Abstract
The CO2 released from respiring cells in woody tissues of trees can contribute to one of three fluxes: efflux to the atmosphere (EA), internal xylem sap transport flux (FT), and storage flux (∆S). Adding those fluxes together provides an estimate of actual stem respiration (RS).We know that the relative proportion of CO2 in those fluxes varies greatly among tree species, but we do not yet have a clear understanding of the causes for this variation. One possible explanation is that species differ in stem radial CO2 conductance (gc). A high gc would favor the EA pathway and a low gc would favor the FT pathway. However, gc has only been measured once in situ and only in a single tree species. We measured gc using two methods in stems of Fraxinus mandshurica Rupr. (ash) and Betula platyphylla Suk. (birch) trees in situ, along with RS, EA, FT and ∆S. Stem radial CO2 conductance was substantially greater in ash trees than in birch trees. Corresponding to that finding, in ash trees over 24 h, EA constituted the entire flux of respired CO2, and FT was negative, indicating that additional CO2, probably transported from the root system via the xylem, was also diffusing into the atmosphere. In ash trees, FT was negative over the entire 24 h, and this study represents the first time that has been reported. The addition of xylem-transported CO2 to EA caused EA to be 9% higher than the actual RS over the diel measurement period. Birch trees, which had lower gc, also had a more commonly seen pattern, with EA accounting for about 80% of the CO2 released from local cell respiration and FT accounting for the remainder. The inorganic carbon concentration in xylem sap was also lower in ash trees than in birch trees: 2.7 versus 5.3 mmol L−1, respectively. Our results indicate that stem CO2 conductance could be a very useful measurement to help explain differences among species in the proportion of respired CO2that remains in the xylem or diffuses into the atmosphere.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The CO2 released by respiring cells in woody tissues can diffuse to the atmosphere through bark (CO2 efflux, EA), be transported in xylem sap (transport flux, FT) or remain in the stem (storage flux, ∆S) (McGuire and Teskey 2004). Thus, the rate of stem respiration (RS) can be calculated as RS = EA + FT + ∆S. The relative distribution of RS to EA, FT and ∆S differ widely among tree species. In recent studies, over 24 h, the lowest distribution of RS to EA was 0.45 in Platanus occidentalis (McGuire and Teskey 2004), and the highest was 1.00 in Quercus pyrenaica (Salomón et al. 2016). The lowest distribution of RS to FT was − 0.4 in Q. pyrenaica (Salomón et al. 2016), and the highest was 0.55 in Populus deltoides (McGuire and Teskey 2004).The calculated ∆S flux was small in all studies, usually less than 1–3% of EA and FT combined.
Many more studies have measured EA than RS. As for RS, these studies have also shown that EA differs greatly among tree species. For example, mean annual EA in Larix gmelinii was 44% greater than that of Pinus koraiensis growing in adjacent stands under very similar air temperatures (Yang et al. 2012a).In another study, mean stem CO2 efflux, standardized to 10 °C, in Quercus acutissima, Pinus massoniana and Pinus taeda growing at the same site was 1.87, 1.12 and 0.90 µmol m−2 s−1, respectively (Yang et al. 2012b), a twofold difference between species that was not related to temperature. Species differences in EA may reflect actual differences in rates of respiration among trees. However, we speculate that differences in EA could also reflect species differences in anatomical and morphological characteristics that affect stem radial CO2 conductance (gc). Little is known about species variation in gc because it has only been measured on one species in one study (Steppe et al. 2007). No measurements have compared gc between species.
In all measurements thus far, the CO2 concentration [CO2] in stems has been much higher than the [CO2] in the atmosphere. Across multiple studies, the [CO2] measured in tree stems of different species ranged from 1.6 to 26%, substantially higher than the atmospheric [CO2] of 0.04% (Teskey et al. 2008). The causes of high variability in stem [CO2] among species have not been identified. This variation may reflect large differences among species in rates of CO2 evolution from respiring cells or differences in the number, activity, or location of respiring cells in the stem. It may also reflect species differences in diffusional resistance. The high stem [CO2] observed in all species relative to atmospheric [CO2] indicates that CO2 released from respiring cells in woody tissues cannot readily move from the stem into the atmosphere. The locations of the barriers to diffusion have not yet been clearly identified. However, the cuticle or barkon the surface of stems was shown to strongly reduce the exchange of CO2 and O2 (Lendzian 2006), and the xylem itself can also be a barrier to gaseous diffusion (Sorz and Hietz 2006). These factors are likely to vary among species and could affect gc, which in turn would affect the quantity of CO2 remaining within the stem or diffusing into the atmosphere. We already know that sap pH, temperature and sap flux density affect the concentration of CO2 in the stem, which in turn affects the diffusional gradient for CO2 to the atmosphere; however, we have not yet evaluated the role of gc in the balance between EA, FT and ∆S fluxes.
In this study, we calculated stem radial CO2 conductance as well as RS, EA, FT and ∆S in Fraxinus mandshurica Rupr. (ash) and Betula platyphylla Suk. (birch) trees. Xylem anatomy is ring-porous in Fraxinus and diffuse-porous in Betula. We hypothesized that there would be differences in stem radial CO2 conductance between the two species and that those differences would affect stem [CO2] and the relative proportions of respired CO2 distributed to EA and FT.
Materials and methods
Three ash trees and three birch trees growing outdoors in the Northeast Forestry University Arboretum (E126°38′, N45°43′) were used in this study. Measurements were made on ash trees with diameters between 13.1 and 16.1 cm and birch trees with diameters between 11.5 and 13.5 cm (Table 1).
Stem temperature and sap flow
Holes 5 mm in diameter were drilled to a depth of 1 cm on the north and south sides of the stem at 1.3 m above the ground. Thermostats (TR-1106, T&D Corp., Japan) were installed in the holes and sealed with rubber cement. Stem temperature was measured and recorded every 10 min with a datalogger (Tr-71U, T&D Corp. Japan).
Sap flow velocity (cm s−1) was measured with pre-calibrated thermal dissipation sensors (TDP-30, DynaMax, Houston, TX, USA) installed at 1.3 m aboveground on the north and south sides of the stem on each tree. Sensors were protected from solar heating by wrapping stems with reflective insulation. Sap flow velocity was measured and recorded every 10 min with a datalogger (CR10X, Campbell Scientific, Logan, UT, USA). After all other stem measurements were complete; a radial core was taken from each tree with an increment borer (10-100-1007, Haglöf, Sweden) and used to calculate sapwood area. Sapwood area was used to convert measurements of sap flow velocity to sap flux density (J, g cm−2 h−1) and sap flow (fS, L h−1) according to the equations of Granier (1985).
CO2/H2O gas exchange
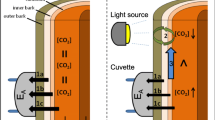
The efflux of CO2 and H2O from the stem surface was measured in situ using a chamber that surrounded a portion of the stem. Chamber construction followed that described in McGuire and Teskey (2004). Chambers were constructed around each stem at a height of 0.5 m. First, to form the support rings at the top and bottom of the chamber, the stem was tightly wrapped with two elastic rubber tubes (20 mm diameter), which were placed 0.5 m apart vertically. Rubber cement was used to bond the rubber tube to the bark to form a gas-tight seal, and the cut ends of each tube were sealed together with waterproof silicone glue. Then, double-sided adhesive tape was applied to cover the entire surface of each the support ring, and polyethylene film was wrapped around the tree over the support rings and attached to the adhesive tape. The junction of the two vertical edges of the film was also sealed with tape. The chamber was connected to an infrared CO2/H2O analyzer (IRGA) (Model 7000; Li-Cor, Lincoln, NE, USA) via plastic tubing inserted through the support rings. Air was supplied to the chamber with a pump (model 6400-907; Li-Cor). The IRGA was operated in a differential configuration, i.e., it measured [CO2] and [H2O] of the air flowing both into the chamber (reference) and out of the chamber (sample). The CR10X datalogger was used to control three solenoid valves (E2-1-HC-1200VDC, Versa Products, Paramus, NJ, USA) to switch the gas flow to three chambers to measure three trees in sequence. The gas flow rate was measured with a mass flow meter (AWM5000, Honeywell, St. Morris, NJ, USA). Ash trees were measured on September 3, 2008, and birch trees were measured on September 13, 2008. The two species could not be measured simultaneously because the trees of the two species were not adjacent to each other, and we did not have duplicate equipment, so the trees were measured on two dates, 10 days apart, that had similar environmental conditions; warm and sunny with few clouds and no precipitation. Over 24 h, mean stem temperature was 20.8 °C in ash trees and 17.6 °C in birch trees, and soil volumetric water content at 20 cm depth was 18.47% in ash and 18.79% in birch on the measuring day. Each set of three trees was measured every 30 min over 24 h. Data were logged every minute and averaged and recorded with the datalogger at the end of each measurement (last 6 of 10 min averaged for each tree in each 30-min cycle).
Stem respiration and its component fluxes
Total stem respiration (RS) was calculated following McGuire and Teskey (2004) as RS = EA + FT + ΔS. Stem CO2 efflux (EA) was calculated as
where fA is the mass air flow rate through the chamber, v is the sapwood volume of the stem surrounded by the chamber, and Δ[CO2] is the difference in the CO2 concentration of air entering and exiting the chamber.
To determine changes in the quantity of CO2 stored in the xylem (ΔS) and the quantity of CO2 transported in xylem sap (FT), stem internal CO2 concentration [CO2] was measured. To measure [CO2], holes with a diameter of 20 mm and depth of 50 mm were drilled above and below the efflux chamber. Non-dispersive infrared (NDIR) CO2 sensors (GMM221, Vaisala, Finland) were placed in the holes to measure gaseous [CO2] in the xylem. The sensors were sealed to the tree with flexible putty adhesive (Blu-Tack, Bostik, Victoria, Australia) to prevent direct gas exchange between the xylem and the atmosphere. The sensors were connected to the data logger and measurements were made every minute and averaged and recorded at 10-min intervals.
The total dissolved inorganic carbon in the stem ([\({\text{CO}}_{2}^*\)], i.e., gaseous and dissolved CO2, µmol L−1), was calculated from measurements of [CO2], stem temperature, and sap pH using Henry’s Law (Butler 1991; McGuire and Teskey 2002). To obtain sap pH, we used a pressure chamber (SKPM1400-50, Skye Instruments, Llandrindod Wells, UK) to express sap from several detached twigs of each tree during the measurement period. The pH of expressed sap was measured immediately with a pH microelectrode (Model PHS-3C, Shanghai INESA Scientific Instrument, Shanghai, PRC) and averaged for each tree (Table 1). Aubrey et al. (2011) observed that pH was similar when measured in sap expressed from stems andtwigs, and it remained relatively constant through a diel period.
The change in xylem [\({\text{CO}}_{2}^*\)] over time (storage flux, ΔS) was calculated as
where [\({\text{CO}}_{2}^*\)]st0 and [\({\text{CO}}_{2}^*\)]st1 are the average CO2 concentration for the stem segment (mean of measurements above and below the efflux chamber) at time t0 and t1, Δt = t1 − t0 (s) and L is the amount of water in the stem segment.
An estimate of the water content of the sapwood was needed to determine ∆S. In a test of the sensitivity of ΔS to volumetric water content, changing the value of from 300 to 700 L m−3 caused relatively small changes in the estimate of ∆S, the smallest component of RS (McGuire and Teskey 2004). For this study, we assumed sapwood water content was 50% of sapwood volume, i.e., 500 L m−3,based on the average specific gravity for hardwoods (Panshin and de Zeeuw 1980).
The upward transport of CO2 in the sap (transport flux, FT) was calculated as
where fS is sap flow (L s−1), Δ[\({\text{CO}}_{2}^*\)] is the difference in the CO2 concentration above and below the efflux chamber (above–below), and v is the volume of sapwood surrounded by the efflux chamber.
Stem radial conductance to CO2
The radial conductance of CO2 through the stems was calculated in two ways:
-
1.
Stem CO2 conductance was calculated based on H2O conductance (\( g_{{\mathrm{c}}{\text{-}}{{\mathrm{H}}_{ 2}{\text{O}}}} \), molCO2 m−2s−1) following Wittmann et al. (2006) as
$$ g_{{\mathrm{c}}{\text{-}}{{\mathrm{H}}_{2}{\text{O}}}} = g_{\mathrm{tw}} /1.56, $$(4)where gtw was periderm conductance of water vapor and 1.56 is the ratio of the diffusivity of water vapor to the diffusivity of CO2 in air (Campbell and Norman 1998).
Periderm conductance to water vapor was calculated as
$$ g_{\mathrm{tw}} = E_{{{\mathrm{H}}_{2} {\text{O}}}} /\left( {W_{\mathrm{i}} - W_{\mathrm{a}} } \right), $$(5)where \( E_{{{\mathrm{H}}_{2} {\text{O}}}} \) was stem H2O efflux Wi and Wa were the molar concentrations of water vapor in the stem and the air, respectively. (Wi was assumed to be at saturation.)
Stem H2O efflux (\( E_{{{\mathrm{H}}_{2} {\text{O}}}}, \) mmol m−2 s−1) was calculated as
$$ E_{{{\mathrm{H}}_{2} {\text{O}}}} = f_{\mathrm{A}} *\Delta \left[ {{\text{H}}_{2} {\text{O}}} \right]/a, $$(6)where fA was the air flow rate through the stem chamber (mol s−1), a was the surface area of the stem surrounded by the chamber (m−2), and Δ[H2O] was the difference in H2O concentration of the air entering and exiting the chamber (mmol mol−1).
-
2.
Stem CO2 conductance was also calculated based on the difference in CO2 concentration between the xylem and the atmosphere (\( g_{{{\mathrm{c}}{\text{-}}{\mathrm{CO}}_{2} }} \), m s−1) following Steppe et al. (2007):
$$ g_{{{\mathrm{c-CO}}_{ 2} }} = E_{\mathrm{A - S}} /\left[ {{\text{CO}}_{2} } \right]_{\mathrm{D}} , $$(7)where EA-S was stem CO2 efflux on a stem surface area basis (µmol m−2 s−1) and [CO2]D was the difference in CO2 concentration between air in the xylem and the atmosphere (µmol mol−1) calculated as
$$ \left[ {{\text{CO}}_{2} } \right]_{\mathrm{D}} = \left( {\left[ {{\text{CO}}_{2} } \right]_{\mathrm{xylem}} {-}\left[ {{\text{CO}}_{2} } \right]_{\mathrm{air}} } \right)/22.4 \times 1000, $$(8)where [CO2]xylem is the mean [CO2] in the xylem measured with the NDIR sensors (µmol mol−1), [CO2]air was the [CO2] of the air entering the efflux chamber measured with the IRGA (µmol mol−1), 22.4 is the molar volume of gas (L mol−1), and 1000 converted values of [CO2]D to units of µmol m−3.
Statistical analyses
A multiple linear regression model was used to relate EA with stem temperature (TS) and sap flux density (J):
where β0,β1, β2 and β3 are regression coefficients, TS is stem temperature, J is sap flux density and \( \varepsilon \) is the random error term which followed a normal distribution N (µ, \( \upsigma^{2} \)). The relative weight of each parameter was calculated using the R package RELAIMPO (Gromping 2006). A one-way ANOVA was used to detect differences in \( g_{{\mathrm{c}}{\text{-}}{{\mathrm{H}}_{ 2}{\text{O}}}} \) and \(g_{{{\mathrm{c}}{\text{-}}{\mathrm{CO}}_{2} }}\) between ash and birch trees (n = 3).
Results
Stem radial conductance to CO2
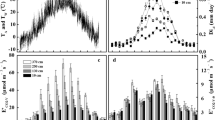
Over 24 h, mean stem CO2 conductance calculated from stem H2O efflux (\( g_{{\mathrm{c}}{\text{-}}{{\mathrm{H}}_{ 2}{\text{O}}}} \)) was greater in ash trees than in birch trees (p < 0.01). It was 0.87 mol m−2 s−1 in ash trees and 0.06 mol m−2 s−1 in birch trees. Diel variation in \( g_{{\mathrm{c}}{\text{-}}{{\mathrm{H}}_{ 2}{\text{O}}}} \) was also much larger in ash trees than in birch trees (Fig. 1). It ranged from 0.56 to 1.35 mmol m−2 s−1 in ash trees. In comparison, \( g_{{\mathrm{c}}{\text{-}}{{\mathrm{H}}_{ 2}{\text{O}}}} \) varied from only 0.05 to 0.09 mmol m−2 s−1 in birch trees. Corresponding with this finding, diel variation in stem H2O efflux was also much larger in ash trees than in birch trees. In ash trees, stem H2O efflux ranged from 12 to 38 mmol m−2 s−1. In birch trees, stem H2O efflux varied between 4 and 10 mmol m−2 s−1.
The diel pattern of mean stem H2O efflux (a), stem CO2 conductance based on H2O conductance (\( g_{{\mathrm{c}}{\text{-}}{{\mathrm{H}}_{ 2}{\text{O}}}} \)) (b), or CO2 conductance (\(g_{{{\mathrm{c}}{\text{-}}{\mathrm{CO}}_{2} }}\)) (c) in ash trees and birch trees. Vertical bars indicate standard error
Mean stem CO2 conductance calculated from the difference in [CO2] between stem and atmosphere (\(g_{{{\mathrm{c}}{\text{-}}{\mathrm{CO}}_{2} }}\)) was greater in ash trees than in birch trees: 1.48 e−06 versus 0.67 e−06 m s−1 (p < 0.05). Diel variation in \(g_{{{\mathrm{c}}{\text{-}}{\mathrm{CO}}_{2} }}\) was also greater in ash trees than in birch trees (Fig. 1). In ash trees, \(g_{{{\mathrm{c}}{\text{-}}{\mathrm{CO}}_{2} }}\) ranged between 1.30 e−06 and 1.71 e−06 m s−1 in ash trees and between 0.58 e−06 and 0.74 e−06 m s−1 in birch trees.
Between-species comparison of the component fluxes of stem respiration and sap [CO2]
Stem CO2 efflux was the main component of RS over 24 h in both species (Fig. 2, Table 2). Mean 24-h EA was 83.9 µmol m−3 s−1 in the ash trees and 67.0 µmol m−3 s−1 in the birch trees. In ash trees, which had higher stem CO2 conductance, EA exceeded RS indicating that part of the CO2 diffusing from the stem into the measurement cuvette was from xylem-transported CO2. In birch trees, which had lower stem CO2 conductance, EA represented 80% of RS with the remaining 20% accounted for by FT. Over the 24-h measurement period, mean FT was negative in ash trees (− 7.3 µmol m−3 s−1) and positive in birch trees (21.5 µmol m−3 s−1).Storage flux was a very small part of the total flux over 24 h, representing < 1% of RS in both species.
Diel patterns of a, d mean stem respiration (RS) and mean component fluxes: efflux to atmosphere (EA), transport flux (FT), and storage flux (∆S) and b, e mean sap flux density and stem temperature of the stem segment enclosed in the chamber, and c, f mean xylem sap CO2 concentration [\({\text{CO}}_{2}^*\)] above (upper) and below (lower) the chamber. Left panels, ash; right panels, birch. Symbols represent mean of three trees per species. Error bars indicate standard error
Data were also examined in 6-h periods to determine whether there were diel differences between species in RS and the component fluxes (Table 2). Stem respiration was substantially higher in birch trees than ash trees from 06:00 to 18:00 h, when most sap flow was occurring. However, EA was lower in birch trees than in ash trees during that time. A substantial difference between the two species was that in ash trees FT was negative at all times during the diel period, while it was positive in birch trees. From 06:00 to 18:00 h, in birch trees FT accounted for − 37% of the CO2 released from respiring cells and EA accounted for − 63% of the CO2. In contrast, for ash trees in the same time period, FT was negative, caused by the diffusion outward of a combination of locally respired CO2 and transported CO2, which resulted in depletion of CO2 in the xylem sap and inflated EA estimates. In trees of both species, diel variation was larger in FT compared with the other fluxes because FT was − 0 at night when sap was not flowing. Mean maximum FT was approximately − 13 µmol m−3 s−1 in ash trees, and 43 mol m−3 s−1 in birch trees, with the peak occurring between 12:00 and 18:00 h in conjunction with maximum sap flux density, which was 6.5 g cm−2 h−1 in ash trees and 15.4 g cm−2 h−1 in birch trees.
Total dissolved inorganic carbon in xylem sap [\({\text{CO}}_{2}^*\)] was also lower in ash trees than in birch trees (Table 2). Over 24 h, mean [\({\text{CO}}_{2}^*\)] was 2.7 mmol L−1 in ash trees and 5.3 mmol L−1 in birch trees. Diel variation in [\({\text{CO}}_{2}^*\)] was also observed. In ash trees, the mean maximum [\({\text{CO}}_{2}^*\)] was 3.1 mmol L−1 and occurred between 18:00 and 24:00 h. In birch trees, the mean maximum [\({\text{CO}}_{2}^*\)] was 5.6 mmol L−1 and occurred later at night, between 00:00 and 06:00 h.
Correlating TS and J with EA
A regression analysis was done for the 06:00–18:00 time period, i.e., when sap was flowing, to compare the effect of TS and J on EA in the two species. During that time, TS and J explained most of the variation in EA for both species, with a combined r2 of 0.90 for the ash trees and 0.87 for the birch trees (Table 3). In birch trees, TS alone accounted for 88% of the variation in EA, while J only accounted for 11%. In contrast, in birch trees TS accounted for 43% of the variation in EA, while J accounted for 57%.
Discussion
This study has shown for the first time that the CO2 conductance through the stem (gc) substantially differed between two tree species, with lower gc in birch trees than in ash trees. The bark of birch trees forms multiple layers of large contiguous thin sheets, which are single layers of phellem (Schonherr and Ziegler 1980). The low radial water and CO2 transport measured in birch suggests that these heavily suberized cell layers were a barrier to the diffusion of both water vapor and CO2. In contrast, the bark of ash does not form large sheets, but is thick and spongy with many longitudinal ridges and furrows (Zhang 2010).This anatomy may have been more conducive to the escape of water vapor and CO2 and could be a reason why EA was a much greater proportion of RS in ash trees than in birch trees. In addition to bark anatomy, several other factors could affect gc including wood anatomy, xylem water content, and stem temperature (Lendzian 2006; Sorz and Hietz 2006).
We observed diel variation in gc using both methods of calculation, although it was much more pronounced in the calculation based on H2O efflux and more pronounced in ash trees than in birch trees. The cause of that variation is unknown, but we suggest that it may have been related to changes stem or bark water content or water potential or to the diffusion gradient for water vapor and CO2 from stem to atmosphere. The difference between the two approaches was probably a major cause of the differing lengths of the pathway for H2O and CO2. However, because the stem gc measurement using CO2 is the more direct measurement, which avoids the assumption that water and CO2 move through the stem proportionally, and the greater diel consistency in the measurements suggest that \(g_{{{\mathrm{c}}{\text{-}}{\mathrm{CO}}_{2} }}\) is the better approach for comparing stem conductance among species. It is likely that gc not only varies among species, but that it also varies in different parts of trees and in trees of different sizes. Stems and branches have very different bark and cambial thickness and morphology, live cell volume, radial path length for the movement of CO2 from respiring cells in the xylem, and [CO2] (Teskey et al. 2008). High variation in EA was observed in simultaneous measurements of branches and stems of Abies amabilis trees, which was attributed to differences in the permeability of bark layers to CO2 (Sprugel 1990). Cavaleri et al. (2006) reported that EA at 25 °C of branches of tropical trees decreased with decreasing branch diameter and increased with canopy height for the same branch diameter.
Within-species variation in gc has also been reported (Steppe et al. 2007). We found some variation in stem gc among trees of the same species, but much less than the sixfold variation in \(g_{{{\mathrm{c}}{\text{-}}{\mathrm{CO}}_{2} }}\) found in three trees of Populus deltiodes of similar size growing on the same site reported by Steppe et al. (2007). They also found that the Populus trees with lower \(g_{{{\mathrm{c}}{\text{-}}{\mathrm{CO}}_{2} }}\) had lower EA and higher stem [\({\text{CO}}_{2}^*\)] compared to those with higher \(g_{{{\mathrm{c}}{\text{-}}{\mathrm{CO}}_{2} }}\), which had higher EA and lower stem [\({\text{CO}}_{2}^*\)]. In our study, there was a similar relationship between stem gc and stem [\({\text{CO}}_{2}^*\)]. In ash trees, high EA due to high gc likely resulted in low stem [\({\text{CO}}_{2}^*\)] and low FT, while in birch, low EA, high stem [\({\text{CO}}_{2}^*\)] and high FT corresponded with low gc. These findings support our hypothesis that species differences in stem gc affects the proportion of respired CO2 distributed to EA versus FT.
Many studies have reported differences in EA among species. For example, EA normalized to 15 °C varied from 0.5 to 2.7 nmol C mol−1 C sapwood s−1 among Pinus banksiana, Picea mariana and Populus tremuloides trees (Lavigne and Ryan 1997). While there are many possible reasons for these differences, including the number of live cells in the xylem and inner bark, stem oxygen concentration, and the respiratory activity of those cells, our results suggest that gc contributes to species differences in EA.
Teskey and McGuire (2007) found that, on average, 45% of EA in large P. occidentalis stems was from xylem-transported CO2, i.e., was from the diffusion of CO2 that originated below the site of measurement and was unrelated to the current rate of stem respiration. We observed that during the day in ash trees, EA was greater than RS, which indicated that high stem radial conductance allowed the CO2 that had been transported from lower in the stem or roots to flux outward to the atmosphere. This conclusion is also supported by the observed decrease in stem [\({\text{CO}}_{2}^*\)] with height in ash trees. Assuming that the gc of the ash stems was similar from the 0.5 m measurement height to the base of the stem, it is likely that the source of the transported CO2 was the root system. Aubrey and Teskey (2009) observed that − 67% of root-respired CO2 was transported into the stem in flowing sap in P. deltoides trees, indicating that root-respired CO2 could be a significant contributor to stem FT. They speculated that a large quantity of CO2 transported from roots into the stem would confound measurements of stem respiration based on EA alone and also those based on EA + FT + ΔS. Our study has provided empirical results to support that contention. Dissolved CO2 in the sap and FT in birch was greater than in ash. The CO2 transported in the xylem can be fixed, and the fixed CO2 delivered in the sap accounts for 2–9% of the CO2 produced via photosynthesis (Hari et al. 1991). It is possible that more CO2 could be re-fixed into the leaves for reuse in birch than in ash.
The results of this study suggest that gc can vary substantially among species, but it is important to note that the two species were measured 10 days apart. Even though the environmental conditions on the 2 days were similar, if environment has a large influence on gc it might have affected the results. We also do not know how gc changes with morphological development and whether species will have lesser or greater differences in gc at different stages of development or different ages. The values of gc may also change seasonally. Although there is still a great deal to learn about stem conductance, we conclude that measurements of gc have great potential to provide a better understanding stem respiration and the flux of respired CO2.
References
Aubrey DP, Teskey RO (2009) Root-derived CO2 efflux via xylem stream rivals soil CO2 efflux. New Phytol 184:35–40
Aubrey DP, Boyles JG, Krysinsky LS, Teskey RO (2011) Spatial and temporal patterns of xylem sap pH derived from stems and twigs of Populus deltoides L. Environ Exp Bot 71:376–381. https://doi.org/10.1016/j.envexpbot.2011.02.006
Butler JN (1991) Carbon dioxide equilibria and their applications. Lewis, Chelsea
Campbell GS, Norman JM (1998) An introduction to environmental biophysics. Springer, New York
Cavaleri MA, Oberbauer SF, Ryan MG (2006) Wood CO2 efflux in a primary tropical rain forest. Glob Change Biol 12(12):2442–2458
Granier A (1985) Une nouvelle méthode pour la mesure du flux de sève brute dans le tronc des arbres. Ann Sci For 42:193–200
Gromping U (2006) Relative importance for linear regression in R: the package relaimpo. J Stat Softw 17:1–27
Hari P, Nygren P, Korpilahti E (1991) Internal circulation of carbon within a tree. Can J For Res 21(4):514–515
Lavigne MB, Ryan MG (1997) Growth and maintenance respiration rates of aspen, black spruce and jack pine stems at northern and southern BOREAS sites. Tree Physiol 17(8–9):543–551
Lendzian KJ (2006) Survival strategies of plants during secondary growth: Barrier properties of phellems and lenticels towards water, oxygen, and carbon dioxide. J Exp Bot 57:2535–2546
McGuire MA, Teskey RO (2002) Microelectrode technique for in situ measurement of carbon dioxide concentrations in xylem sap of trees. Tree Physiol 22:807–811
McGuire MA, Teskey RO (2004) Estimating stem respiration in trees by a mass balance approach that accounts for internal and external fluxes of CO2. Tree Physiol 24:571–578
Panshin AJ, de Zeeuw C (1980) Textbook of wood technology, 4th edn. McGraw-Hill, New York
Salomón RL, Valbuena-Carabaña M, Gil L, McGuire MA, Teskey RO, Aubrey DP, González-Doncel I, Rodríguez-Calcerrada J (2016) Temporal and spatial patterns of internal and external stem CO2 fluxes in a sub-Mediterranean oak. Tree Physiol. https://doi.org/10.1093/treephys/tpw029
Schonherr J, Ziegler H (1980) Water permeability of Betula periderm. Planta 147:345–354
Sorz J, Hietz P (2006) Gas diffusion throughwood: implications for oxygen supply. Trees Struct Funct 20:34–41
Sprugel DG (1990) Components of woody-tissue respiration in young Abies amabilis Forbes trees. Trees Struct Funct 4:88–98
Steppe K, Saveyn A, McGuire MA, Lemeur R, Teskey RO (2007) Resistance to radial CO2 diffusion contributes to between-tree variation in CO2 efflux of Populus deltoides stems. Funct Plant Biol 34:785–792
Teskey RO, McGuire MA (2007) Measurement of stem respiration of sycamore (Platanus occidentalis L.) trees involves internal and external fluxes of CO2 and possible transport of CO2 from roots. Plant Cell Environ 30:570–579
Teskey RO, Saveyn A, Steppe K, McGuire MA (2008) Origin, fate and significance of CO2 in tree stems. New Phytol 177:17–32
Wittmann C, Pfanz H, Loreto F, Centritto M, Pietrini F, Alessio G (2006) Stem CO2 release under illumination: corticular photosynthesis, photorespiration or inhibition of mitochondrial respiration? Plant, Cell Environ 29:1149–1158
Yang JY, Teskey RO, Wang CK (2012a) Stem CO2 efflux of ten species in temperate forests in northeastern China. Trees Struct Funct 26:1225–1235
Yang QP, Xu M, Chi YG, Zheng YP, Shen RC, Li PX, Dai HT (2012b) Temporal and spatial variations of stem CO2 efflux of three species in subtropical China. J Plant Ecol 5:229–237
Zhang ZX (2010) Dendrology—the north, 2nd edn. China Forestry Publishing House, Beijing
Author information
Authors and Affiliations
Corresponding author
Additional information
Project funding: The work was supported by the National Natural Science Foundation of China (31670476 and 31100284) and the Fundamental Research Funds for the Central Universities (2572016CA02).
The online version is available at http://www.springerlink.com
Corresponding editor: Tao Xu.
Rights and permissions
About this article
Cite this article
Wang, X., Mao, Z., McGuire, M.A. et al. Stem radial CO2 conductance affects stem respiratory CO2 fluxes in ash and birch trees. J. For. Res. 30, 21–29 (2019). https://doi.org/10.1007/s11676-018-0737-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-018-0737-z