Abstract
The synergistic effects of irradiance and salinity on leaf angle, the photochemical efficiency of photosystem II and photosynthetic pigment composition of mangroves were studied in a factorial experiment. Seedlings of Aegiceras corniculatum (L.) Blanco (Myrsinaceae) and Avicennia marina (Forstk.) Vierh var. australasica (Walp.) Moldenke (Avicenniaceae) were grown under salinity treatments (0, 5, 25, 50, 75, and 100% artificial seawater), in full sunlight or under shade cloth (transmitting 30 or 70% sunlight), during summer and autumn. Significant species’ differences and effects of salinity and growth irradiance were found for key measures. Depressions in Fv/Fm due to salinity and growth irradiance were chronic, they were least in 25% seawater and in 30% sunlight, and greater in low and high salinity, and higher irradiance. A diurnal depression of Fv/Fm was superimposed on the chronic depression, and was greater for Ae. corniculatum than Av. marina. Increases in leaf angle; and increases in the size, and de-epoxidation state of the xanthophyll cycle pigment pool afforded protection from adverse effects of excess excitation energy. Adverse effects of the highest salinities on β,β-carotene and β,ɛ-carotene biosynthetic pathways were suggested, particularly in Ae. corniculatum. The ecological significance of differences in species’ extent and temporal patterns of response are discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Variation in mangrove community structure along physicochemical gradients may depend on species’ differences in physiological tolerances, including responses of photosynthesis to irradiance and salinity (Snedaker 1982; Ball and Critchley 1982; Ball 1988; McKee 1995). It has been hypothesised that species-specific strategies for photoprotection may determine canopy dominance (Lovelock and Clough 1992), and differences in mangroves’ capacities for photosynthesis and protective dissipation of excess excitation energy along salinity gradients may impact on plant performance and the distribution of species (Ball 1996).
Exposure to excess irradiance can lead to photoinhibition, which is characterised by a light-dependent reduction in the intrinsic quantum yield of photosynthesis and a loss of photosystem II (PSII) activity (Osmond 1994). Photosynthesis of mangrove leaves becomes light-saturated at incident photon flux densities of 40% sunlight or less (Ball and Critchley 1982; Andrews et al. 1984; Carter et al. 1990; Cheeseman et al. 1991; Cheeseman 1994; Youssef and Saenger 1998). Hence, irradiance may often be excessive. In mangroves, photoinhibition occurs under high irradiance in canopies in the field, but the degree to which photoinhibition is sustained varies (Attiwill and Clough 1980; Ball and Critchley 1982; Björkman et al. 1988; Cheeseman et al. 1991, 1997; Kitao et al. 2003). There have been no laboratory-based studies of photoinhibition under varying growth irradiance.
Photoprotection allows downregulation of the photosynthetic apparatus to balance light energy receipt and use (Osmond 1994). In mangroves, inhibition of zeaxanthin (Z) formation has been associated with more sustained depression of Fv/Fm (the potential maximal photochemical efficiency of photosystem II) in seedlings (Demmig-Adams et al. 1989). Consistent with a key role of the xanthophyll cycle in photoprotection, in mature canopies pool sizes were lower in the shade compared with sunlit leaves and in leaves with steeper angles, and large pool size was accompanied by higher midday de-epoxidation state (Lovelock and Clough 1992). Steep angles of display in exposed leaves also help maintain photosynthetic efficiency (Björkman et al. 1988).
In mangroves, effects of increasing salinity on photosynthetic carbon assimilation may be detrimental (Ball and Farquhar 1984; Sobrado 1999b), beneficial (Naidoo and von Willert 1995; Werner and Stelzer 1990) or negligible (Pezeshki et al. 1990). Salinity-dependent limitations to photosynthetic carbon metabolism may increase the potential for photoinhibition (Powles 1984). However, responses reported for mangroves vary. In field studies of mature canopies, greater depressions of Fv/Fm in habitats with hyposaline compared with seawater salinities (Naidoo et al. 2002), contrast with no differences in Fv/Fm in habitats with hypersaline compared with seawater salinities (Sobrado and Ball 1999). Although glasshouse-grown seedlings showed lower Fv/Fm when grown in 100% seawater compared with 10% seawater (Björkman et al. 1988), another study found no significant effects on Fv/Fm of an increase in the salinity of the sodium chloride solutions in which seedlings were grown (Sobrado 1999a). There have been no laboratory-based studies of photoprotection under varying salinity in mangroves. However, a study of mature canopies found no differences in xanthophyll cycle pool size or de-epoxidation state in habitats with hypersaline compared with seawater salinities (Sobrado and Ball 1999).
My study (Christian 1999) tested the hypotheses of Lovelock and Clough (1992) and Ball (1996), as stated above. The study species Ae. corniculatum and Av. marina are sympatric in southeastern Australia (West et al. 1985; Busby and Bridgewater 1986), where they tend to dominate mangrove communities in the upper and lower reaches of estuaries, respectively (Clarke and Hannon 1970; Owen 1978). Usually, Av. marina occurs as a tree or shrub in the overstorey, and Ae. corniculatum shrubs occur in monospecific stands or as an understorey beneath Av. marina (Christian 1999).
A factorial experiment was used to test three hypotheses: (1) interactions between high light and adverse salinity exacerbate photoinhibition, (2) this effect is greater in Ae. corniculatum than Av. marina and (3) differences in leaf display and chlorophyll (chl) and carotenoid composition contribute to species-specific strategies of photoprotection along salinity and light gradients.
Materials and methods
Plant material
Propagules of Ae. corniculatum and Av. marina were collected in August and October 1993, respectively, at Cullendulla Creek, Batemans Bay, New South Wales (35°42′S, 150°12′E). Propagules were grown in sand culture in 50% (v/v) seawater, made by diluting artificial seawater with tap water, in a glasshouse in Canberra (35°18′S, 149°12′E). Day and night air temperatures were approximately 25°C and 15°C, respectively, and seedlings received approximately 70% sunlight. When the first pair of leaves was fully expanded and shedding of cotyledons commenced in Av. marina, cotyledons remaining on Av. marina were removed. Fresh weights of all seedlings were measured. Seedlings were replanted in sand in perforated pots (140 mm diameter, approximately 1 L volume). Each pot was placed in a 2-L tub and immersed in 50% seawater with added nutrient solution (10% Hoagland’s; Hewitt 1966).
Experimental procedure
A split-plot experiment was conducted outdoors from 20 January to 1 May 1994. Restricted randomisation was used. Three shade shelters were used in each of three north-south oriented blocks. Shade treatments were achieved using no shading (100% sunlight) or shade-cloth transmitting either 30 or 70% sunlight. Species (Av. marina and Ae. corniculatum) and salinity treatment (0, 5, 25, 50, 75 or 100% seawater) were assigned to one of 12 positions in each shelter. Three pots containing seedlings of similar fresh weight were assigned to each position. Pots were assigned to one of three harvests (H1: 0 days; H2: 52 days; and H3: 101 days), as part of a growth analysis study (Christian 1999).
Once plants were positioned, salinities were brought to treatment levels in increments of 10% seawater or less by replacement of bathing solutions twice daily. Concentrations of Hoagland’s solution were increased concurrently to 30%. Thereafter, pots were aerated through a 16 mm microspray jet inserted in the sand. Solutions were topped-up with tap water daily and were replaced weekly.
Conditions
Daily global solar radiation data for Canberra were obtained from the Bureau of Meteorology (Radiation Network Exposure Data). Photosynthetic photon flux densities incident on a horizontal surface (PPFD) were measured with a quantum sensor (model 190s, LI-COR, Lincoln, Nebraska) above the leading shoot apex of each plant at intervals through the day on 9 February, 23 March, and 21 and 27 April. Daily maximum and minimum air temperatures in each shelter were recorded from 11 February 1994.
Leaf selection
For each H3 plant a fully expanded, north to west facing, exposed leaf, which had developed under the experimental treatment was tagged for all measurements. New leaves were tagged when growth led to shading. Not all 108 plants were tagged because under adverse treatments plants did not grow enough new large leaves.
Chlorophyll fluorescence measurements
Chlorophyll fluorescence was measured in situ on sunny days. On three days (2 February, 23 March, and 21 April) I made consecutive one- to two-hourly measurements throughout the day from predawn until dusk. Predawn and midday measurements were also made on 28 April, concurrent with sampling for pigment analyses.
The dark-adapted minimum (Fo) and maximum (Fm) chlorophyll fluorescence were measured with a field-portable, time-resolving fluorimeter (Plant Efficiency Analyser, Hansatech Instruments Ltd., King’s Lynn, Norfolk, UK). Leaves were first dark-adapted and then exposed for 5 s to saturating red light. The ratio of variable to maximal fluorescence Fv/Fm, was calculated as (Fm−Fo)/Fm. Measurements made predawn represent the true dark-adapted state. During daylight, dark-adaptation was for 5 min, allowing relaxation of fluorescence quenching associated with thylakoid membrane energisation (Krause et al. 1983; Demmig et al. 1987).
Leaf display and photosynthetic pigments
The midrib angle of each tagged leaf was measured with a protractor and shot-line. Predawn and at midday, one to four 0.95 cm2 discs were punched from each leaf, frozen in liquid nitrogen and stored at −80°C. Discs were powdered in liquid nitrogen and extracted for 5 min with 100% AR grade acetone in the presence of NaHCO3. The solvent was then diluted with water to 80% acetone and extracted for 10 min more. The sample was centrifuged (5 min, 5,000 rpm, 2°C) and the supernatant collected and stored under N2(g) at −80°C.
Chlorophyll concentrations were determined by absorption spectroscopy (Ultraspec II spectrophotometer, Pharmacia LKB, Uppsala, Sweden) using wavelengths and extinction coefficients as in Porra et al. (1989). The filtered (0.45 μm) supernatant was analysed for carotenoid pigments using the high-performance liquid chromatography (HPLC) method of Gilmore and Yamamoto (1991). Solvent A1 (acetonitrile–methanol–Tris–HCl buffer 0.1 M pH 8.0, 72:8:3) was used. Absorption was measured at 440 nm using a chromatograph with a variable wavelength detector (Waters model 490). Spherisorb ODS-1 nonendcapped columns (5 μm particle size, 250 mm × 4.6 mm, Alltech Associates Aust. Pty. Ltd.) and guard columns were used. Elution of pigments was as described in Roden and Ball (1996). All sample injections were 40 μl. Pigment concentrations were calculated using calibrations based on standards (as described in Robinson et al. 1993).
The de-epoxidation state (DPS) of xanthophyll cycle pigments, violaxanthin (V), antheraxanthin (A) and zeaxanthin (Z) was calculated as (Z+0.5A)/(V+A+Z). The xanthophyll cycle pool size (VAZ/chl) was calculated as (V+A+Z)/(chl a+b).
Statistical analyses
Data were analysed using GenStat (5th edition, releases 3.1 through to 7.2, Rothamsted Experimental Station 1994 and VSN International). The description of factorial models is as in Wilkinson and Rogers (1973). Fixed effects were Shade, Species, Salinity and Hour (where repeated measures are compared for two occasions). Random effects were block, shelter, position and time.
Where the variance was heterogeneous the data scales were transformed to equalise variances. In the case of Fv/Fm, the constant 0.84 was taken as an upper limit and when necessary, the following transformation was used:
The data scale is important for determining whether synergistic effects of treatment factors occur. For example, when two factors show multiplicative effects (i.e., an interaction), on a linear scale:
transformation to a logarithm scale:
makes the relationship additive.
For balanced data, analyses of variance were used. For unbalanced data, linear mixed models were fitted using restricted maximum likelihood estimation (REML; Patterson and Thompson 1971). The full model was fitted and terms were then successively dropped from nested submodels where changes in deviance tests (χ2 test) were not significant (p<0.05), to arrive at the final, most parsimonious sub-model. The analysis of deviance for the final model is summarised (in the figure and table captions) as: the residual deviance (RD) and degrees of freedom (df) for the final model; and the change in RD and df of the submodel when each term was dropped, and its χ2 significance level.
Repeated measures made on different days were pooled as there was no evidence for autocorrelation between dates within a subject. Repeated measures made on more than two occasions through the day (e.g., PPFD) were explored by plotting the response through time. Where all subjects showed consistent patterns, responses of each were modelled by fitting nonlinear curves to the data (Payne et al. 1994). A summary measure was then derived for each fitted curve, which was analysed as above.
Models of significant (p<0.05) treatment effects are presented graphically for the highest order factors and interactions that were significant. For data that did not require transformation, figures show the means and average standard errors. Where the variate was transformed, results are presented as backtransformed means and 95% confidence intervals [i.e., mean (lower CI, upper CI)]. Where all effects were not significant the null model was fitted using REML to obtain the correct error term, and the grand mean and 95% CI are given in the text.
Equations describing the relationships between Fv/Fm and several measures of photoprotection were examined by fitting linear mixed models to the data collected on 28 April. Fixed effects of treatment factors (Shade, Species, Salinity and Hour), three covariates (leaf angle, VAZ/chl and DPS), and all possible two-way interactions were modelled (random effects: block/shelter/position/time). The variates were transformed where necessary. Marginal predicted values of the response variate were estimated for given values of the explanatory variates as in Welham et al. (2004).
Results
Experimental conditions
The mean daily global radiation over the experimental period was 19.5 MJ m−2 day−1 (6.9, 32.1). The 30, 70 and 100% sunlight treatments, received PPFDs at midday of 349 (283, 421), 927 (818, 1,043) and 1,263 (1,135, 1,398) μmol m−2 s−1; and total daily photon receipt of 11.3 (7.4, 16), 26.7 (20.5, 33.6) and 39.4 (31.8, 47.8) mol m−2, respectively. There were no significant effects of Shade on the maximum [26.9°C (26.7, 27.1)] or minimum [8.65°C (8.55, 8.75)] air temperatures within each shelter.
Chlorophyll fluorescence
Repeated measurements throughout the day indicated that Fv/Fm was reduced through the morning, and recovered in the afternoon (data not presented). The minima for Fv/Fm occurred at midday [1206 hours EST (1140, 1234)], and the time of occurrence was not significantly affected by any factor. Variation in Fo was large and differences with time of day were not significant (data not presented).
Predawn, on average, Av. marina showed a 2% lower Fv/Fm than Ae. corniculatum (Fig. 1a). However, at midday on average, Ae. corniculatum showed an 11% lower Fv/Fm than Av. marina. On average, Fv/Fm was 14% lower in plants grown in full sunlight compared with 30% sunlight (Fig. 1b). On average, compared with 25% seawater, Fv/Fm was reduced by 7% in freshwater and 13% in 100% seawater (Fig. 1c). Additive effects of shading and salinity on log-transformed Fv/Fm data indicate multiplicative effects on the original scale, and a greater adverse effect of high growth irradiance under adverse salinity. The frequent need for transformation of the Fv/Fm data indicates that variation in this ratio was greatest when photoinhibition was greatest. This may be because of variation between plants in the extent of resistance to photoinhibition, and/or because of the low signal-to-noise ratio which arises when chlorophyll fluorescence is most quenched.
Effects of a Species.Hour (ΔRD=14.4, df=1), b Shade (ΔRD=11.4, df=2), and c Salinity (ΔRD=15.4, Δdf=5) on Fv/Fm in Ae. corniculatum and Av. marina seedlings grown in a three-way factorial pot trial under three shading and six salinity treatments. Backtransformed means and 95% CIs for the final linear mixed model (residual deviance = 116.58, df=315) fitted to ln(0.84 – Fv/Fm) using REML. Data for three dates (23 March, 21 and 28 April 1994) were pooled
Leaf display and photosynthetic pigments
Leaf angles were increasingly vertical with increasing growth irradiance (Fig. 2a) and increasing salinity (Fig. 2b), but did not differ significantly with species. The salinity level did not affect the response to growth irradiance. The total chl concentration per unit leaf area was maximal in 25% seawater and decreased in high and low salinity (Fig. 2c), but was not significantly affected by shading, species or time of day. The molar ratio of chl a to b [3.07 (2.71, 3.43)] was not significantly affected by any of the treatments.
Effects of a Shade (ΔRD=10.31, Δdf=2) and b Salinity (ΔRD=12.30, Δdf=5) on leaf angles (from the horizontal). Significantly different means for the final linear mixed model (residual deviance = 539.23, df=78) fitted using REML. c Effect of Salinity (ΔRD=12.08, Δdf=5) on the total leaf chlorophyll per unit leaf area (28 April 1994). Backtransformed means and 95% CIs for the final linear mixed model (residual deviance = 434.05, df=106) fitted to (chl a+b (μmol m−2))1/2 using REML
Xanthophyll cycle pool sizes relative to chl increased with increasing growth irradiance (Fig. 3a), due to an increase in xanthophylls per unit leaf area (not presented). Pool sizes in Av. marina were lowest in 25% seawater and increased in lower and higher salinities (Fig. 3b). The two species had similar pool sizes in 0–25% seawater; however, for Ae. corniculatum pool sizes increased and then decreased with further increases in salinity. The salinity response was partly driven by changes in chl per unit leaf area (Fig. 2c); and Av. marina had greater concentrations of xanthophylls per unit leaf area than Ae. corniculatum (data not presented). The DPS increased at midday compared with predawn (Fig. 4a and b). Aegiceras corniculatum had lower DPS predawn and higher DPS at midday than Av. marina (Fig. 4a). At midday, DPS increased with increasing growth irradiance (Fig. 4b). The DPS was higher in 100% seawater than at other salinities (Fig. 4c).
Effects of a Shade.Hour (ΔRD=8.693, Δdf=2), and b Species.Salinity (ΔRD=18.37, Δdf=5) on xanthophyll cycle pool size relative to total chlorophyll (VAZ/chl), . Backtransformed means and 95% CIs for the final linear mixed model (residual deviance = 83.46, df=92) fitted to ln(100 + [VAZ/chl {mmol mol−1}]) using REML
Effects of a Species.Hour (ΔRD=8.127, Δdf=1), b Shade.Hour (ΔRD=10.57, df=2), and c Salinity (ΔRD=17.62, Δdf=5) on the de-epoxidation state of the xanthophyll cycle pigments (DPS=[Z+0.5A]/[V+A+Z]). Significantly different means from the linear mixed model (residual deviance = 255.08, df=102) fitted using REML
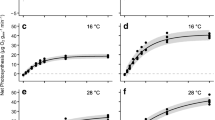
The ratio of β-carotene (βC) to chl varied significantly with salinity and time (Fig. 5a), but not species or shade treatment. Concentrations of βC per unit area [9.76 μmol m−2 (7.73, 12.04)] showed no significant effects of any factors (for square-root transformed data). The ratio of neoxanthin (Neo) to chl decreased at midday compared with predawn (Fig. 5b). Ratios were highest in moderate salinities and decreased in 100% seawater (Fig. 5c), but did not differ significantly with species. These patterns were associated with lower Neo concentrations per unit leaf area (data not presented) at midday compared with predawn, and in 100% seawater.
Effects of a Salinity.Hour (ΔRD = 12.16, df = 5) on the ratio of β-carotene to total chlorophyll (βC/chl). Significantly different means from linear mixed model (residual deviance = 795.10, df=99) fitted using REML. Effects of b Hour (ΔRD=4.701, Δdf=1), and c Salinity (ΔRD=16.59, Δdf=5) on the ratio of neoxanthin to total chlorophyll (Neo/chl). Backtransformed means and 95% CIs for the linear mixed model (residual deviance = 74.32, df=104) fitted to ln(10+(Neo/chl [mmol mol−1]) using REML
The ratio of lutein (L) to chl increased with increasing growth irradiance and in shaded treatments ratios decreased at midday compared with predawn (Fig. 6a). In Av. marina, ratios were lowest in 25% seawater and increased in lower and higher salinities (Fig. 6b). The two species had similar ratios in 0–25% seawater. In Ae. corniculatum, ratios increased and then decreased with further increases in salinity. These patterns were associated with lower L concentrations per unit leaf area (data not presented) at midday compared with predawn, in Ae. corniculatum compared with Av. marina, and with increasing salinity.
Relationships between measures of photoinhibition and photoprotection
Decreases in leaf angle, increases in xanthophyll cycle pool size under high growth irradiance, and increases in DPS, particularly in Ae. corniculatum, were associated with decreases in Fv/Fm (Table 1). Hour affected only the intercept and not the slope of these relationships and Salinity had no significant effects. The models indicated that for a given Ae. corniculatum seedling growing under high irradiance, individuals with lower leaf angle, larger xanthophyll cycle pool size and higher DPS had lower Fv/Fm (Fig. 7). If the seedling was grown under low irradiance the effect of pool size was less and if the mangrove was an Av. marina seedling, the effect of DPS was less.
Predicted values of transformed Fv/Fm for given values of a range of measures of photoprotection, for the models given in Table 1. Dotted lines show the 95% confidence intervals in all cases. a Effect of leaf angle at midday for leaves of Ae. corniculatum in 100% sunlight with the mean xanthophyll cycle pool size and mean de-epoxidation state. b Effect of xanthophyll cycle pool size under three shading treatments at midday for leaves of Ae. corniculatum with an angle of 45° and the mean de-epoxidation state. c Effect of de-epoxidation state in Ae. corniculatum and Av. marina at midday for leaves with an angle of 45° and the mean xanthophyll cycle pool size
Discussion
Photoinhibition in leaves developed under different salinity and irradiance treatments
High growth irradiance led to sustained depressions in Fv/Fm, consistent with depressions of Fv/Fm in sunlit leaves compared with shaded leaves in mature canopies of Ae. corniculatum and Av. marina (Björkman et al. 1988). Salinity extremes also led to persistent depressions of Fv/Fm, consistent with at least 10% lower Fv/Fm for seedlings of Av. marina grown in 100% seawater compared with 10% seawater, under high irradiance (Björkman et al. 1988). Consistent with Hypothesis 1, these effects of high growth irradiance and salinity were multiplicative. Such ‘chronic’ photoinhibition (sensu Osmond 1994) due to high light and salinity in mangroves has been attributed to a regulatory, protective increase in the rate constant for radiationless energy dissipation in the light-harvesting antennae (Björkman et al. 1988).
Sustained depressions in the photochemical efficiency of PSII were greater in Av. marina than Ae. corniculatum, contrary to Hypothesis 2. However, transient diurnal reductions were superimposed on the sustained depressions in Fv/Fm and were greater in Ae. corniculatum than Av. marina. This transient component is consistent with responses observed in leaves of Av. marina (Attiwill and Clough 1980) and Rhizophora stylosa (Cheeseman et al. 1997) canopies in the field, and for glasshouse-grown Avicennia germinans (Sobrado 1999b). Midday reductions in the efficiency of PSII in high light were recovered shortly after sunset (Cheeseman et al. 1997) or predawn (Sobrado 1999b) and were thought to result from protective downregulation rather than damage. The responses of the two species, therefore, differ most in their temporal pattern and lie on a continuum with Ae. corniculatum showing responses more similar to the pattern of diurnal photoinhibition observed by Cheeseman et al. (1997) and Av. marina showing responses more similar to the chronic photoinhibition observed by Björkman et al. (1988).
Photoprotection mechanisms
Chlorophyll a/b ratios were typical of sun plants (Anderson et al. 1988). Lower leaf chl content in salinity extremes may represent a protective strategy in which the capture of photons is reduced to match the capacity for energy transduction and use, as has been suggested for sunlit leaves of Xylocarpus granatum in the field (Kitao et al. 2003). Lower chlorophyll may also reduce leaf absorptance and enable avoidance of high leaf temperatures when stomatal conductance is low (Havaux and Tardy 1999).
Steeper leaf angles under high growth irradiance and in more saline treatments may be adaptive in avoiding exposure to excess photons (Björkman et al. 1988) and leaf temperatures (Andrews et al. 1984) when the sun is near the zenith. Additive effects of shading and salinity on leaf angle, and a small slope of the relationship between log-transformed Fv/Fm and leaf angle suggest that temperature was the more important causal driver.
My models (Table 1, Fig. 7) reflected the role of steep leaf angles in avoiding photoinhibition, and a role for the xanthophyll cycle in protective downregulation, and were consistent with Hypothesis 3. The species- and shading-dependent nature of the relationships presumably reflects the importance of lumen pH and other factors in the development of nonradiative dissipation and depression of Fv/Fm (Gilmore and Yamamoto 1992, 1993; Gilmore and Govindjee 1999; Müller et al. 2001). Nonlinear relationships between reductions in Fv/Fm and the extent of de-epoxidation of the xanthophyll cycle pool are in accordance with other reports (Adams et al. 1994; Cheng 2003).
Xanthophyll cycle pool sizes and DPS reflected historic exposures to excess irradiance, and excess irradiance at the time of sampling, respectively, as in other studies (e.g., Laing et al. 1995). In general, depression of Fv/Fm was associated with increases in the pool size and increases in the midday DPS of the xanthophyll cycle pigments, suggesting a key role for Z (and A) in the protective dissipation of excess excitation energy. The pattern of response of xanthophyll cycle pool size to salinity suggested an increase in the capacity for Z-associated protective dissipation of excess excitation energy in salinity extremes, except at the highest salinity in Ae. corniculatum.
Aegiceras corniculatum and Av. marina showed similar responses of the xanthophyll cycle pool size to growth irradiance and had similar leaf angles. Species differences in DPS, and its effect on Fv/Fm were key factors. This is in contrast to mature canopies of Rhizophoraceae, for which species’ differences in strategies for photoprotection were manifest as differences in pool size or leaf angle (Lovelock and Clough 1992), suggesting phylogeny or ontogeny may influence strategies of photoprotection. The lesser diurnal depression and relaxation of depression of Fv/Fm, and lesser diurnal de-epoxidation and re-epoxidation of xanthophylls in Av. marina than Ae. corniculatum, suggests a greater protective, longer-term down-regulation in Av. marina and more extreme but transient protective response under excess excitation in Ae. corniculatum (Adams et al. 1994).
Predawn depression of Fv/Fm in Av. marina and high salinity may be related to the presence of de-epoxidised xanthophylls (as observed in another study of Av. marina, Sobrado and Ball 1999), and sustained engagement of xanthophyll cycle-dependent thermal dissipation (Adams et al. 1994; Verhoeven et al. 1997). Such sustained engagement in the dark could occur if the trans-thylakoid pH gradient was maintained by ATP hydrolysis (Gilmore and Yamamoto 1992; Gilmore and Björkman 1995). In leaves of Av. marina and seedlings grown under salinity extremes, a sustained higher DPS should result in more rapid induction of nonradiative dissipation of excess excitation energy. In leaves of Ae. corniculatum and seedlings grown under moderate salinity, lower predawn DPS may result in slower development of nonphotochemical quenching under conditions of excess excitation, requiring minutes to hours for de-epoxidation (Bilger et al. 1989; Demmig-Adams 1990; Thayer and Björkman 1990; Adams et al. 1994).
An adverse effect of high salinity on the β,β-carotene pathway was suggested by lowered levels of VAZ in Ae. corniculatum and lowered levels of Neo in both species in 100% seawater. Greater Z-dependent quenching might therefore be expected in Av. marina than in Ae. corniculatum in 100% seawater. Reductions in Neo at midday and in high salinity have implications for the synthesis of abscisic acid (Lee and Milborrow 1997; Niyogi 1997; Schwartz et al. 1997).
An adverse effect of high salinity on the β,ɛ-carotene pathway in Ae. corniculatum was suggested by lowered levels of L in this species in 100% seawater, which may have adverse effects on the capacity for nonradiative dissipation of excess excitation energy (Pogson et al. 1998). Increasing growth irradiance resulted in an accumulation of L relative to chl, as in previous studies (Thayer and Björkman 1990; Niyogi 1997), which may enhance nonphotochemical quenching (Niyogi 1997; Pogson et al. 1998). Diurnal midday declines in L under low growth irradiance suggest the possible operation of a lutein-epoxide cycle (Bungard et al. 1999).
Possible ecological significance of species’ differences
The two sympatric species showed different extents of chronic and diurnal photoinhibition, diurnal de-epoxidation of the xanthophyll cycle pigments, and effects of high salinity on L and xanthophyll cycle pool sizes. Species’ differences may be of ecological significance, and be indicative of their relative performance along salinity and irradiance gradients in habitats where they co-occur.
The greater long-term down-regulation and lesser diurnal depression of the photochemical efficiency of PSII in leaves of Av. marina suggest this species will be favoured under conditions of persistent excess photon dose, for example, during periods of consecutive sunny days, in exposed environments, and at high salinities. However, this strategy may result in lower net carbon gain under low excitation pressure, such as in the understorey or in habitats with moderate salinities. Greater diurnal depressions in the photochemical efficiency of PSII, but lesser long-term downregulation in leaves of Ae. corniculatum suggest this species will be favoured under conditions where excess excitation is less frequent, for example during cloudy weather, in shaded environments, and at low salinities. However, this strategy may result in lower net carbon gain under high excitation pressures, such as in exposed sites or in habitats with high salinities.
The chlorophyll fluorescence characteristics of leaves provide a rapid assay of the activity of PSII, and a useful noninvasive tool for monitoring the responses of plants to stress in numbers appropriate to addressing ecological questions (Ball et al. 1994). Confidence in upscaling of the leaf level patterns observed here to whole plants and communities requires additional measurements of processes at intermediate scales (Jarvis 1995; Körner 1995). I have therefore explored the relationship between the responses reported here and species-specific interactive effects of light and salinity on the net assimilation rate for the whole canopy, and the growth, survivorship and distribution of the two species in the field (Christian 1999).
References
Adams IWW, Demmig-Adams B, Verhoven AS, Barker DH (1994) “Photoinhibition” during winter stress: involvement of sustained xanthophyll cycle-dependent energy dissipation. Aust J Plant Physiol 22:261–276
Anderson JM, Chow WS, Goodchild DJ (1988) Thylakoid membrane organisation in sun/shade acclimation. Aust J Plant Physiol 15:11–26
Andrews TJ, Clough BF, Muller GJ (1984) Photosynthetic gas exchange properties and carbon isotope ratios of some mangroves in north Queensland. In: Teas HJ (ed) Physiology and management of mangroves, vol 9. Junk, The Hague, pp 15–23
Attiwill PM, Clough BF (1980) Carbon dioxide and water vapour exchange in the white mangrove. Photosynthetica 14:40–47
Ball MC (1988) Salinity tolerance in the mangroves Aegiceras corniculatum and Avicennia marina. I. Water use in relation to growth, carbon partitioning, and salt balance. Aust J Plant Physiol 15:447–464
Ball MC (1996) Comparative ecophysiology of mangrove forest and tropical lowland moist rainforest. In: Mulkey SS, Chazdon RL, Smith AP (eds) Tropical forest ecophysiology. Chapman and Hall, New York, pp 461–496
Ball MC, Critchley C (1982) Photosynthetic responses to irradiance by the grey mangrove Avicennia marina grown under different light regimes. Plant Physiol 70:1101–1106
Ball MC, Farquhar GD (1984) Photosynthetic and stomatal responses of the grey mangrove, Avicennia marina, to transient salinity conditions. Plant Physiol 74:7–11
Ball MC, Butterworth JA, Roden JS, Christian R, Egerton JJG (1994) Applications of chlorophyll fluorescence to forest ecology. Aust J Plant Physiol 22:311–319
Bilger W, Björkman O, Thayer SS (1989) Light-induced spectral absorptance changes in relation to photosynthesis and the epoxidation state of xanthophyll cycle components in cotton leaves. Plant Physiol 91:542–551
Björkman O, Demmig B, Andrews JT (1988) Mangrove photosynthesis: response to high-irradiance stress. Aust J Plant Physiol 15:43–61
Bungard RA, Ruban AV, Hibberd JM, Press MC, Horton P (1999) Unusual carotenoid composition and a new type of xanthophyll cycle in plants. Proc Natl Acad Sci USA 96:1135–1139
Busby JR, Bridgewater PB (1986) A preliminary atlas of mangrove species in Australia. Australian Government Publishing Service, Canberra
Carter DR, Cheeseman JM, Clough BF, Lovelock CE, Sim RG, Ong JE (1990) Photosynthetic characteristics of the mangrove, Bruguiera parviflora (Roxb.) Wright and Arn., under natural conditions. In: Baltscheffsky M (ed) Current research in photosynthesis, vol IV. Kluwer, Dordrecht, pp 859–862
Cheeseman JM (1994) Depressions of photosynthesis in mangrove canopies. In: Baker NR, Bowyer JR (eds) Photoinhibition of photosynthesis: from molecular mechanisms to the field. Bios Scientific, Oxford, UK, pp 377–389
Cheeseman JM, Clough BF, Carter DR, Lovelock CE, Eong OJ, Sim RG (1991) The analysis of photosynthetic performance in leaves under field conditions: a case study using Bruguiera mangroves. Photosynth Res 29:11–22
Cheeseman JM, Herenden LB, Cheeseman AT, Clough BF (1997) Photosynthesis and photoprotection in mangroves under field conditions. Plant Cell Environ 20:579–588
Cheng L (2003) Xanthophyll cycle pool size and composition in relation to the nitrogen content of apple leaves. J Exp Bot 54:385–393
Christian R (1999) The distribution of two sympatric mangrove species and interactive effects of salinity and irradiance. PhD thesis, Australian National University, Canberra
Clarke LD, Hannon NJ (1970) The mangrove swamp and salt marsh communities of the Sydney district. III. Plant growth in relation to salinity and waterlogging. J Ecol 58:351–369
Demmig B, Winter K, Krüger A, Czygan F-Z (1987) Photoinhibition and zeaxanthin formation in intact leaves. A possible role for the xanthophyll cycle in the dissipation of excess light energy. Plant Physiol 84:218–224
Demmig-Adams B (1990) Carotenoids and photoprotection in plants: a role for the xanthophyll zeaxanthin. Biochim Biophys Acta 1020:1–24
Demmig-Adams B, Winter K, Krüger A, Czygan F-C (1989) Zeaxanthin synthesis, energy dissipation and photoprotection of photosystem II at chilling temperatures. Plant Physiol 90:894–898
Gilmore AM, Björkman Ö (1995) Temperature-sensitive coupling and uncoupling of ATPase-mediated nonradiative energy dissipation: similarities between chloroplasts and leaves. Planta 197:646–654
Gilmore AM, Govindjee (1999) How higher plants resond to excess light: energy dissipation in photosystem II. In: Singhal GS, Renger G, Sopory SK, Irrgang K-D, Govindjee (eds) Concepts in photobiology. Navara Publishing, New Delhi, India
Gilmore AM, Yamamoto HY (1991) Resolution of lutein and zeaxanthin using a non-endcapped, lightly carbon-loaded C18 high-performance liquid chromatographic column. J Chromatogr 543:137–145
Gilmore AM, Yamamoto HY (1992) Dark induction of zeaxanthin-dependent nonphotochemical quenching mediated by ATP. Proc Natl Acad Sci USA 89:1899–1903
Gilmore AM, Yamamoto HY (1993) Linear models relating xanthophylls and lumen acidity to non-photochemical fluorescence quenching. Evidence that antheraxanthin explains zeaxanthin-independent quenching. Photosynth Res 35:67–78
Havaux M, Tardy F (1999) Loss of chlorophyll with limited reduction of photosynthesis as an adaptive response of Syrian barley landrace to high-light and heat stress. Aust J Plant Physiol 26:569–578
Hewitt HEJ (1966) Sand and water culture methods used in the study of plant nutrition. Commonwealth Agricultural Bureaux, Farnham Royal, Bucks, England
Jarvis PG (1995) Scaling processes and problems. Plant Cell Environ 18:1079–1089
Kitao M, Utsugi H, Kuramoto S, Tabuchi R, Fujimoto K, Lihpai S (2003) Light-dependent photosynthetic characteristics indicated by chlorophyll fluorescence in five mangrove species native to Pohnpei Island, Micronesia. Physiol Plant 117:376–382
Körner C (1995) Towards a better experimental basis for upscaling plant responses to elevated CO2 and climate warming. Plant Cell Environ 18:1101–1110
Krause GH, Briantis J-M, Vernotte C (1983) Characterisation of chlorophyll fluorescence quenching in chloroplasts by fluorescence spectroscopy at 77K. I. ΔpH-dependent quenching. Biochim Biophys Acta 723:169–175
Laing WA, Greer DH, Schnell TA (1995) Photoinhibition of photosynthesis causes a reduction in vegetative growth rates of dwarf bean (Phaseolus vulgaris) plants. Aust J Plant Physiol 22:511–520
Lee H-S, Milborrow BV (1997) Endogenous biosynthetic precursors of (+)- Abscisic acid. IV. Biosynthesis of ABA from [2Hn] carotenoids by a cell-free system from avocado. Aust J Plant Physiol 24:715–726
Lovelock CE, Clough BF (1992) Influence of solar radiation and leaf angle on leaf xanthophyll concentrations in mangroves. Oecologia 91:518–525
McKee KL (1995) Interspecific variation in growth, biomass partitioning, and defensive characteristics of neotropical mangrove seedlings: response to light and nutrient availability. Am J Bot 82:299–307
Müller P, Li X-P, Niyogi KK (2001) Nonphotochemical quenching. A response to excess light energy. Plant Physiol 125:1558–1566
Naidoo G, von Willert DJ (1995) Diurnal gas exchange characteristics and water-use efficiency of three salt-secreting mangroves at low and high salinities. Hydrobiologia 295:13–22
Naidoo G, Tuffers AV, von Willert DJ (2002) Changes in gas exchange and chlorophyll fluorescence characteristics of two mangroves and a mangrove associate in response to salinity in the natural environment. Trees 16:140–146
Niyogi KK (1997) The roles of specific xanthophylls in photoprotection. Proc Natl Acad Sci USA 94:14162–14167
Osmond CB (1994) What is photoinhibition? Some insights from comparisons of shade and sun plants. In: Baker NR, Bowyer JR (eds) Photoinhibition of photosynthesis: molecular mechanisms to the field. Bios Scientific, Oxford, UK, pp 1–24
Owen PC (1978) Estuaries. In: Gunn RH (ed) Biophysical background studies, vol 2. CSIRO, Melbourne, pp 100–117
Patterson HD, Thompson R (1971) Recovery of inter-block information when block sizes are unequal. Biometrika 58:545–554
Payne RW et al. (1994) Genstat 5 release 3 reference manual. Clarendon, Oxford
Pezeshki SR, DeLaune RD, Patrick JR (1990) Differential response of selected mangroves to soil flooding and salinity: gas exchange and biomass partitioning. Can J For Res 20:869–874
Pogson BJ, Niyogi KK, Björkman O, DellaPenna D (1998) Altered xanthophyll compositions adversely affect chlorophyll accumulation and nonphotochemical quenching in Arabidopsis mutants. Proc Natl Acad Sci USA 95:13324–13329
Porra RJ, Thompson WA, Kriedemann PE (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta 975:384–394
Powles SB (1984) Photoinhibition of photosynthesis induced by visible light. Annu Rev Plant Physiol 35:15–34
Robinson SA, Lovelock CE, Osmond CB (1993) Wax as a mechanism for protection against photoinhibition—a study of Cotyledon orbiculata. Bot Acta 106:307-312
Roden JS, Ball MC (1996) The effect of elevated [CO2] on growth and photosynthesis of two Eucalyptus species exposed to high temperatures and water deficits. Plant Physiol 111:909–919
Schwartz SH, Tan BC, Gage DA, Zeevaart JAD, McCarty DR (1997) Specific oxidative cleavage of carotenoids by VP14 of maize. Science 276:1872–1874
Snedaker SC (1982) Mangrove species zonation: why? In: Sen DN, Rajpurchit KS (eds) Contribution to the ecology of halophytes, vol 2. Junk, The Hague, pp 111–125
Sobrado MA (1999a) Drought effects on photosynthesis of the mangrove, Avicennia germinans, under contrasting salinities. Trees 13:125–130
Sobrado MA (1999b) Leaf photosynthesis of the mangrove Avicennia germinans as affected by NaCl. Photosynthetica 36:547–555
Sobrado MA, Ball MC (1999) Light use in relation to carbon gain in the mangrove, Avicennia marina, under hypersaline conditions. Aust J Plant Physiol 26:245–251
Thayer SS, Björkman O (1990) Leaf xanthophyll content and composition in sun and shade determined by HPLC. Photosynth Res 23:331–343
Verhoeven AS, Demmig-Adams B, Adams IWW (1997) Enhanced employment of the xanthophyll cycle and thermal energy dissipation in spinach exposed to high light and N stress. Plant Physiol 113:817–824
Welham S, Cullis B, Gogel B, Gilmour A, Thompson R (2004) Prediction in linear mixed models. Aust N Z J Stat 46:325–347
Werner A, Stelzer R (1990) Physiological responses of the mangrove Rhizophora mangle grown in the absence and presence of NaCl. Plant Cell Environ 13:243–255
West RJ, Thorogood CA, Walford TR, Williams RJ (1985) An estuarine inventory for New South Wales, Australia. Division of Fisheries, Department of Agriculture, New South Wales
Wilkinson GN, Rogers CE (1973) Symbolic description of factorial models for analysis of variance. Appl Stat 22:392–399
Youssef T, Saenger P (1998) Photosynthetic gas exchange and water use in tropical and subtropical populations of the mangrove Aegiceras corniculatum. Mar Freshwater Res 49:329–334
Acknowledgements
I thank Marilyn Ball for initiating the project, MB and Julian Ash for guidance in the course of completing the research, Ross Cunningham and Christine Donnelly of the Statistical Consulting Unit, ANU, for assisting with experimental design and data analyses. I also thank the Australian National Aquarium for providing artificial seawater, Alison Saunders for assisting with maintaining the experiment, Jen Butterworth for assistance with HPLC, and Paul Adam, Aaron Ellison, Nicola Fortune, Richard Holdaway, Cath Lovelock, and Amber Parker for their comments on the work. This research was conducted while I was supported by an Australian Postgraduate Research Award
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Christian, R. Interactive effects of salinity and irradiance on photoprotection in acclimated seedlings of two sympatric mangroves. Trees 19, 596–606 (2005). https://doi.org/10.1007/s00468-005-0419-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-005-0419-2