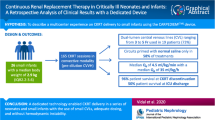
Abstract
Background
The Cardio-Renal Pediatric Dialysis Emergency Machine (CA.R.P.E.D.I.E.M.®) device is a continuous kidney replacement therapy (CKRT) equipment dedicated to neonates and small infants. This study aimed to assess the effectiveness, feasibility, outcomes, and technical considerations relating to CARPEDIEM® use.
Methods
This retrospective multicenter study included 19 newborns and six infants receiving CARPEDIEM® in five French pediatric and neonatal intensive care units. Laboratory parameters were collected at the initiation and end of the first CARPEDIEM® session. Results are presented as median [IQR] (range).
Results
At initiation, age was 4 days [2–13] (1–1134) with a body weight of 3.3 kg [2.5–4] (1.3–11.1). Overall, 131 sessions and 2125 h of treatment were performed. Treatment duration per patient was 42 h [24–91] (8–557). Continuous veno-venous hemofiltration (CVVH) was performed in 20 children. Blood flow rate was 8 mL/kg/min [6–9] (3–16). The effluent flow rate for CVVH was 74 mL/kg/h [43–99] (28–125) and net ultrafiltration (UF) 6 mL/kg/h [2–8] (1–12). In the five children treated by hemodialysis, the blood and dialysate flow rates were 6 mL/kg/min [5–7] (4–7) and 600 mL/h [300–600] (120–600), respectively, while session duration was 8 h [6–12] (2–24). Most infants required a catheter between 4.5 and 6.5 French. Hemodynamic instability with a need for volume replacement occurred in 31 sessions (23%). Thrombocytopenia was observed in 29 sessions (22%). No hemorrhage occurred; all the patients survived the sessions, but only eight patients (32%) were alive at hospital discharge.
Conclusions
These data confirm that the use of CARPEDIEM® is safe and effective in critically ill neonates and infants.
Graphical abstract

A higher resolution version of the Graphical abstract is available as Supplementary information
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute kidney injury (AKI) is a common complication in neonatal intensive care and is associated with higher mortality, particularly in neonates (31.3%) and children requiring dialysis (27.1%) [1, 2]. When conservative measures fail, AKI management requires kidney replacement therapy (KRT) to control fluid overload (FO) and metabolic abnormalities. The modality choice for dialysis is based on the patient’s characteristics, the performance of the dialysis type, and the local institutional resources [3]. Since 1986, continuous kidney replacement therapy (CKRT) is performed in neonates with AKI, to treat severe FO that cannot be optimally managed by peritoneal dialysis (PD) and to allow higher ultrafiltration (UF) [4].
In 2012, Ronco et al. reported the development of new CKRT equipment which has recently been approved by the Food and Drug Administration (FDA): the Cardio-Renal Pediatric Dialysis Emergency Machine (CA.R.PE.DI.E.M.®) [5]. This machine was developed for newborns and small infants weighing 2.0–9.9 kg [6], and the flow rate of the miniaturized blood pump varies from 2 to 50 mL/min. The use of CARPEDIEM® in continuous veno-venous hemodialysis (CVVHD) mode rather than in continuous veno-venous hemofiltration (CVVH) seems to enhance the purification of small solutes [7]. To date, two studies have reported the use of CARPEDIEM® in neonates and small infants.
In CVVHD, successful blood purification for small solutes was obtained in 95 consecutive treatments in 13 neonates and small children [8]. A similar result was reported in 26 neonates and small children having received 165 CVVH sessions [9].
This study aimed to retrospectively assess the effectiveness, feasibility, outcomes, and technical considerations relating to the use of CARPEDIEM® in French pediatric and neonatal intensive care units.
Methods
Patients
This multicenter retrospective study consists of a case series of 19 newborns and six infants recruited in five French pediatric/neonatal intensive care units (Nice n = 9, Paris-Saclay n = 7, Lyon n = 4, Bordeaux n = 4, Toulouse n = 1). All children who received CARPEDIEM® treatments between December 2018 and January 2022 were included.
Laboratory data collection
Serum creatinine, serum potassium, blood urea nitrogen, hemoglobin, and platelet levels were regularly assessed as part of the routine follow-up of these patients with multiple organ dysfunctions. Estimated glomerular filtration rate (eGFR) was estimated using the 2009 Schwartz formula [10]. Laboratory parameters were collected at two time points during the follow-up of each patient: initiation and end of the first CARPEDIEM® session. The percentage of FO (% FO) was calculated for each patient using the method described by Goldstein et al. (% FO = (CKRT initiation weight − baseline weight) × 100) [11]. The Sethi Tibrewal Agrawal Raina waZir (STARZ) neonatal score was used to quantitatively calculate the risk of AKI in neonates [12]. Due to the retrospective nature of the study, chemical clearances were not measured.
CARPEDIEM®
The CARPEDIEM® machine (Medtronic, Inc., Dublin, Ireland) was used for all treatments, and each center followed its local practices for dialysis prescription. The dialysis modality and the use of CARPEDIEM® machine were decided at the physician’s discretion.
Several circuits were available with different surface areas (0.075, 0.147, and 0.245 m2) and extracorporeal volumes (27.2 mL, 33.5 mL, and 41.5 mL), which were chosen according to weight and body surface area. CARPEDIEM® circuits were changed every 24 h. Different modalities were performed: CVVH, CVVHD, and prolonged intermittent KRT. HbioFluids + ® (Medtronic) containing 32 mmol/L of sodium bicarbonate and 2.5 mmol/L of potassium was used as replacement solution. The potassium concentration was adapted in some patients according to their needs. The dialysis downtime (%) was calculated for treatment duration of 24 h, by calculating the number of prescribed hours/delivered hours using the following formula: [Treatment duration achieved (hours)/24 h] × 100. The net UF (mL/kg/h) was calculated using the following formula: Total UF volume/ (Hospital admission weight (kg) × CKRT duration (hours)).
Statistical analysis
Results are presented as medians with inter-quartile range [IQR] and range (min–max) for patient characteristics and laboratory routine parameters. Statistical Mann–Whitney tests were used to compare patient characteristics of the survivor and non-survivor groups. Survival was defined as survival at hospital discharge. The laboratory parameters at initiation and end of the first session were compared using a Wilcoxon test (non-parametric paired tests). The Chi-square test was used to evaluate complications (clotting and circuit dysfunction) according to catheter size, catheter localization, anticoagulation, blood flow, and platelet transfusion variables. In all cases, p-values below 0.05 were considered statistically significant using GraphPad Prism software 9.3.1 (GraphPad, La Jolla, CA, USA).
Results
Patient characteristics
Relevant demographic and clinical features of the 25 patients (15 males) at the time of CARPEDIEM® initiation are presented in Tables 1 and 2. Nine neonates were preterm (36%), and two were very preterm (25 and 28 weeks of gestational age). Six neonates presented a birth weight of less than 2 kg, three of whom had in utero growth restriction.
CARPEDIEM® initiation
CARPEDIEM® was initiated at a median [IQR] (range) age of 4 days [2-13] (1–1134) in children with a body weight of 3.3 kg [2.5–4.0] (1.3–11.1). CARPEDIEM® was prescribed to treat AKI with FO in 15 patients (60%), chronic kidney disease (CKD) stage 5 in five patients (20%), inborn error of metabolism (IEM) with hyperammonemia in three patients (12%), and electrolyte abnormalities in two patients (8%). FO was 18% [7-25] (4–32) (Table 1). The CKD stage 5 group included three children with polycystic kidney disease, one with an oxalosis, and one with hemolytic-uremic syndrome. For two of them, CARPEDIEM® was initiated before the peritoneal dialysis catheter implantation. For the others, CARPEDIEM® was used as a replacement therapy after peritoneal dysfunction, until the switch to hemodialysis. For the patient weighing 11 kg, the CARPEDIEM® machine was used because the conventional device was not available. For these children, CKRT was performed with CVVHD modality for three of them, and with CVVH modality for the two others.
Most of the children were critically ill with a need for mechanical ventilation in 21 patients (84%), vasoactive medications in 20 (80%), and intravenous furosemide in 11 (44%) (Table 1).
CARPEDIEM® circuits and parameters
A total of 131 sessions and 2125 h of treatment were realized. The duration of treatment per patient was 42 h [24–91] (8–557), and the duration of one session was 19 h [8-24] (2–24) (Table 3). A trend toward a longer treatment duration was found in the CKD stage 5 group (50 h [30–192] (11–215)) compared to the AKI group (40 h [24–77] (8–557), p = 0.56).
CVVH was used in 20 children (80%; predilution n = 8; postdilution n = 12), with a blood flow rate of 8 mL/kg/min [6-9] (3–16), an effluent flow rate of 74 mL/kg/h [43–99] (28–125), and a net UF flow of 6 mL/kg/h [3-9] (1–12). Supplemental Table 1 provides details on the CARPEDIEM® circuits and parameters used according to the primary diagnosis.
In the five children treated in hemodialysis mode, the blood and dialysate flow rates were 6 mL/kg/min [5-7] (4–7) and 600 mL/h [300–600] (120–600), respectively, and session duration was 8 h [6,7,8,9,10,11,12] (2–24). The net UF flow was 9 mL/kg/h [8-11] (8–12).
For patients with IEM, the effluent flow rate was 82 and 104 mL/kg/h for those treated with CVVH, and the dialysis flow rate was 120 mL/h for the patient treated with CVVHD.
Vascular access was obtained through the internal jugular vein in 18 patients (72%), the left subclavian vein in five patients (20%), the umbilical vein in one patient (4%), and the femoral vein in another patient (4%). The French line sizes of the catheters used were 4.5 Fr (32%), 5.5 Fr (24%), 6 Fr (16%), 6.5 Fr (24%), and 8 Fr (4%). To prevent filter clotting, circuits were anticoagulated using continuous heparin therapy at a dose of 10 UI/kg/h [8-12] (3–19) in 22 patients (Table 3). For the three other patients, one did not have any anticoagulation, and two benefitted from a loading dose of heparin at CARPEDIEM® initiation.
The circuit was primed with normal saline in 54 sessions (41%), 4% albumin or isofundine in 69 sessions (52%), and packed red blood cells in 8 sessions (in one patient weighing 2.5 kg). In children for whom the extracorporeal circuit exceeded 10% of the infant’s total blood volume, crystalloid or colloid was used to prime the circuit for all but one patient for whom packed erythrocytes were used. For the patients who did not receive blood priming, packed erythrocytes could be used before the CKRT initiation according to the transfusion indication protocol to prevent or treat anemia.
CARPEDIEM® outcomes
All patients survived the CARPEDIEM® sessions, but 17 (68%) died from their primary severe disease. The proportion of patients who survived was higher in the CKD stage 5 group (50%) than in the AKI group (13%). After the first CARPEDIEM® session, creatinine and urea plasma levels decreased significantly from 131 [98–181] (46–216) to 82 µmol/L [62–104] (14–185) (p < 0.0001) and 8.3 mmol/L [5.4–11.7] (2.3–19.0) to 5.1 [2.9–7.1] (0.8–16.4) (p < 0.0001), respectively (Fig. 1A and B). Serum potassium levels decreased significantly from 4.9 [4.1–6.3] (2.8–9.6) to 4.2 mmol/L [3.8–4.7] (3.1–5.6) (p = 0.002). Hyperkaliemia management was possible using CARPEDIEM®, and kaliemia remained stable in normal ranges (Fig. 1C). For the patients treated for hyperammonemia (two with CVVH and one with CVVHD), a minimum of tenfold decrease in ammonia was found in each patient after 24 h of treatment (Fig. 1D). Two of them were still alive at hospital discharge.
Comparison of serum creatinine (A), blood urea nitrogen (B), serum potassium (C), and ammonia (D) levels between the initiation and end of the first CARPEDIEM® session. Each dot on the graph represents the laboratory parameter of each patient at the initiation and the end of the first treatment session with CARPEDIEM®. The red line corresponds to the median of all patients. Statistical analyses were performed using Wilcoxon test: *p < 0.05; **p < 0.01; ***p = 0.001; ****p < 0.0001
CARPEDIEM® complications
Over the 131 sessions, UF was stopped momentarily and infusion was administered in 31 sessions (23%) due to hemodynamic instability. The dialysis downtime was 79% [54–91] (33–100). Clotting was observed in 25 sessions (19%), catheter dysfunction in three sessions (2%), pressure dysfunction due to a machine pressure pod issue in seven sessions (5%), and restitution failure in four sessions (3%). Table 4 reports complications according to catheter localization and size, anticoagulation, and blood flow rate. Thrombocytopenia was observed in 29 sessions (22%) representing 20 patients but was already present before treatment initiation in 15 patients due to their primary disease. No hemorrhage was reported.
Discussion
This French cohort study evaluating CARPEDIEM® use in 19 newborns and six small infants showed that blood purification, FO, and metabolic disorders such as hyperammonemia can be managed successfully, without severe complication, using this machine.
Herein, the survival rate at hospital discharge was low, but all patients survived the CARPEDIEM® course. The high mortality rate can be explained by the severity of the primary disease, a majority of patients presenting with multi-organ failure and neurological symptoms secondary to hypoxic ischemic encephalopathy. Ronco et al. reported that a weight over 3 kg was associated with survival [9], a result that could not be confirmed in the current study. However, CARPEDIEM® initiation occurred at a significantly older age in the survivors of the present cohort, likely due to the relatively high number of CKD stage 5 patients present in the survivor group. Although elevated FO% has been shown to be correlated with high mortality [13,14,15], the FO% herein, which was close to the 14% previously reported [9], was not significantly different between survivors and non-survivors, likely due to missing data.
In the current study, the CVVH modality was used for the majority of sessions and induced a significant decrease in blood creatinine, urea nitrogen, and hyperkaliemia after the first session. The effluent flow rate required during CVVH has not been defined and remains controversial [16,17,18]. Although the international KDIGO statements and the French Society of Intensive Care and Pediatric Emergency recommend an effluent volume of 25–35 mL/kg/h [19, 20], the CVVH substitution range used herein was larger. The median effluent flow rate was higher for patients with IEM in order to achieve a higher and faster removal of toxic metabolites. Such high flow rates may be associated with electrolyte complications although none were found in the present study. Based on these results, it appears that the effluent flow rate must be adapted to the primary diagnosis. The blood flow rate, however, was consistent with the recommended blood flow of 3–10 mL/kg/min obtained from animal studies [21], which seems to be more appropriate than the higher blood flow rates (10–12 mL/kg/min) reported when using adult devices in neonates [22].
In the present study, most infants required lines between 4.5 Fr (Vygon®, 6 cm) and 6.5 Fr while one required an 8 Fr line, located mainly in the internal jugular vein. Previous studies showed better outcomes in CKRT when using large catheters [23]. However, in a recent in vitro study, higher blood flow rates were obtained when using small 4 French and 5 French catheters with a small three-roller CARPEDIEM® pump (13 and 29 mL/min, respectively) rather than with an adult two-roller pump (10 and 20 mL/min, respectively) [24]. The present results and those from the Italian cohort confirm that purification can be obtained successfully using small catheters and pumps in a dedicated pediatric device [9].
Devices other than CARPEDIEM® are currently in use or being developed for dialysis in infants and neonates. The Newcastle Infant Dialysis and Ultrafiltration System (NIDUS® Allmed, England) was designed in the UK for CKRT and is currently only available for research purposes. It can be used in infants weighing 0.8 to 8 kg with a single lumen vascular access [25]. In a comparative study evaluating dialysis in 10 children weighing 1.8 to 5.9 kg, Coulthard et al. reported that using NIDUS® in CVVHD mode provided higher clearances and allowed for better UF control compared to PD and intermittent hemodialysis using an adult device [25]. Before the approval of CARPEDIEM® by the FDA, the adult machine Aquadex® (Baxter Corporation, Minneapolis, Minnesota) was adapted to children in the USA. The UF pump has a range of 0 to 500 mL/h and an accuracy of ± 10% [26]. In a case series of 12 children using Aquadex®, Askenazi et al. reported a blood priming of the circuit in 79% of the sessions according to the size of the extracorporeal volume of 33 mL, with few complications [26]. Compared to previous adult devices, Goldstein et al. reported a higher survival in infants treated with CARPEDIEM® [11].
In urea cycle disorders and organic acidemia, ammonia serum levels > 200 μmol/l (341 μg/dl) are associated with poor neurological outcomes and can lead to hepatic encephalopathy and irreversible cerebral damage. In such cases, KRT should be quickly considered when medical treatment fails. Guidelines for hyperammonemia management recommend using high-dose CVVHD with Qb 30–50 mL/min as first-line treatment, when available [27]. In metabolic diseases, CVVHDF and CVVHD have been shown to enable a more rapid elimination of toxic metabolites than PD, and the usual dose for CVVHD or CVVHDF in patients with IEM is a dialysis flow of 460 mL/kg/h or an effluent volume of 8000 mL/1.73m2/h [27,28,29]. For most infants, CARPEDIEM® may not be able to achieve those high doses and as fast an epuration as intermittent dialysis devices [30]; however, the risk/effectiveness balance, taking into account age and weight, should be considered. Herein, there was at least a tenfold decrease in ammonia in the three neonates with hyperammonemia at the end of the first CARPEDIEM® session which lasted 17, 23, and 24 h. In these three patients, CKRT modality did not seem to affect treatment effectiveness or survival.
It is important to note that, although destabilization with a need for volume replacement was the most common complication that required intervention herein, it is difficult to conclude if this was due to an imprecise UF control or to the clinical severity of the patients (80% had vasoactive treatment), even though the smallest extracorporeal circuit is applied when using CARPEDIEM®. Moreover, thrombocytopenia was the second most common complication reported. Although no hemorrhagic complication was noted, patients received platelet transfusions in about a fifth of the sessions. In CKRT, thrombocytopenia is a frequent complication and has been reported in 42.9% of sessions in neonates [31]. When using other extracorporeal circuits such as extracorporeal membrane oxygenation, a recent meta-analysis reported thrombocytopenia in 21% of adults [32]. Although the etiology of thrombocytopenia during extracorporeal treatment is multifactorial, the presence of inflammatory stress and oxidative mediators is likely to contribute to decreased platelet counts and increase the risk of thrombocytopenia. Other factors including medication, sepsis, and use of anticoagulation with heparin, which are found in critically ill patients such as those herein, have also been associated with an increased risk of thrombocytopenia [33]. Such risk factors could explain the high proportion of thrombocytopenia observed in the present cohort. Moreover, in CKRT, a possible destruction or retention of platelets in the hemofilter may also induce thrombocytopenia. Indeed, although using a larger filter and a higher blood flow, Mulder et al. measured platelet levels in adults before and after the CKRT filter and estimated that 625 × 109 platelets were lost daily across the filter [34]. Importantly, recent studies have shown that thrombocytopenia due to CKRT is associated with poor outcomes such as higher mortality, lower kidney recovery, and increased risk of secondary infections [35,36,37]. When considering the use of CARPEDIEM®, physicians thus need to consider such possible complications. More broadly, ethical considerations based on comorbidities should be discussed among neonatologists and nephrologists and communicated to parents prior to CARPEDIEM® initiation [38].
The present study represents the first French national cohort evaluating the CARPEDIEM® machine and the first to assess its use in such low-birth-weight premature neonates. The study, however, has some limitations, particularly due to the small number of patients and the presence of missing data due to its retrospective design. These are classical limitations when working in the field of pediatric orphan diseases, and even more so when assessing an off-label use of novel therapies (CARPEDIEM® use in infants under 2 kg).
Conclusion
The present data confirm that the use of CARPEDIEM® is safe and effective in neonates and infants. Long-term kidney assessment remains to be evaluated, and studies comparing the efficacy of CARPEDIEM® with that of PD and other adult devices are needed.
Data availability
The data is available if requested from the corresponding author.
References
Sutherland SM, Ji J, Sheikhi FH, Ling XB et al (2013) AKI in hospitalized children: epidemiology and clinical associations in a national cohort. Clin J Am Soc Nephrol 8:1661–1669. https://doi.org/10.2215/CJN.00270113
Jetton JG, Boohaker LJ, Sethi SK, Askenazi DJ et al (2017) Incidence and outcomes of neonatal acute kidney injury (AWAKEN): a multicentre, multinational, observational cohort study. Lancet Child Adolesc Health 1:184–194. https://doi.org/10.1016/S2352-4642(17)30069-X
de Galasso L, Picca S, Guzzo I (2020) Dialysis modalities for the management of pediatric acute kidney injury. Pediatr Nephrol 35:753–765. https://doi.org/10.1007/s00467-019-04213-x
Ronco C, Brendolan A, Bragantini L, La Greca G et al (1986) Treatment of acute renal failure in newborns by continuous arterio-venous hemofiltration. Kidney Int 29:908–915. https://doi.org/10.1038/ki.1986.85
Ronco C, Garzotto F, Brendolan A, Goldstein SL et al (2014) Continuous renal replacement therapy in neonates and small infants: development and first-in-human use of a miniaturised machine (CARPEDIEM). Lancet 383:1807–1813. https://doi.org/10.1016/S0140-6736(14)60799-6
Ronco C, Garzotto F, Ricci Z (2012) CA.R.PE.DI.E.M. (cardio-renal pediatric dialysis emergency machine): evolution of continuous renal replacement therapies in infants A personal journey. Pediatr Nephrol 27:1203–1211. https://doi.org/10.1007/s00467-012-2179-8
Lorenzin A, Garzotto F, Alghisi A, Ronco C et al (2016) CVVHD treatment with CARPEDIEM: small solute clearance at different blood and dialysate flows with three different surface area filter configurations. Pediatr Nephrol 31:1659–1665. https://doi.org/10.1007/s00467-016-3397-2
Vidal E, Cocchi E, Paglialonga F, Ronco C et al (2019) Continuous veno-venous hemodialysis using the Cardio-Renal Pediatric Dialysis Emergency MachineTM: first clinical experiences. BPU 47:149–155. https://doi.org/10.1159/000494437
Garzotto F, Vidal E, Ricci Z, Ronco C et al (2020) Continuous kidney replacement therapy in critically ill neonates and infants: a retrospective analysis of clinical results with a dedicated device. Pediatr Nephrol 35:1699–1705. https://doi.org/10.1007/s00467-020-04562-y
Schwartz GJ, Work DF (2009) Measurement and estimation of GFR in children and adolescents. Clin J Am Soc Nephrol 4:1832–1843. https://doi.org/10.2215/CJN.01640309
Goldstein SL, Vidal E, Ricci Z, Ronco C et al (2022) Survival of infants treated with CKRT: comparing adapted adult platforms with the CarpediemTM. Pediatr Nephrol 37:667–675. https://doi.org/10.1007/s00467-021-05180-y
Sethi SK, Raina R, Rana A, Wazir S et al (2022) Validation of the STARZ neonatal acute kidney injury risk stratification score. Pediatr Nephrol 37:1923–1932. https://doi.org/10.1007/s00467-021-05369-1
Gorga SM, Sahay RD, Askenazi DJ, Selewski DT et al (2020) Fluid overload and fluid removal in pediatric patients on extracorporeal membrane oxygenation requiring continuous renal replacement therapy: a multicenter retrospective cohort study. Pediatr Nephrol 35:871–882. https://doi.org/10.1007/s00467-019-04468-4
Selewski DT, Cornell TT, Lombel RM, Heung M et al (2011) Weight-based determination of fluid overload status and mortality in pediatric intensive care unit patients requiring continuous renal replacement therapy. Intensive Care Med 37:1166–1173. https://doi.org/10.1007/s00134-011-2231-3
Alobaidi R, Morgan C, Basu RK, Bagshaw SM et al (2018) Association between fluid balance and outcomes in critically ill children: a systematic review and meta-analysis. JAMA Pediatr 172:257–268. https://doi.org/10.1001/jamapediatrics.2017.4540
Fayad AI, Buamscha DG, Ciapponi A (2016) Intensity of continuous renal replacement therapy for acute kidney injury. Cochrane Database Syst Rev 10:CD010613. https://doi.org/10.1002/14651858.CD010613.pub2
Naorungroj T, Neto AS, Zwakman-Hessels L, Bellomo R et al (2021) Early net ultrafiltration rate and mortality in critically ill patients receiving continuous renal replacement therapy. Nephrol Dial Transplant 36:1112–1119. https://doi.org/10.1093/ndt/gfaa032
Murugan R, Kerti SJ, Chang C-CH, Bellomo R et al (2019) Association of net ultrafiltration rate with mortality among critically ill adults with acute kidney injury receiving continuous venovenous hemodiafiltration: a Secondary Analysis of the Randomized Evaluation of Normal vs Augmented Level (RENAL) of Renal Replacement Therapy Trial. JAMA Netw Open 2:e195418. https://doi.org/10.1001/jamanetworkopen.2019.5418
Khwaja A (2012) KDIGO clinical practice guidelines for acute kidney injury. NEC 120:c179–c184. https://doi.org/10.1159/000339789
Vinsonneau C, Allain-Launay E, Blavau C, Vong LV (2015) Épuration extrarénale en réanimation adulte et pédiatrique - La SFAR. Société Française d’Anesthésie et de Réanimation. https://sfar.org/epuration-extrarenale-en-reanimation-adulte-et-pediatrique/. Accessed 4 May 2022
Werner HA, Herbertson MJ, Seear MD (1994) Functional characteristics of pediatric veno-venous hemofiltration. Crit Care Med 22:320–325. https://doi.org/10.1097/00003246-199402000-00025
Kaempfen S, Dutta-Kukreja P, Mok Q (2017) Continuous venovenous hemofiltration in children less than or equal to 10 kg: a single-center experience. Pediatr Crit Care Med 18:e70–e76. https://doi.org/10.1097/PCC.0000000000001030
Hackbarth R, Bunchman TE, Chua AN, Goldstein SL et al (2007) The effect of vascular access location and size on circuit survival in pediatric continuous renal replacement therapy: a report from the PPCRRT registry. Int J Artif Organs 30:1116–1121. https://doi.org/10.1177/039139880703001212
Garzotto F, Zaccaria M, Vidal E, Ronco C et al (2019) Choice of catheter size for infants in continuous renal replacement therapy: bigger is not always better. Pediatr Crit Care Med 20:e170–e179. https://doi.org/10.1097/PCC.0000000000001825
Coulthard MG, Crosier J, Griffiths C, Lambert HJ et al (2014) Haemodialysing babies weighing <8 kg with the Newcastle infant dialysis and ultrafiltration system (Nidus): comparison with peritoneal and conventional haemodialysis. Pediatr Nephrol 29:1873–1881. https://doi.org/10.1007/s00467-014-2923-3
Askenazi D, Ingram D, White S, Fathallah-Shaykh S et al (2016) Smaller circuits for smaller patients: improving renal support therapy with AquadexTM. Pediatr Nephrol 31:853–860. https://doi.org/10.1007/s00467-015-3259-3
Raina R, Bedoyan JK, Lichter-Konecki U, Warady BA et al (2020) Consensus guidelines for management of hyperammonaemia in paediatric patients receiving continuous kidney replacement therapy. Nat Rev Nephrol 16:471–482. https://doi.org/10.1038/s41581-020-0267-8
Arbeiter AK, Kranz B, Wingen A-M, Büscher R et al (2010) Continuous venovenous haemodialysis (CVVHD) and continuous peritoneal dialysis (CPD) in the acute management of 21 children with inborn errors of metabolism. Nephrol Dial Transplant 25:1257–1265. https://doi.org/10.1093/ndt/gfp595
Celik M, Akdeniz O, Ozgun N, Ozbek MN et al (2019) Short-term results of continuous venovenous haemodiafiltration versus peritoneal dialysis in 40 neonates with inborn errors of metabolism. Eur J Pediatr 178:829–836. https://doi.org/10.1007/s00431-019-03361-4
Snauwaert E, Van Biesen W, Raes A, Vande Walle J et al (2017) Accumulation of uraemic toxins is reflected only partially by estimated GFR in paediatric patients with chronic kidney disease. Pediatr Nephrol 33:315–323. https://doi.org/10.1007/s00467-017-3802-5
Nishimi S, Sugawara H, Onodera C, Oyama K et al (2019) Complications during continuous renal replacement therapy in critically ill neonates. Blood Purif 47(Suppl 2):74–80. https://doi.org/10.1159/000496654
Jiritano F, Serraino GF, ten Cate H, Lorusso R et al (2020) Platelets and extra-corporeal membrane oxygenation in adult patients: a systematic review and meta-analysis. Intensive Care Med 46:1154–1169. https://doi.org/10.1007/s00134-020-06031-4
Thachil J, Warkentin TE (2017) How do we approach thrombocytopenia in critically ill patients? Br J Haematol 177:27–38. https://doi.org/10.1111/bjh.14482
Mulder J, Tan HK, Bellomo R, Silvester W (2003) Platelet loss across the hemofilter during continuous hemofiltration. Int J Artif Organs 26:906–912. https://doi.org/10.1177/039139880302601006
Griffin BR, Jovanovich A, You Z, Lorusso R et al (2019) Effects of baseline thrombocytopenia and platelet decrease following renal replacement therapy initiation in patients with severe acute kidney injury. Crit Care Med 47:e325–e331. https://doi.org/10.1097/CCM.0000000000003598
Griffin BR, Ten Eyck P, Faubel S, Bellomo R et al (2022) Platelet decreases following continuous renal replacement therapy initiation as a novel risk factor for renal nonrecovery. Blood Purif 51:559–566. https://doi.org/10.1159/000517232
Griffin BR, Wu C, O’Horo JC, Kashani K et al (2021) The association of platelet decrease following continuous renal replacement therapy initiation and increased rates of secondary infections. Crit Care Med 49:e130–e139. https://doi.org/10.1097/CCM.0000000000004763
Ranchin B, Plaisant F, Demède D, Bacchetta J et al (2021) Review: Neonatal dialysis is technically feasible but ethical and global issues need to be addressed. Acta Paediatr 110:781–788. https://doi.org/10.1111/apa.15539
Acknowledgements
The authors would like to thank the physicians involved in this clinical study aiming at improving our management practices with the use of CARPEDIEM®. The authors would also like to thank Véréna Landel (Direction de la Recherche en Santé, HCL) for help in manuscript preparation.
Funding
D. De Luca has received research assistance and speaker fees from MEDTRONIC Inc., outside of the present work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics
The study was approved by an ethics committee (Comité d’Ethique des Recherches non Interventionelles Université Côte d’Azur, session 23 September 2020, approval N°2020–68) and respected all local and European relevant regulations. This study was performed with the appropriate participants’ informed consent in compliance with the Helsinki Declaration.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Battista, J., De Luca, D., Eleni Dit Trolli, S. et al. CARPEDIEM® for continuous kidney replacement therapy in neonates and small infants: a French multicenter retrospective study. Pediatr Nephrol 38, 2827–2837 (2023). https://doi.org/10.1007/s00467-022-05871-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-022-05871-0