn and − 0.89 MPa at midday (Silva et al. 2010). However, despite showing similar responses, the rehydration of P. stipulacea was completed three days earlier than in M. tenuiflora.
The recovery of Ψw precedes, and is usually faster than, that of gas exchange parameters, occurring within hours to a few days (Ruehr et al. 2019; Duan et al. 2020). For this reason, water relations of drought-stressed trees can benefit even from short rainfall events (Dietrich and Kahmen 2019). This is particularly important in the Caatinga dry forest, where plants experience low and irregular rainfall (Sampaio 1995; Dombroski et al. 2011). The sooner the plant water status is normalized, the faster the full recovery of photosynthetic activity will be (Yordanov et al. 2000), as demonstrated in this study for P. stipulacea. To withstand droughts, plants have evolved a series of adaptive strategies. An important mechanism is the accumulation of compatible solutes like proline, soluble sugars and amino acids, which helps to maintain cellular homeostasis and promotes osmotic adjustment (Liao et al. 2018). Therefore, the equal ability of M. tenuiflora and P. stipulacea to decrease their Ψw can be explained by the biochemical changes observed in these plants at the highest stress level.
Osmotic adjustment may take up to three weeks to achieve (Spieß et al. 2012). Nevertheless, after only seven days of drought, both species showed increased proline, soluble sugar and soluble amino acid contents. Proline had by far the highest increase, presumably because of its importance both as an osmolyte and as an antioxidant (Hu et al. 2015; Khaleghi et al. 2019). Plants under drought stress must contend not only with the loss of cell turgor, but also with the overproduction of reactive oxygen species, which increases the risk of irreversible damages to major macromolecules such as lipids, proteins and carbohydrates by oxidative stress (Gallé and Feller 2007). With regards to soluble sugars, short periods of drought induce the accumulation of readily metabolisable carbohydrates (Spieß et al. 2012). Thus, the increased contents observed here may indicate an important adaptation to prepare for recovery. During drought, soluble sugars are important for osmoregulation and as signalling molecules to induce stress responses. But once no longer needed for these purposes, they can be essential to supply carbon and energy for repair and regrowth (Khaleghi et al. 2019). This could also explain the accumulation of soluble amino acids, which would support subsequent protein synthesis (Taiz et al. 2015).
Decreases in photosynthetic pigments are commonly reported in drought-stressed plants and attributed to a slow biosynthesis or a fast degradation of chlorophylls (Fox et al. 2018; Haider et al. 2018; Liao et al. 2018). Similar to our findings, there seems to be no major impact on the chlorophyll content of certain tree species (Frosi et al. 2016; Freitas et al. 2018; Duan et al. 2020), which suggests a lesser impact on their photosynthetic apparatus (Gallé et al. 2007). The increase in chlorophyll a and, consequently, in the chlorophyll a/b ratio of M. tenuiflora under stress could also indicate an adjustment of photosystem (PS) stoichiometry towards a higher PSI/PSII ratio, reducing light harvesting and avoiding photoinhibition (Liu et al. 2011). In P. stipulacea, because there was no significant change in chlorophyll levels, and net photosynthesis recovered much earlier, it is presumed that these functional units may have been downregulated but preserved during drought.
Altogether, the biochemical changes in M. tenuiflora and P. stipulacea contributed to the rapid and complete recovery of their photosynthetic activities after rehydration. Such recovery is an indicator of drought tolerance and demonstrates a high physiological plasticity (Schimpl et al. 2019), emphasizing the ability of these plants to withstand drought events. To prevent extreme water loss in leaf tissues during the stress period, both species reduced transpiration by closing their stomata, which in some circumstances affects CO2 uptake (Yordanov et al. 2000; Haider et al. 2018; Liao et al. 2018). Peguero-Pina et al. (2018) observed that the reduction in stomatal conductance of Quercus ilex L. consisted in a limitation to carbon assimilation. Although it was initially considered the possibility of drought-induced stomatal limitations to photosynthesis, it was later confirmed that this was not the case in this study, indicating exclusively the occurrence of mesophyll or biochemical limitations.
At the highest stress level, the reduction in net photosynthesis, despite increased CO2 availability, could be attributed to a low gas use efficiency of chloroplasts (Ashraf and Harris 2013). Increased resistance in the mesophyll layer could reduce CO2 diffusion to carboxylation sites (Elferjani et al. 2021). In addition, plants under increasing water deficits, if still exposed to light, are likely to experience photoinhibition (Zargar et al. 2017). Drought can lead to the degradation of the D1 protein, a key subunit of PSII, thus causing the inactivation of its reaction centre (Ashraf and Harris 2013). In this case, the photosynthetic activity of drought-sensitive plants might not be fully recovered (Ruehr et al. 2019). Conversely, Gallé and Feller (2007) reported enhanced photoprotection in Fagus sylvatica L. after a reversible downregulation of PSII accompanied by an increase in the dissipation of excess excitation energy. This helped to maintain a functional photosynthetic apparatus, allowing the complete recovery upon rewatering, as observed here for both species.
While the magnitude of the effects of stomatal and non-stomatal limitations on photosynthetic capacity cannot be easily distinguished (Ashraf and Harris 2013), we found that M. tenuiflora and P. stipulacea are mainly affected by the latter, because at no time was photosynthesis limited by low CO2 availability. Besides, there were no positive correlations between stomatal conductance and intercellular CO2 concentration (data not shown). At the highest stress level, the fact that photosynthesis was almost completely suppressed explains, in part, the increases in CO2 availability, given that this substrate was no longer being used. However, since stomatal conductance was restricted, this increase was probably due to respiration rather than a high influx of CO2. In any case, the extremely low carboxylation efficiency observed at that point could be the result of declines in mesophyll conductance and/or in the activity of ribulose-1,5-bisphosphate carboxylase/oxygenase (Zhou et al. 2014; Elferjani et al. 2021).
Based on several studies, Elferjani et al. (2021) estimated that stomatal, mesophyll and biochemical limitations accounted for 49%, 39% and 12%, respectively, of the reductions in photosynthesis of Populus spp. Nevertheless, the impacts of short-term drought stress on these proportions vary greatly among tree species (Zhou et al. 2014). Apparently, drought stress affects stomatal conductance and net photosynthesis at the same rate, leading to a high correlation between these parameters. However, the extent of stomatal closure was insufficient to limit CO2 influx into the sub-stomatal chamber, unlike that observed for another Caatinga species (Dombroski et al. 2014). Therefore, neither of the species studied here presented stomatal limitations to photosynthesis, which is in accordance with Alves and Freire (2019) for M. tenuiflora. Unfortunately, to the best of our knowledge, there are no studies on the effect of drought on photosynthesis of P. stipulacea, which highlights the need for further research.
In addition to altering photosynthetic activity, drought stress affected growth patterns. Changes in biomass production and allocation can play an important role in adaptation to future drought events (Gallé and Feller 2007; Niinemets 2016). We found that previously stressed plants invested less in shoot (M. tenuiflora and P. stipulacea) and root (P. stipulacea) growth. Thus, even a relatively short drought period of seven days can induce morphological adaptations in these species. Despite reporting a strong compensation growth upon rewatering, Spieß et al. (2012) observed that long-term drought had a negative effect on the subsequent growth of Quercus robur L., affecting mainly the shoots. Curiously, root growth in P. stipulacea is more reduced than shoot growth, as suggested by Barros et al. (2019). A meta-analysis revealed that, under drought stress, roots are not as affected as leaves and stems, and their biomass generally increases to facilitate water uptake (Eziz et al. 2017). However, this is more common for herbaceous than woody species, and may not even occur under extreme water deficit (Xu et al. 2010; Eziz et al. 2017). Moreover, there are reports on the increase of shoot/root ratios during drought (Santiago et al. 2001; Bueno et al. 2021). In contrast, Barros et al. (2020) found no differences in the shoot/root ratio of four woody species after rehydration, as shown here for M. tenuiflora.
Conclusions
The drought tolerance of Mimosa tenuiflora and Piptadenia stipulacea is associated with their ability to respond quickly and effectively to this stress. These species maintain a low leaf water potential throughout the day by accumulating compatible solutes, thus allowing a rapid and full recovery of water status upon rewatering. Nonetheless, because the rehydration of P. stipulacea occurs at a faster rate, its photosynthetic activity recovers earlier. Although both species minimize water loss by closing their stomata, neither showed stomatal limitations to photosynthesis. The inhibition of this process during drought is probably related to mesophyll limitations as well as to a reversible downregulation of photosystems, along with, in the case of M. tenuiflora, adjustments of their stoichiometry. Moreover, water deficit triggers morphological adaptations in these species, leading to reduced subsequent growth, mainly of shoots in M. tenuiflora and roots in P. stipulacea. Future studies may help to elucidate the gene expression and antioxidant enzyme activity underlying this drought tolerance.
Change history
17 June 2022
The original version is updated due to missing of the project funding details, guest editor and corresponding editor details.
References
Alves FJB, Freire ALO (2019) Gas exchange of Mimosa tenuiflora (Willd.) poiret under water deficit and rewatering. J Agric Stud 7(4):297–308
Antezana-Vera SA, Marenco RA (2021) Sap flow rates of Minquartia guianensis in central Amazonia during the prolonged dry season of 2015–2016. J for Res 32(5):2067–2076
Ashraf M, Harris PJC (2013) Photosynthesis under stressful environments: an overview. Photosynthetica 51(2):163–190
Barros JPA, Souza LSB, Silva TGF, Moura MSB, Silva LF (2019) Partitioning and modeling of biomass in caatinga legume seedlings in different water conditions. Floresta Ambient 26(4):e20180348
Barros V, Melo A, Santos M, Nogueira L, Frosi G, Santos MG (2020) Different resource-use strategies of invasive and native woody species from a seasonally dry tropical forest under drought stress and recovery. Plant Physiol Biochem 147:181–190
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39(1):205–207
Benedito CP, Ribeiro MCC, Torres SB, Guimarães IP, Oliveira KJB (2017) Overcome dormancy, temperatures and substrates on germination of Mimosa tenuiflora Willd seeds. Semin-Cienc Agrar 38(1):125–134
Benedito CP, Ribeiro MCC, Paiva EP, Medeiros HLS (2019) Dormancy overcoming and germination test in Piptadenia stipulacea (Benth.) ducke seeds. Rev Cienc Agron 50(2):338–344
Bueno A, Pritsch K, Simon J (2021) Responses of native and invasive woody seedlings to combined competition and drought are species-specific. Tree Physiol 41(3):343–357
Campos DA, Andrade EM, Castanho ADA, Feitosa RC, Palácio HQA (2020) Biomass dynamics in a fragment of Brazilian tropical forest (caatinga) over consecutive dry years. Appl Sci 10(21):7813
Choat B, Brodribb TJ, Brodersen CR, Duursma RA, Lopez R, Medlyn BE (2018) Triggers of tree mortality under drought. Nature 558:531–539
Dietrich L, Kahmen A (2019) Water relations of drought-stressed temperate trees benefit from short drought-intermitting rainfall events. Agric Meteorol 265:70–77
Dombroski JLD, Praxedes SC, Freitas RMO, Pontes FM (2011) Water relations of caatinga trees in the dry season. S Afr J Bot 77(2):430–434
Dombroski JLD, Freitas RMO, Tomczak VE, Pinto JRS, Farias RM (2014) Ecophysiology of water stressed Handroanthus impetiginosus (Mart. ex. DC) Mattos) seedlings. Sci for 42(101):155–163
Duan HL, Wang DF, Wei XH, Huang GM, Fan HB, Zhou SX, Wu JP, Liu WF, Tissue DT, Wan SZ (2020) The decoupling between gas exchange and water potential of Cinnamomum camphora seedlings during drought recovery and its relation to ABA accumulation in leaves. J Plant Ecol 13(6):683–692
DuBois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28(3):350–356
Elferjani R, Benomar L, Momayyezi M, Tognetti R, Niinemets Ü, Soolanayakanahally RY, Théroux-Rancourt G, Tosens T, Ripullone F, Bilodeau-Gauthier S, Lamhamedi MS, Calfapietra C, Lamara M (2021) A meta-analysis of mesophyll conductance to CO2 in relation to major abiotic stresses in poplar species. J Exp Bot 72(12):4384–4400
Eziz A, Yan Z, Tian D, Han WX, Tang ZY, Fang JY (2017) Drought effect on plant biomass allocation: a meta-analysis. Ecol Evol 7(24):11002–11010
Falqueto AR, Silva Júnior RA, Gomes MTG, Martins JPR, Silva DM, Partelli PL (2017) Effects of drought stress on chlorophyll a fluorescence in two rubber tree clones. Sci Hortic 224:238–243
Fox H, Doron-Faigenboim A, Kelly G, Bourstein R, Attia Z, Zhou J, Moshe Y, Moshelion M, David-Schwartz R (2018) Transcriptome analysis of Pinus halepensis under drought stress and during recovery. Tree Physiol 38(3):423–441
Freitas VMB, Scalon SPQ, Dresch DM, Bastos SS, Souza APR (2018) Influence of exogenous application of abscisic acid on gas exchanges in Hymenaea courbaril L (Fabaceae) seedlings subjected to water deficit. Floresta 48(2):163–172
Frosi G, Barros VA, Oliveira MT, Santos M, Ramos DG, Maia LC, Santos MG (2016) Symbiosis with AMF and leaf Pi supply increases water deficit tolerance of woody species from seasonal dry tropical forest. J Plant Physiol 207:84–93
Gallé A, Feller U (2007) Changes of photosynthetic traits in beech saplings (Fagus sylvatica) under severe drought stress and during recovery. Physiol Plant 131(3):412–421
Gallé A, Haldimann P, Feller U (2007) Photosynthetic performance and water relations in young pubescent oak (Quercus pubescens) trees during drought stress and recovery. New Phytol 174(4):799–810
Gomes FP, Oliva MA, Mielke MS, Almeida AAF, Leite HG, Aquino LA (2008) Photosynthetic limitations in leaves of young Brazilian Green Dwarf coconut (Cocos nucifera L ‘nana’) palm under well-watered conditions or recovering from drought stress. Environ Exp Bot 62(3):195–204
Haider MS, Kurjogi MM, Khalil-ur-Rehman M, Pervez T, Songtao J, Fiaz M, Jogaiah S, Wang C, Fang J (2018) Drought stress revealed physiological, biochemical and gene-expressional variations in Yoshihime peach (Prunus persica L) cultivar. J Plant Interact 13(1):83–90
Hu Y, Wang B, Hu TX, Chen H, Li H, Zhang W, Zhong Y, Hu HL (2015) Combined action of an antioxidant defence system and osmolytes on drought tolerance and post-drought recovery of Phoebe zhennan S Lee Saplings. Acta Physiol Plant 37(4):84
Jiao PP, Wu ZH, Wang X, Jiang ZB, Wang YQ, Liu H, Qin R, Li ZJ (2021) Short-term transcriptomic responses of Populus euphratica roots and leaves to drought stress. J for Res 32(2):841–853
Karimi S, Yadollahi A, Arzani K, Imani A, Aghaalikhani M (2015) Gas-exchange response of almond genotypes to water stress. Photosynthetica 53(1):29–34
Khaleghi A, Naderi R, Brunetti C, Maserti BE, Salami SA, Babalar M (2019) Morphological, physiochemical and antioxidant responses of Maclura pomifera to drought stress. Sci Rep 9:19250
Liao T, Wang Y, Xu CP, Li Y, Kang XY (2018) Adaptive photosynthetic and physiological responses to drought and rewatering in triploid Populus populations. Photosynthetica 56(2):578–590
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382
Lima AT, Meiado MV (2018) Effect of hydration and dehydration cycles on Mimosa tenuiflora seeds during germination and initial development. S Afr J Bot 116:164–167
Liu CC, Liu YG, Guo K, Fan DY, Li GQ, Zheng YR, Yu LF, Yang R (2011) Effect of drought on pigments, osmotic adjustment and antioxidant enzymes in six woody plant species in karst habitats of southwestern China. Environ Exp Bot 71(2):174–183
Niinemets Ü (2016) Uncovering the hidden facets of drought stress: secondary metabolites make the difference. Tree Physiol 36(2):129–132
Peguero-Pina JJ, Mendoza-Herrer Ó, Gil-Pelegrín E, Sancho-Knapik D (2018) Cavitation limits the recovery of gas exchange after severe drought stress in holm oak (Quercus ilex L.). Forests 9(8):443
Pritzkow C, Szota C, Williamson VG, Arndt SK (2020) Phenotypic plasticity of drought tolerance traits in a widespread eucalypt (Eucalyptus obliqua). Forests 11(12):1371
Ruehr NK, Grote R, Mayr S, Arneth A (2019) Beyond the extreme: recovery of carbon and water relations in woody plants following heat and drought stress. Tree Physiol 39(8):1285–1299
Sampaio EVSB (1995) Overview of the Brazilian caatinga. In: Medina E, Mooney HA, Bullock SH (eds) Seasonally dry tropical forests. Cambridge University Press, Cambridge, pp 35–63
Santiago LS, Bonal D, De Guzman ME, Ávila-Lovera E (2016) Drought survival strategies of tropical trees. In: Goldstein G, Santiago L (eds) Tropical tree physiology. Springer, Cham, pp 243–258
Santiago AMP, Nogueira RJMC, Lopes EC (2001) Crescimento em plantas jovens de Mimosa caesalpiniifolia Benth., cultivadas sob estresse hídrico. Ecossistema 26(1):23–30
Schimpl FC, Ferreira MJ, Jaquetti RK, Martins SCV, Goncalves JFC (2019) Physiological responses of young Brazil nut (Bertholletia excelsa) plants to drought stress and subsequent rewatering. Flora 252:10–17
Serra-Maluquer X, Mencuccini M, Martínez-Vilalta J (2018) Changes in tree resistance, recovery and resilience across three successive extreme droughts in the northeast Iberian Peninsula. Oecologia 187(1):343–354
Silva EC, Silva MFA, Nogueira RJMC, Albuquerque MB (2010) Growth evaluation and water relations of Erythrina velutina seedlings in response to drought stress. Braz J Plant Physiol 22(4):225–233
Spieß N, Oufir M, Matušíková I, Stierschneider M, Kopecky D, Homolka A, Burg K, Fluch S, Hausman JF, Wilhelm E (2012) Ecophysiological and transcriptomic responses of oak (Quercus robur) to long-term drought exposure and rewatering. Environ Exp Bot 77:117–126
Stan KD, Sanchez-Azofeifa A, Duran SM, Guzmán QJA, Hesketh M, Laakso K, Portillo-Quintero C, Rankine C, Doetterl S (2021) Tropical dry forest resilience and water use efficiency: an analysis of productivity under climate change. Environ Res Lett 16(5):054027
Suresh K, Nagamani C, Kantha DL, Kumar MK (2012) Changes in photosynthetic activity in five common hybrids of oil palm (Elaeis guineensis Jacq.) seedlings under water deficit. Photosynthetica 50(4):549–556
Taiz L, Zeiger E, Møller IM, Murphy A (2015) Plant physiology and development. Sinauer Associates Inc, Sunderland, p 761
Thammanu S, Marod D, Han H, Bhusal N, Asanok L, Ketdee P, Gaewsingha N, Lee S, Chung J (2021) The influence of environmental factors on species composition and distribution in a community forest in Northern Thailand. J for Res 32(2):649–662
Xu Z, Zhou G, Shimizu H (2010) Plant responses to drought and rewatering. Plant Signal Behav 5(6):649–654
Yemm EW, Cocking EC, Ricketts RE (1955) The determination of amino-acids with ninhydrin. Analyst 80(948):209–214
Yordanov I, Velikova V, Tsonev T (2000) Plant responses to drought, acclimation, and stress tolerance. Photosynthetica 38(2):171–186
Zargar SM, Gupta N, Nazir M, Mahajan R, Malik FA, Sofi NR, Shikari AB, Salgotra RK (2017) Impact of drought on photosynthesis: molecular perspective. Plant Gene 11(Part B):154–159
Zhou S, Medlyn B, Sabaté S, Sperlich D, Prentice IC, Whitehead D (2014) Short-term water stress impacts on stomatal, mesophyll and biochemical limitations to photosynthesis differ consistently among tree species from contrasting climates. Tree Physiol 34(10):1035–1046
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Project funding: This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001.
Guest editor: Yanbo Hu
Corresponding editor: Yanbo Hu
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
de Sousa Leite, T., Oliveira de Freitas, R.M., da Silva Dias, N. et al. The interplay between leaf water potential and osmotic adjustment on photosynthetic and growth parameters of tropical dry forest trees. J. For. Res. 34, 177–186 (2023). https://doi.org/10.1007/s11676-022-01495-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-022-01495-0