Abstract
The [CO2] in the xylem of tree stems is typically two to three orders of magnitude greater than atmospheric [CO2]. In this study, xylem [CO2] was experimentally manipulated in saplings of sycamore (Platanus occidentalis L.) and sweetgum (Liquidambar styraciflua L.) by allowing shoots severed from their root systems to absorb water containing [CO2] ranging from 0.04% to 14%. The effect of xylem [CO2] on CO2 efflux to the atmosphere from uninjured and mechanically injured, i.e., wounded, stems was examined. In both wounded and unwounded stems, and in both species, CO2 efflux was directly proportional to xylem [CO2], and increased 5-fold across the range of xylem [CO2] produced by the [CO2] treatment. Xylem [CO2] explained 76–77% of the variation in pre-wound efflux. After wounding, CO2 efflux increased substantially but remained directly proportional to internal stem [CO2]. These experiments substantiated our previous finding that stem CO2 efflux was directly related to internal xylem [CO2] and expanded our observations to two new species. We conclude that CO2 transported in the xylem may confound measurements of respiration based on CO2 efflux to the atmosphere. This study also provided evidence that the rapid increase in CO2 efflux observed after tissues are excised or injured is likely the result of the rapid diffusion of CO2 from the xylem, rather than an actual increase in the rate of respiration of wounded tissues.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Previously, we observed a direct relationship between the rate of CO2 efflux from tree stems and [CO2] in the xylem (Teskey and McGuire 2002). CO2 efflux to the atmosphere from the stem appeared to arise from a combination of CO2 released by local respiring cells and CO2 that was transported through the xylem to the site of measurement. These results suggest errors in most previous in situ efflux-based estimates of woody tissue respiration because CO2 moving in the transpiration stream may alter xylem [CO2] and affect the rate of diffusion of CO2 from woody tissues to the atmosphere. Carbon dioxide concentrations in the xylem of trees were first measured in the early part of the twentieth century, yet to date only a few estimates have been made (Bushong 1907; MacDougal and Working 1933; Chase 1934; Jensen 1967; Eklund 1990, 1993; Hari et al. 1991; Levy et al. 1999; Teskey and McGuire 2002). In these studies, xylem [CO2] ranged from less than 1% to over 26%, i.e., the internal [CO2] in the stem was two to three orders of magnitude greater than atmospheric [CO2]. The high concentration of CO2 in xylem indicates significant barriers to diffusion of CO2 from the xylem to the atmosphere.
Rapid increases in CO2 efflux from injured woody tissues, often called “wound respiration,” may be related to high [CO2] in xylem. The effect of traumatic stimuli on measurements of woody tissue respiration has been debated for many years, and remains poorly understood (Sprugel and Benecke 1991). Some of the earliest reports of stem respiration noted that the rate of CO2 efflux from excised woody tissue appeared greater than from intact tissue (Boysen-Jensen 1933; Johansson 1933). An increase in CO2 efflux from woody tissue following wounding has been observed (Oohata et al. 1967; Levy et al. 1999) and is usually interpreted as an increase in respiration caused by injury to cells. However, excising or cutting into a branch or stem may simply reduce the barriers to diffusion of CO2 from xylem, allowing more CO2 to escape into the atmosphere. In this study we experimentally manipulated stem xylem [CO2] in saplings of sycamore (Platanus occidentalis L.) and sweetgum (Liquidambar styraciflua L.) to further investigate the relationship between xylem [CO2] and stem CO2 efflux and to examine the effect of xylem [CO2] on the “wound respiration” phenomenon.
Materials and methods
We conducted this experiment during the growing season in the months of June through October, 2002 at Whitehall Forest, an experimental forest of the University of Georgia near Athens, Georgia. Entire saplings of sycamore (P. occidentalis L.) and sweetgum (L. styraciflua L.) with diameters of 1.4–2.2 cm and heights of 2–3 m were severed from their roots in early morning. These species were chosen because they have diffuse-porous xylem anatomy that provided a large area of sapwood for water conduction and measurement of xylem [CO2]. The stems were not cut under water, but were immediately placed in a 500 ml graduated cylinder of water with one of three [CO2] treatment levels. We sealed the top of each cylinder with Parafilm. The [CO2] treatment levels, referred to as low, medium, and high, were created by bubbling air with either ambient, 10% or 20% [CO2] through 10 l containers of deionized water for 2–3 h prior to the initiation of the experiment. The actual measured [CO2] of the low, medium, and high solutions were approximately 0.04, 8.8, and 14.1%, respectively. One replication of the experiment was conducted on a given day. A replication consisted of three sapling shoots of either sweetgum or sycamore, each subjected to a different [CO2] treatment level. The shoots transpired outdoors in sunlight for approximately 4 h to allow the treated water to move into the portion of the stem above the graduated cylinder. We monitored and refilled the cylinders as necessary. Subsequently, we measured [CO2] in each stem ∼30 cm above the graduated cylinder using a CO2 microelectrode (model MI-720, Microelectrodes, Bedford, N.H., USA) as described in McGuire and Teskey (2002). Stem temperatures were measured with thermocouples and ranged from 20.7 to 37.0°C over the course of the experiment, with ≤2.0°C variation among stems within each replication. The experiment was repeated five times for each species.
To measure CO2 efflux from the stems to the atmosphere, we placed a 6 cm diameter ×11.5 cm long cylindrical PVC cuvette around each stem, 5 cm above the graduated cylinder. The cuvette was secured to the stem at both ends with gaskets constructed of 2 cm thick closed cell foam, providing a gas-tight seal. Air from a compressed gas cylinder with near-ambient [CO2] was supplied to the cuvettes at a rate of 0.5 l min−1 using mass flow controllers (model FMA 5514, Omega Engineering, Stamford, Conn., USA). CO2 efflux into the cuvettes was measured with an infrared gas analyzer (IRGA) (model LI-6262, LiCor, Lincoln, Neb., USA) in open configuration and the rate of efflux was determined using standard calculations (Long and Hallgren 1985). Data from the microelectrode and IRGA measurements were used to assess the effect of xylem [CO2] on efflux-based calculations of stem respiration.
After measuring the rate of CO2 efflux from unwounded stems, we removed the cuvettes and drilled three 5.5 mm diameter holes through each stem. We quickly replaced the cuvettes over the holes on the stems, usually within 1 min, and monitored CO2 efflux for another 15 min. Data from this part of the experiment were used to assess the effect of xylem [CO2] on putative wound respiration.
Data were compared within species using linear regression and among treatment levels using one-way analysis of variance and a paired t-test (SigmaStat 2.03, Systat Software, Point Richmond, Calif., USA).
Results
All trees retained turgor and were able to take up appreciable quantities of water during the 4 h treatment period. Based on visual observations of the cylinders, uptake ranged from ∼400 to 700 ml in sweetgum saplings and ∼200 to 400 ml in sycamore saplings. In both species, absorption of the treatment water was effective in changing xylem [CO2] (Fig. 1a). We observed a consistent difference in stem xylem [CO2] among the low, medium, and high treatment levels. Xylem [CO2] in sweetgum was consistently greater than in sycamore at each treatment level. The treatment produced a range of mean [CO2] from 1.4 (low), to 3.6 (medium) and 5.7% (high) in sycamore stems, and from 4.2 (low) to 8.2 (medium) and 10.8% (high) in sweetgum stems (Fig. 1). In both species, mean [CO2] in the stems subjected to the low treatment level was substantially greater than that in the applied solution (0.04%) or in the atmosphere (0.037%), indicating that CO2 released by respiring cells in the stem was retained in the xylem.
Mean xylem [CO2] in stems (a) and mean CO2 efflux from stems (b) of sycamore and sweetgum saplings infused with water with [CO2] of 0.4% (low), 8.8% (medium), or 14.1% (high). Different letters on same graph indicate significant differences within species (n=5, α=0.05). Bars are standard errors of the means
Mean stem CO2 efflux was proportional to mean xylem [CO2] (Fig. 1b) and was significantly different among the three treatment levels in sweetgum and at the high level in sycamore. Comparing between the low and high treatment levels, mean stem CO2 efflux was approximately 7 times greater at the high level, changing from 0.3 to 2.3 μmol m−2 s−1 in sycamore and from 0.6 to 4.4 μmol m−2 s−1 in sweetgum.
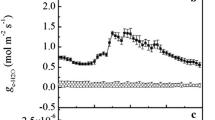
In individual saplings, substantial differences in xylem [CO2] were evident within and among treatment levels (Fig. 2a,b). Across all levels, xylem [CO2] ranged from 0.4 to 7.6% in sycamore and 1.1 to 17.5% in sweetgum. The greatest variation was at the high treatment level, where stem [CO2] differed by as much as 3.6% among individual sycamore stems and 12.9% among sweetgum stems. Differences in xylem [CO2] between species may have been caused by several factors, including possible restrictions to uptake due to embolisms at the cut end of the stem, variation in cross-sectional area of xylem and variation in total leaf area. Differences among replications within species were likely the result of variation in environmental factors, i.e. temperature, vapor pressure deficit, and radiation, that affected stomatal conductance and transpiration. Temperature may have also affected the solubility of CO2 in water. Irrespective of the effectiveness of absorption of the treatment water, CO2 efflux from the stems was closely correlated with xylem [CO2] in both species (Fig. 2a,b). Plotting the combined data from all treatment levels and both species reveals a remarkable consistency in the linear relationship across a 4-fold range of CO2 efflux (Fig. 2c).
Relationship between mean xylem [CO2] and mean stem CO2 efflux of stems of sycamore saplings (a), and sweetgum saplings (b) infused with water with [CO2] of 0.4% (low), 8.8% (medium), or 14.1% (high). Each point represents efflux from an individual sapling at its measured xylem [CO2]. Combined data (c) from graphs a and b, illustrating the consistency of the relationship between stem CO2 efflux and xylem [CO2]. Line is the linear regression between efflux and xylem [CO2]. (Note different scales in subplots)
After wounding, there was a substantial increase in CO2 efflux from the stems of both species (Fig. 3). Across treatment levels, mean post-wound efflux increased 4- to 5-fold in sycamore and 5- to 7-fold in sweetgum, compared with mean pre-wound efflux (Fig. 3 cf. Fig. 1b). For the entire dataset, the difference between pre-and post-wound efflux was statistically significant (n=30, t=−8.903, P=<0.001). A consistent pattern was also evident in post-wound efflux among the [CO2] treatment levels, i.e., greater post-wound efflux at higher [CO2] treatment levels (Fig. 3a); however, there was only one statistically significant difference. Similar to pre-wound measurements, post-wound efflux from individual stems remained highly correlated with internal [CO2] in both species (Fig. 4). Xylem [CO2] alone explained 84% and 81% of variation in stem CO2 efflux after wounding.
a Mean post-wound CO2 efflux from stems of sycamore and sweetgum saplings infused with water with [CO2] of 0.4% (low), 8.8% (medium), or 14.1% (high). Different letters indicate significant differences within species (n=5, α=0.05, ns non-significant). Bars are standard errors of the means. b Mean percent increase in CO2 efflux after wounding
Relationship between mean xylem [CO2] and mean post-wound stem CO2 efflux of stems of sycamore saplings (a), and sweetgum saplings (b), infused with water with [CO2] of 0.4% (low), 8.8% (medium), or 14.1% (high). Each point represents efflux from an individual sapling at its measured xylem [CO2]. Combined data (c) from graphs a and b, illustrating the consistency of the relationship between stem CO2 efflux and xylem [CO2]. Line is the linear regression between efflux and xylem [CO2]. (Note different scales in subplots)
The difference between pre- and post-wound efflux of individual stems increased as xylem [CO2] increased (Fig. 5). We attribute this response to enhanced diffusion of CO2 caused by the increase in the concentration gradient from stem to atmosphere as stem xylem [CO2] increased.
Discussion
This experiment verified that CO2 can be transported in the xylem of trees. The effect appeared to be additive at the higher [CO2] treatment levels, and was reflected in CO2 efflux from the stem. Interestingly, within each treatment level, stem [CO2] was much greater in sweetgum compared to sycamore, which may indicate a greater proportion of live cell volume in the sweetgum stems. Alternatively, [CO2] in the sweetgum stems may have been greater than in the sycamore stems because they took up more treatment water. The difference in xylem [CO2] between sycamore and sweetgum stems cannot be attributed to differences in the rate of CO2 diffusion from the xylem to the atmosphere, i.e., greater permeability of sycamore bark, because the rate of CO2 efflux was lower in sycamore than sweetgum, and directly proportional to internal [CO2] in both species (Fig. 2).
The [CO2] measured in the stems was sometimes less than the [CO2] in the treatment water. Several factors, alone or in combination may have caused this difference, including xylem cavitation or blockage leading to incomplete replacement of the pre-existing xylem water with the treatment water, loss of CO2 to radial diffusion to the atmosphere, or photosynthetic fixation by green tissue in the stems. In some cases, most notably in sweetgum subjected to the medium and high [CO2] levels, the variation in [CO2] among stems within the same treatment level was quite large. The effect of treatment on stem [CO2] was much more consistent in sycamore stems.
Our direct manipulation of [CO2] in the xylem of sapling stems confirmed the overwhelming effect of CO2 transported in xylem sap on the rate of CO2 efflux to the atmosphere. Prior to wounding, CO2 efflux in both species was linearly correlated with xylem [CO2] across the range measured, substantiating our previous findings in large Liriodendron tulipifera and Quercus alba stems that CO2 efflux is affected by internal stem [CO2] and that CO2 is transported in xylem sap (Teskey and McGuire 2002). These results suggest that estimates of stem respiration based on measurements of CO2 efflux are confounded by high internal [CO2].
Similar to the initial measurements, efflux of CO2 from the stems after wounding was proportional to xylem [CO2] in both species. Fifteen minutes after wounding, CO2 efflux was 4 to 7 times greater than prior to wounding, and the difference in pre- and post-wound efflux increased with increasing stem [CO2]. The large immediate increase in CO2 efflux from wounded woody tissue appears to differ from the much slower response to trauma observed over days or weeks associated with cell repair and callus formation (Uritani and Asahi 1980). In this experiment, trauma affected only a small portion of cells in the sample enclosed in the cuvette. Approximately 2% of the total tissue volume and 4% of the surface area of the measured sample was injured. Injured but functioning cells and uninjured neighboring cells may have increased their rate of respiration, but to account for the rapid increase in CO2 efflux from the stem, those cells would need to respire at a tremendous rate, equivalent to an instantaneous increase of 100 to 200 times (surface area basis) or 200 to 400 times (volume basis) greater than their pre-wound rate. Rapid diffusion of CO2 from the xylem when barriers to diffusion were removed by wounding is a more likely explanation for the increase in CO2 efflux and explains why efflux after wounding was proportional to xylem [CO2]. The high internal [CO2] in stems observed in this experiment are consistent with previous reports of stem [CO2] (Bushong 1907; MacDougal and Working 1933; Chase 1934; Jensen 1967; Eklund 1990, 1993; Hari et al. 1991; Levy et al. 1999; Teskey and McGuire 2002). These high values, relative to atmospheric [CO2], indicate that there are barriers to radial diffusion of CO2 from stems. Wounding woody tissues alters or removes these barriers, and CO2 efflux is substantially greater as a result of the diffusion of CO2 from the cut or open surfaces. Our results indicate that respiration estimates calculated from efflux measurements made on excised woody tissues (Levy and Jarvis 1998; McCutchan and Monson 2001; Burton and Pregitzer 2002; Desrochers et al. 2002) may have been affected by diffusion of CO2 from cut surfaces. Rapid diffusion from xylem may also explain the initial high CO2 efflux observed in tree roots shortly after excision (Rakonczay et al. 1997). It is likely that the diffusion of dissolved CO2 from xylem sap of excised tissues continues at progressively slower rates for hours or days, so that an unknown proportion of CO2 released during these measurements should not be attributed to the immediate rate of respiration. Similarly, if respiration measurements are made on intact stems in situ, but the barriers to diffusion on the stem surface are diminished by removing a portion of the bark, an error from increased release of xylem CO2 will result, in addition to the error caused by high [CO2] in the xylem.
By increasing the [CO2] in the xylem within a realistic range observed in trees, we increased stem CO2 efflux, typically interpreted as the rate of stem respiration, 5-fold (Fig. 2). Therefore, we conclude that xylem [CO2] affects the rate of efflux from stems regardless of the actual rate of respiration and that CO2 efflux arises from a combination of CO2 produced locally and CO2 transported to the site of measurement from an unknown location lower in the stem, root, or the soil. These experiments verified and expanded our recent evidence of this phenomenon observed in other tree species (Teskey and McGuire 2002). This study also provided evidence that the rapid increase in CO2 efflux observed after tissues are excised or injured is likely the result of the rapid diffusion of CO2 from the xylem, rather than an actual increase in the rate of respiration of wounded tissues.
References
Boysen-Jensen P (1933) Respiration I stamme og grene af traer. Sven Skogsvards Tidskr 31:239–241
Burton AJ, Pregitzer KS (2002) Measurement carbon dioxide concentration does not affect root respiration of nine tree species in the field. Tree Physiol 22:67–72
Bushong FW (1907) Composition of gas from cottonwood trees. Kans Acad Sci Trans 21:53
Chase WW (1934) The composition, quantity, and physiological significance of gases in tree stems. Minnesota Agricultural Experiment Station Technical Bulletin 99, University of Minnesota, Minneapolis
Desrochers A, Landhausser SM, Lieffers VJ (2002) Coarse and fine root respiration in aspen (Populus tremuloides). Tree Physiol 22:725–732
Eklund L (1990) Endogenous levels of oxygen, carbon dioxide and ethylene in stems of Norway spruce trees during one growing season. Trees 4:150–154
Eklund L (1993) Seasonal variations of O2, CO2, and ethylene in oak and maple stems. Can J For Res 23:2608–2610
Hari P, Nygren P, Korpilahti E (1991) Internal circulation of carbon within a tree. Can J For Res 21:514–515
Jensen KF (1967) Measuring oxygen and carbon dioxide in red oak trees. U.S. Forest Service Research Note NE-74. USDA Forest Service, Northeastern Forest Experiment Station
Johansson N (1933) Om foervedade stammars andning, dess faststaellande och betydelse. Sven Skogsvards Tidskr 31:242–249
Levy PE, Jarvis PG (1998) Stem CO2 fluxes in two Sahelian shrub species (Guiera senegalensis and Combretum micranthum). Funct Ecol 12:107–116
Levy PE, Meir P, Allen SJ, Jarvis PG (1999) The effect of aqueous transport of CO2 in xylem sap on gas exchange in woody plants. Tree Physiol 19:53–58
Long SP, Hallgren J-E (1985) Measurement of CO2 assimilation by plants in the field and in the laboratory. In: Coombs J, Hall DO, Long SP, Scurlock JMO (eds) Techniques in bioproductivity and photosynthesis. Pergamon, Oxford, UK, pp 62–94
MacDougal DT, Working EB (1933) The pneumatic system of plants, especially trees. Publication 441. Carnegie Institute of Washington, Washington, D.C.
McCutchan CL, Monson RK (2001) Effects of tissue-type and development on dark respiration in two herbaceous perennials. Ann Bot 87:355–364
McGuire MA, Teskey RO (2002) Microelectrode technique for in situ measurement of carbon dioxide concentrations in xylem sap of trees. Tree Physiol 22:807–811
Oohata S, Shidei T, Tsuji H, Hatakeyama I (1967) Changes in respiratory rates of excised tree organs. Bull Kyoto Univ For 39:100–109
Rakonczay Z, Seiler JR, Kelting DL (1997) Carbon efflux rates of fine roots of three tree species decline shortly after excision. Environ Exp Bot 38:243–239
Sprugel DG, Benecke U (1991) Measuring woody tissue respiration and photosynthesis. In: Lassoie JP, Hinckley TM (eds) Techniques and approaches in forest tree ecophysiology. CRC, Boca Raton, Fla., pp 329–347
Teskey RO, McGuire MA (2002) Carbon dioxide transport in xylem causes errors in estimation of rates of respiration in stems and branches of trees. Plant Cell Environ 25:1571–1577
Uritani I, Asahi T (1980) Respiration and related metabolic activity in wounded and infected tissues. In: Stumpf PK, Conn EE (eds) The biochemistry of plants: a comprehensive treatise, vol 2. Academic, New York, pp 463–485
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Teskey, R.O., McGuire, M.A. CO2 transported in xylem sap affects CO2 efflux from Liquidambar styraciflua and Platanus occidentalis stems, and contributes to observed wound respiration phenomena. Trees 19, 357–362 (2005). https://doi.org/10.1007/s00468-004-0386-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-004-0386-z