Abstract
Acute kidney injury (AKI) is an increasingly frequent complication among hospitalized children. It is associated with high morbidity and mortality, especially in neonates and children requiring dialysis. The different renal replacement therapy (RRT) options for AKI have expanded from peritoneal dialysis (PD) and intermittent hemodialysis (HD) to continuous RRT (CRRT) and hybrid modalities. Recent advances in the provision of RRT in children allow a higher standard of care for increasingly ill and young patients. In the absence of evidence indicating better survival with any dialysis method, the most appropriate dialysis choice for children with AKI is based on the patient’s characteristics, on dialytic modality performance, and on the institutional resources and local practice. In this review, the available dialysis modalities for pediatric AKI will be discussed, focusing on indications, advantages, and limitations of each of them.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute kidney injury (AKI) is an increasingly frequent complication among hospitalized patients. In particular, AKI defined according to Kidney Disease Improving Global Outcomes (KDIGO) criteria, occurs in one in three children hospitalized worldwide and is particularly common in the critical care setting [1, 2]. In a prospective study involving patients admitted to Pediatric Intensive Care Unit (PICU), AKI developed in 26.7% of critically ill children [3]. A similar increasing trend has also been noted for severe AKI requiring renal replacement therapies (RRT) [2, 4]. AKI is also associated with higher morbidity and mortality. The reported mortality rate of hospitalizations complicated by AKI is 15.3% compared to 0.6% among non-AKI hospitalizations (p = 0.001) [5]. Furthermore, mortality is significantly higher among neonates (31.3%) and children requiring dialysis (27.1%) [5] and reaches rates as high as 30–50% in critically ill patients receiving RRT [6]. In addition, children with AKI experience longer hospitalizations, prolonged PICU stay, greater need for mechanical ventilation [3, 7, 8] and increased risk for subsequent renal abnormalities [9, 10]. Development of technology and a higher standard of care allow treating critically ill and young patients.
In this review, we will discuss the different modalities of RRT available for the treatment of children with dialysis-requiring AKI, considering indications, advantages, and limitations of each dialytic modality.
Renal replacement therapy for AKI
In the last two decades, RRT options for AKI have expanded from peritoneal dialysis (PD) and intermittent hemodialysis (HD) to continuous renal replacement therapy (CRRT) and hybrid approaches [11,12,13].
The choice of dialytic approach modality should be based on the patient’s characteristics, on dialytic modality performance [14] and on the institutional resources and local practice.
See Table 1 for a concise comparison between dialytic modalities for the treatment of pediatric AKI.
Peritoneal Dialysis
Introduction
Historically, PD has been the primary RRT modality employed in pediatric care for the treatment of AKI [14]. In the past decades, the use of PD for AKI, however, has declined considerably in favor of other types of extracorporeal (EC) therapies [15]. In fact, there is no evidence to suggest significant differences in mortality between PD and EC dialytic modalities in AKI [16]. Recently, there has been a renewed interest in PD to manage AKI patients, even in developed countries [17].
Advantages
Acute PD is the modality of choice in newborns, including babies weighing < 1000 g [18], and in infants who develop AKI following surgery for congenital heart disease [19]. It is generally safe and effective in children after cardiopulmonary bypass, with some researchers utilizing it as a prophylactic therapy as well [19, 20]. Furthermore, acute PD has generally been considered the preferred therapy if there is isolated kidney failure in small children, including glomerular diseases, acute tubular necrosis due to ischemia and/or drugs and hemolytic uremic syndrome [14, 21]. PD is a dynamic dialysis process which is more physiological and less pro-inflammatory than EC treatments [22]. The gradual and continuous nature of solute and fluid removal allows large volumes of ultrafiltration (UF), making PD feasible also in hemodynamically unstable patients. Although fluid removal is unpredictable, using the adequate catheter and technique, appropriate solute clearances and UF can be achieved providing adequate space for fluid administration and for nutrition [21]. Dialysate containing dextrose can also act as a source of supplemental calories, especially in infants for whom hypoglycemia with fluid restriction may be a problem [23]. Moreover, PD does not require vascular access, allowing preservation of vessels for future procedures. Finally, PD is a simple and safe technique that is universally available and which, as with intermittent HD, can be performed outside the PICU [21, 24, 25]. It can be performed in units with no HD expertise [21, 26] and with minimal infrastructural support [21].
Providing PD may be threefold to fivefold less expensive than providing HD or CRRT, making the former a more viable RRT modality in developing countries [27].
Disadvantages
The gradual solute clearance and the unpredictable UF may result in unacceptably slow correction of life-threatening hyperkalemia or severe acute pulmonary edema. Slow clearance of small solutes makes PD less effective than HD or CRRT in children with inborn errors of metabolism or hypercatabolic conditions [28]. In addition, to achieve effective UF, solutions with high concentrations of dextrose (2.5% or 4.25%) may be required and, as a consequence, hyperglycemia can result, especially in young infants [17, 21]. Moreover, warming the dialysate is difficult without the use of an automatic cycler, influencing both effective solute clearance and hemodynamic stability [14]. PD requires an intact peritoneal cavity and hence is relatively contraindicated in children with recent abdominal surgeries, abdominal cellulitis, inguinal hernia, diaphragmatic hernia, paralytic ileus and peritonitis [21]. Patients with pulmonary conditions may have worsening symptoms due to increased abdominal dialysate volumes [29]. Furthermore, patients with ventriculo-peritoneal shunts or prune-belly syndrome have been successfully dialyzed with PD, but do present increased potential complications. Finally, manual PD may need higher nursing workload depending on the frequency of cycles [14].
Prescription
The PD prescription needs to be individualized according to patient size and clinical condition. The most important point, especially in small children, is to start with low dwell volume (10 ml/kg) in order to avoid leakage caused by the PD solution–induced rise in intraperitoneal pressure [17]. If no leakage occurs, the exchange volume can be gradually increased to improve solute and fluid removal. A final fill volume no greater than 800 ml/m2 is recommended in infants, while larger volumes (1100 ml/m2) may be reached in older children [17, 21]. The initial exchange duration (including inflow, dwell, and drain times) should be of around 60–90 min, but it may be reduced to improve UF. Different regimens adapted from chronic PD (acute intermittent PD, continuous PD, tidal PD, high-volume PD, and continuous flow PD) have been tested for acute treatment in children. Strong evidence about the best performing scheme is still not available [21]. In selected patient populations with deficient lactate conversion, such as critically ill children with shock, liver dysfunction, and metabolic disorders, bicarbonate-buffer solutions may be preferred (see next section) [17, 21]. Serum concentrations of electrolytes should be measured at least daily and, after the first 12–24 h, supplementation of sodium and potassium may be required in the dialysis solution [17]. The recent report of Vasudevan offers a useful scheme prescription for acute PD initiation in children [21].
Equipment
The required equipment for performing an effective PD are a functioning PD catheter, dialysis fluids, and connecting tubing and drainage bags. For manual PD, the commercially available delivery systems are the PD-Paed system (Fresenius Medical Care, BadHomburg, Germany), and the Dialy-Nate system/Gesco Dialy-nate (Utah Medical Products, Midvale, UT, USA) [17]. For automated PD, the SleepSafe Harmony (Fresenius Medical Care, BadHomburg, Germany) or the HomeChoice (Baxter Healthcare, Deerfield, Illinois, USA) is a feasible commercial option. PD solutions for acute dialysis are generally available with dextrose concentrations of 1.5%, 2.5%, and 4.25% (1.36%, 2.27%, or 3.86% are equivalent if glucose is measured), with an osmolality of 346, 396, and 485 mOsmol/l, respectively [17]. Until recently, lactate was the only buffer available for PD solutions. The use of double-chamber PD solutions has produced dialysis fluid bags containing either bicarbonate or a mixture of bicarbonate and lactate buffer, such as the Bicavera bags (Fresenius Medical Care, BadHomburg, Germany) or the Physioneal bags (Baxter Healthcare, Deerfield, Illinois, USA). The standard PD solutions contain 132–134 mmol/l of sodium and have two different calcium concentrations: 1.75 mmol/l (high-calcium) and 1.25 mmol/l (low-calcium), respectively [17]. ISPD guidelines suggest the use of a small concentration of potassium (0–2 mmol/l) in dialysate solution for the initial phase of the treatment [17].
Anticoagulation
Systemic anticoagulation is not required but the addition of heparin (125–250 UI/l) to the dialysate solution is suggested when fibrin develops (as during peritonitis episodes) in order to reduce fibrin clot formation in the effluent [17, 20].
Access
Peritoneal dialysis access can be a flexible cuffed catheter, a rigid stylet short-term catheter or an adapted PD catheter (nasogastric tube, surgical drain, dialysis catheter) [30]. A flexible, single- or double-cuff Tenckhoff catheter, either straight or swan neck, is the preferred PD access. It can be inserted surgically (laparoscopic or open technique) or under local anesthesia at the bedside using a modified Seldinger approach (with a guidewire and a peel-away sheath). In this case, the placement of the catheter can be performed by blind percutaneous puncture (not suggested in those patients with previous history of abdominal surgery) or taking advantage of an x-ray image. Injecting contrast solution into the needle during the procedure allows confirmation of needle entry into the peritoneal cavity and to better define the correct site of catheter placement [17]. The flexible Cook Mac-Loc Multipurpose Drainage catheter (Cook Medical Inc., Bloomington, IN, USA), is an alternative to the Tenckoff catheter which can be inserted at bedside in children of all sizes. Low catheter-related complications have also been reported with the short-term Cook catheter. In low-income countries, surgical placement of the PD catheter and/or specific PD catheters may be unavailable. Thus, rigid catheters and improvised catheters are still used, but leakage of dialysate and mechanical complications may develop, and their routine use is not recommended [17].
Complications
The most frequent complications of PD are mechanical and infectious.
Mechanical complications include leaks, catheter displacement, catheter obstruction, and hernias, and they may lead to poor drainage of the dialysis fluid [14, 21]. Frequently, drainage problems are secondary to omentum and/or fibrin clot obstructions. Performing omentectomy at initial catheter insertion may be a valid strategy for reducing outflow obstruction. Few retrospective pediatric studies consider the impact of prophylactic omentectomy suggesting improved catheter survival [31, 32]. Conversely, a recent trial advises that the type of catheter rather than omentectomy may influence the incidence of catheter obstruction [33]. Furthermore, flushing the catheter and preventing fibrin accumulation by increasing the heparin dosage in the dialysate and/or using a fibrinolytic agent (streptokinase or urokinase) is suggested to reduce mechanical complications [21]. If available, a laparoscopic technique may be used to correct poor drainage or replace the malfunctioning catheter [21]. Serious complications, such as bladder or bowel perforation are rare and mostly seen with the use of a rigid catheter [21]. Conversely, peritonitis remains a frequent risk, especially if unsterile manipulation of the catheter occurs. Significant losses in immunoglobulins may increase the risk of infections in these patients [21]. Peritonitis can lead to further increased dialysate protein loss, nutritional compromise, loss of UF capacity, and permanent damage to the peritoneal membrane [14].
Hemodialysis
Introduction
Intermittent and continuous modalities are complementary therapies for treating AKI [34]; however, HD is preferred in specific conditions requiring rapid and effective solute clearance.
Advantages
Hemodialysis may be the preferred mode of treatment in relatively stable patients with adequate vascular access [14]. The main advantage of HD over the other dialysis modalities is the rapid rate of fluid removal and solute clearance [14, 26] (Table 1). This characteristic allows an efficient correction of life-threatening hyperkalemia or acidosis and rapid resolution of pulmonary edema. For the same reason, HD is specifically indicated in children with inborn errors of metabolism and severe hyperammonemia refractory to medical therapy [28]. In these cases, the neurological outcome has been correlated with ammonium reduction and depuration rapidity is essential [35]. In selected cases (thrombocytopenia, coagulopathy, liver failure), the shorter duration of the session, compared to CRRT, enables dialysis to be performed without anticoagulation, reducing the risk of bleeding [36]. Similarly to PD, HD can be performed outside the PICU setting. Finally, the intermittent nature of HD treatment allows down-times for diagnostic and therapeutic procedures.
Disadvantages
Well-functioning vascular access and hemodynamic stability are essential for the provision of HD. Fluid restriction usually is required in oliguric or anuric AKI patients, limiting the amount of daily parenteral nutrition [14]. Finally, whenever heparin is used for anticoagulation of the circuit, the risk of bleeding is necessarily increased.
Prescription
Hemodialysis prescription in pediatric AKI requires frequent adjustments and should consider weight, catabolic state, body composition, UF target, and changes in nutritional requirements. In order to prevent hemodynamic instability, bloodline priming is usually performed when the EC circuit volume (tubing volume + dialyzer volume) exceeds 10% of the child’s blood volume [37]. An HD circuit can be primed with isotonic saline or with albumin or blood [26] (packed red blood cells diluted with saline to a hematocrit of 30 to 40%) [26, 37, 38]. Special considerations on blood flow and dialysate flow are required. The blood flow rate (Qb) is usually 5–8 ml/kg/min but depends on the catheter and patient size. Lower rate (2–3 ml/kg/min with a gradual increase to a maximum of 5 ml/kg/min) is preferred for small infants. Particularly during the first dialysis session, it is important to prevent too rapid a solute removal and the consequent disequilibrium syndrome [26]. For this reason, a urea clearance of less than 3 ml/min/kg is advised [39]. Usually, the length of the session and the blood flow should be tailored in order to obtain a urea reduction rate of 30% for the first session, with a progressive increase up to 70% in the following days. Those patients with severe uremia, acute or acute-on-chronic liver failure, acute or chronic brain injury and those with increased permeability of blood-brain barrier are particularly at risk from dialysis disequilibrium syndrome, and a lower-efficiency dialysis treatment is recommended in these cases [40]. All children should be dialyzed using volume-controlled machines and the weight loss should not exceed 5% of the patient’s body weight, with 1–2% of body weight reduction per hour [37]. In small children, repeated dialysis treatments or switching to CRRT may be the only way to remove more fluid safely. Conversely, for those patients with fluid overload and hemodynamic stability in whom larger UF is required, considerations on the type of filter to be used will be made in the next section. Although no well-established methods for measuring the efficacy of RRT in AKI exist, the KDIGO guidelines suggest that AKI patients treated with HD should obtain a small solute clearance equivalent to a Kt/V value of 1.2 per treatment (approximately corresponding to a urea reduction ratio of at least 0.70) at least three times per week [34].
Equipment
The use of dialyzers with biocompatible membranes for HD and CRRT in patients with AKI is recommended [34]. Biocompatible membranes, producing less complement and decreased oxidative stress, lead to a less frequent onset of hypotension, vasodilatation, leucopenia, hypoxia, and fever. Historically, dialyzers utilized cellulose-based membranes with poor biocompatibility. Technological advances have created modified cellulose-based and synthetic membranes in order to improve biocompatibility [41]. Currently, the majority of clinically used dialyzers are synthetic membranes, such as polysulfone, polyethersulfone, polyacrylonitrile, polyamide, and polymethylmethacrylate [41].
However, despite a couple of trials reporting improved recovery of renal function and a trend towards better survival among patients dialyzed with biocompatible membranes compared to non-biocompatible membranes [42, 43], a systematic review (10 studies, 1100 patients) failed to demonstrate a difference in mortality or recovery of renal function between the two dialysis membranes [44]. Together with the attempts to increase biocompatibility, many efforts have been made to improve dialyzer structures. According to the dimensions of the pores, the membranes are divided into low- and high-flux membranes. High-flux membranes are more water-permeable and allow the clearance of middle molecules such as beta2-microglobulin. Due to their high water permeability, high-flux membranes are characterized by a coefficient of UF (Kuf) > 20 ml/h per mmHg (compared to < 8 ml/h per mmHg for low-flux membranes). This means that in those patients in whom removal of a high amount of fluids is needed, the use of high-flux membranes is advised because it allows maintenance of transmembrane pressure (TMP) within an acceptable range. Nevertheless, although few studies have focused on the membrane flux properties and outcome in AKI, no significant differences were reported in a randomized trial of critically ill adult patients with AKI treated with intermittent HD that compared low-flux versus high-flux synthetic membranes in terms of survival, recovery of renal function, incidence of oliguria, and dialysis duration [45]. Finally, a new generation of high cut-off membranes (the cut-off represents the molecular weight of the smallest solute retained by the membrane), capable of removal of substances in the range of 20 to 60 kDa, have been created [46]. They have greater cytokine removal capacity compared with standard hemofiltration membranes that have cut-off points of 10 to 30 kDa. Haase and colleagues, in a study conducted on ten septic patients with AKI treated with intermittent HD, demonstrated greater cytokine clearance and greater decrease in IL-8 and IL-10 plasma concentrations with high cut-off membranes compared to standard high-flux filters [47]. The negative effect of these membranes on albumin loss and antibiotic clearance during treatment is still under evaluation [46]. Regardless of the membrane utilized, when selecting a hemodialyzer for pediatric use, the dialyzer size needs to be adapted to the size of the patient in order to minimize EC circuit volume and to maintain effective solute removal. The dialyzer surface area should be between 75 and 100% of the patient’s total body surface area (BSA). Dialyzer choice also depends on the priming volume, the maximum Qb, the urea clearance (between 3 and 5 ml/kg/min), and the UF required [26]. See Table 2 for characteristics of the most used dialyzers adapted for infants.
Anticoagulation
Anticoagulation of the EC circuit is required to prevent circuit thrombosis and vascular access occlusion. In the pediatric population, circuit and dialyzer thrombosis is a common clinical problem because children, compared to adults, require narrower central catheter lumens and smaller dialyzers, as well as circuit tubing with lower Qb [36, 48]. Furthermore, children face a high risk of significant blood loss in the event of circuit loss due to the elevated EC circuit volume compared to patient blood volume [48]. Unfractionated heparin (UH) is the most commonly used agent for anticoagulation of the EC HD circuit. It is not expensive and clinical experience on its usage is well established in most dialysis units. The UH protocol consists of an initial bolus (20–30 UI/kg) followed by continuous infusion of 10–20 UI/kg/h [25, 36]. Doses should be individualized according to the patient’s coagulation pattern. Clotting tests used for monitoring heparin therapy are mostly activated partial thromboplastin time (APTTr), range 1.5–2, and activated clotting time (ACT), range 180–200 s [36, 39]. The use of UH is burdened by a series of side effects. Systemic heparinization and prolonged heparin half-life in patients with renal dysfunction may result in severe or prolonged bleeding with an increased risk of hemorrhage [48]. UH use has also been associated with the development of antibody-mediated thrombocytopenia [49]. Alternatively, systemic anticoagulation can be provided with low-molecular-weight heparin (LMWH), heparinoids, hirudin, prostacyclin, serine protease inhibitors, direct thrombin inhibitors, and activated protein C. LMWH is the anticoagulant recommended by the European Best Practice Guidelines for chronic HD, but studies about the use of LMWH for HD in the AKI setting are lacking [34]. LMWH anticoagulation of EC circuit requires measurement of anti-Xa activity and a target range of 0.5–1 IU/ml has been proposed. In those patients with increased risk of bleeding, anticoagulant-free dialysis with frequent pre-filter saline flushes is a common practice [36, 48]. It is also possible to use heparinized saline (5000 IU/l) priming to allow heparin binding to filter and lines followed by a second priming with normal saline to remove heparin before starting dialysis [48]. Heparin-coated membranes could also be useful in prolonging filter life [50]. In case of short filter life with these approaches, citrate is the most commonly used regional anticoagulant [51]. Citrate anticoagulation is reviewed in detail below.
Access
Well-functioning vascular access is essential for the provision of adequate EC RRT. Uncuffed and non-tunneled central venous catheters (CVCs) represent the first choice of vascular access in patients requiring urgent HD. A limited variety of temporary vascular catheters are available for the pediatric population [26, 52] (Table 3). Their placement can be performed at the bedside for short-term catheters, or in an operating room by a surgeon or an interventional radiologist. The choice of appropriate catheter is based on the size, expressed by length and diameter (French), and location. The catheter size depends on patient weight/length (Table 3) [53]. The largest diameter of CVC that can be placed safely should be chosen to optimize blood flows and circuit survival [53]. According to the KDIGO guidelines, the right internal jugular vein should be the first choice as insertion site for dialysis catheter, followed by the femoral vein (FV), the left jugular vein, and the subclavian vein (SV) [34]. Utilization of the neck veins, although limited by anatomy, may be preferable, especially in infants weighing less than 5 kg [14]. Despite these indications, the FV is frequently used for easier placement (69% in the US Registry, 58.8% in our cohort) [6, 54]. SV catheter placement should be avoided for the risk of malfunction and following vein stricture. The very high incidence of stenosis of the SV might preclude permanent access in the form of an arteriovenous graft or fistula if chronic HD is needed [14, 55]. After the HD session, the catheter should be flushed with isotonic saline and filled with diluted heparin (1000 IU/ml). Heparin volume is defined according to manufacturer’s recommendations (usually marked on the catheter itself) [26]. Citrate lock has been tested as an alternative anticoagulant locking solution showing lower incidence of bleeding episodes over heparin, but limited advantages on catheter malfunction or the need for catheter removal for poor flows. Conversely, antimicrobial-containing citrate lock appears superior to heparin lock in the prevention of catheter-related infections [56]. Nevertheless, a recent Cochrane review showed that the use of antimicrobial lock solutions probably reduces catheter-related infections, but makes little or no difference to the risk of thrombosis compared to standard locking solutions [57].
Complications
Hemodialysis is frequently associated with hypotension, mechanical catheter complications, and infections [37]. The disequilibrium syndrome, occurring usually after the first 30–60 min of dialysis, may complicate acute dialysis especially in small infants [26]. Common problems related to vascular access include: kinking of the CVC (far more frequent in infants than adults), thrombus formation and formation of a fibrin sheath, reported in up to 50% of long-term CVCs. Catheter infection risk can be decreased by the use of sterile techniques during CVC placement, hand washing between dialysis patients, and the use of antibacterial antiseptic solutions for exit-site care. Antimicrobial-based locking solutions should be reserved for those at high risk of catheter infection [12].
CRRT
Introduction
CRRT is considered by many clinicians the most appropriate modality for the management of the critically ill patient and is the suggested modality of RRT in hemodynamically unstable patients with AKI [13, 34, 58]. Since the introduction of CRRT in 1980s, its use has increased steadily over the two past decades, including in the pediatric population [53].
Advantages
The critically ill child with multiple organ dysfunction syndrome and hemodynamic instability represents the typical target of CRRT performed in the PICU. The main indications to start CRRT reported in the ppCRRT Registry are metabolic or fluid abnormalities directly related to AKI [6]. These abnormalities can include fluid overload, hyperkalemia, and symptomatic uremia (encephalopathy, pericarditis). Non-renal indications are hyperammonemia associated with an inborn error of metabolism, intoxication, or medication overdose. CRRT shares many principles with HD, but flow rates are significantly slower (Table 1). The gradual fluid removal and solute re-equilibration of CRRT allow for better hemodynamic stability and reduced transcellular solute shifts compared to intermittent EC therapies [58]. Thus, this RRT modality avoids the treatment-induced increase of intracranial pressure described for HD. CRRT compensates lower hourly clearance rates with longer duration of the session. Over 24 h, CRRT can provide solute clearance comparable to that seen during a 4-h HD session [58].
Compared to PD, CRRT can provide far more efficient clearance and easier control of fluid balance (Table 1). The predictable and net volume removal eliminates the need for fluid restriction and allows greater fluid intake necessary for nutrition or blood component therapy. Although CRRT can be technically challenging in neonates and small infants, the recent development of miniaturized circuits and membranes and accurate CRRT machines makes it a feasible option even in this group of patients. The Cardio-Renal Pediatric Dialysis Emergency Machine (CARPEDIEM®), the Newcastle Infant Dialysis and Ultrafiltration System (NIDUS®), and the Aquadex® provide promising results for the treatment of neonates requiring dialysis [59,60,61].
Disadvantages
Although the latest CRRT machines are user-friendly, CRRT is technically challenging and requires expertise [24, 26]. The need for alarm vigilance leads to a greater nursing workload than other RRT modalities. Other significant technical disadvantages of CRRT include the necessity of continuous anticoagulation and the high risk of circuit clotting [14]. Higher costs compared to the other dialysis modalities represent one of the major limitations of CRRT in low-income countries [24, 26] (Table 1).
Prescription
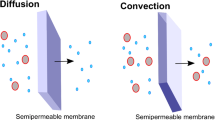
As for intermittent HD, bloodline priming is recommended if the EC circuit volume exceeds 10% of circulating blood volume. Qb should range from 3 to 5 ml/kg/min in neonates to 5–10 ml/kg/min in infants and 100–150 ml/min in older children and adolescents [58]. Bicarbonate-based solutions with varying electrolyte amounts are available for both dialysate and replacement fluids. Recently, the use of a new phosphate-containing replacement fluid (Phoxilium® Gambro Lundia AB, Lund, Sweden) has successfully prevented the onset of hypophosphatemia during CRRT [62]. CRRT can be provided in various forms. Continuous Veno-Venous Hemofiltration (CVVH) uses convection, with ultrafiltrate replaced in part or completely with appropriate replacement fluids; the replacement fluid can be infused before (predilution) and/or after (postdilution) the hemofilter [13, 58]. Predilution is preferred in pediatric patients to reduce the risk of circuit clotting. In continuous veno-venous hemodialysis (CVVHD), the main mechanism of solute clearance is diffusion. Continuous veno-venous hemodiafiltration (CVVHDF) combines diffusive and convective clearance. The modality choice depends on patient needs, center attitude, and anticoagulation protocols. North America data shows that CVVHD was used in 48% of cases [6], while in Europe a predominant use of convective modalities has been reported [54]. Specific advantages concerning the clearance of medium-/high-molecular-weight solutes, including inflammatory mediators, makes CVVH an attractive form of CRRT especially for septic patients [63]. Currently, no randomized trials exist to define the optimal CRRT dose prescription in children. Adult data have clearly showed no benefit with high CRRT dose (greater than 20–25 ml/kg/h) [12, 64,65,66]. However, in clinical practice, the delivered dose may be substantially lower than the prescribed dose: access malfunction or membrane fouling can produce significant daily clearance reduction. Additionally, pre-dilution CVVH can further reduce clearance by 15–35% [52]. Thus, to deliver the recommended minimum dose of 20–25 ml/kg/h, a higher prescription dose is usually required [34]. Conversely, higher CRRT doses may be associated with excessive drug clearance rates and protein losses. Currently, clearances of 2 l/h/1.73 m2 of BSA are normally applied in children [67]. However, weight and BSA are not linearly related and when prescription is referred to BSA treatment, intensity in neonates and infants is 1.5–3 times higher than that of adolescents and adults (2 l/h/1.73 m2 corresponds to 80 ml/kg/h in a neonate and to 35 ml/kg/h in a 70 kg adolescent) [67]. Another important point is the optimal timing for RRT initiation. In the adult population, meta-analyses have produced discordant conclusions on whether “early” compared with “delayed” RRT initiation improves survival, kidney recovery, or reduces ICU or hospital stay [12, 68]. In the pediatric population, fluid overload may represent a trigger to initiate “early” CRRT. The association between fluid overload degree at CRRT start and morbidity and mortality was first characterized in PICU settings and later observed in a wide variety of ICU populations [69, 70]. According to the current evidence, it may be reasonable starting CRRT before fluid accumulation exceeds 10–20% of the patient’s weight at ICU admission.
Equipment
Continuous RRT disposables are specific for every machine and are usually designed for a specific treatment modality. A number of hemofilters and membranes have been developed for use with CRRT (Table 4). Very recently, the results of a double-blind randomized study comparing standard CVVH versus CVVH with high cut-off membranes in critically ill patients with AKI requiring vasopressors have been published. Duration of vasopressor support, cytokine removal, and mortality were not different between the two groups of patients [71].
Anticoagulation
Anticoagulation of the EC circuit is required to prevent clotting of the circuit, preserve filter performance, and prevent blood loss. During CRRT, anticoagulation is most frequently performed with UH or regional anticoagulation. We have extensively discussed heparin anticoagulation in the HD section. Regional citrate anticoagulation (RCA) inhibits the clotting cascade within the EC circuit and it is an ideal option for critically ill patients at high risk for bleeding [34]. The six additional trials on adults (674 patients) published since KDIGO guideline publication, describe superior hemofilter lifespan and fewer adverse effects associated with RCA compared to UH [72]. Since the first reported use of citrate in children in 2002 [73], an increasing amount of data has shown that RCA can be safe and effective in the pediatric population also [74,75,76]. Citrate is a tricarboxylic acid that inhibits thrombin formation by chelating ionized calcium (iCa) [77]. Many commercially produced citrate formulations and solutions are available, and numerous RCA protocols have been developed. Citrate is infused into the access line of the EC circuit as proximally as possible (usually in the pre-blood pump) at rates proportional to blood flow. The citrate infusion rate is titrated to maintain iCa levels in the EC circuit of 0.25–0.35 mmol/l. In order to obtain this target, the citrate level in the EC circuit should be approximately 2–3 mmol/l. A discrete quantity of the small-molecular-weight complexes of iCa-citrate are filtered quite efficiently (20–50% removal) and lost in the effluent fluid. As a result, the blood returning to the patient is calcium-depleted and supplemental IV calcium is mandatory to avoid hypocalcemia. The calcium solution needs to be infused through a separate CVC or, more frequently, by the blood out-flow line, as calcium chloride or gluconate. No data support superiority of one calcium salt over the other. Modern control interfaces offer an integrated calcium management system where the calcium infusion is synchronized with the citrate pump and with the calcium lost in the effluent. Adequate monitoring of a patient’s iCa levels should be performed initially at 30–60 and 120 min; subsequently, every 4–6 h [78]. Total calcium levels and the total to ionized calcium ratio should be measured daily in patients receiving CRRT with RCA to detect citrate accumulation. In particular, in those clinical conditions in which inadequate citrate metabolism may occur (liver failure, septic shock) there is an impaired release of calcium from calcium-citrate complexes, generating a high total to ionized calcium ratio. A value of 2.1 accurately predicts citrate accumulation and a ratio > 2.5 is associated with increased metabolic complications [78]. Recently, RCA has been used safely and successfully in patients with advanced liver disease, as well as in perioperative liver transplant patients [79, 80]. Nevertheless, in these patients, citrate load should be reduced, and calcium monitoring intervals shortened to avoid complications [78].
Access
Current CRRT treatments use a “veno-venous” technique. “Artero-venous” circuits used in the past have been virtually abandoned. As for HD, adequate vascular access is required for efficient provision of CRRT [14]. Before the introduction of miniaturized machines dedicated to neonates and infants, the use of two single-lumen 5-French catheters was discouraged because of poor circuit survival [53]. Recently, successful use of 5 cm dual-lumen-4 French catheter, placed surgically into the femoral vein, has been reported in a neonate of 2.9 kg treated with the CARPEDIEM [81].
Complications
Continuous RRT is associated with catheter complications and infections, hypothermia, and electrolyte imbalance. Development of nutritional deficiency during CRRT must not be underestimated and adequate supplemental protein (as high as 3–4 g/kg per day) should be provided [14]. CRRT prescription has a significant impact on drug dosing. Regular correction of the dose is required and whenever possible drug levels should be monitored.
Conclusions
The dialytic treatment of AKI remains an open challenge and requires experience. Intermittent HD is the most efficient in volume and solute removal, but considering the growing population of hemodynamically unstable and critically ill children, PD and CRRT are more suitable. In particular, recent important improvements in membrane materials, anticoagulation approach, and miniaturized machines have made CRRT the preferred modality in many pediatric centers.
In the last decade, KDIGO criteria and severity illness scores have allowed the comparison of different pediatric populations with AKI. Despite this, we are still not able to answer the relevant question of whether one of the dialysis techniques is superior to the other in terms of outcome. To this end, we are collecting data on the three dialysis modalities in a European registry. Similarly, pediatric studies on the correct dialytic dose for AKI patients are still lacking. Specifically, it would be necessary to better define the impact of dialysis dose on outcome and the effect of high volumes (relative to patient size) on the removal of antibiotics and nutrients.
Conversely, KDIGO criteria, the well-defined role of fluid overload, renal angina index, and biomarkers are allowing identification of the correct timing of intervention. Early initiation of dialysis seems to be critical in order to improve outcome and avoid further complications.
Key summary points
-
The RRT options for pediatric AKI include PD, intermittent HD, continuous RRT (CRRT), and hybrid modalities.
-
The choice of dialytic approach to pediatric AKI is still an open challenge and should be based on the patient’s characteristics, on the physical processes of each technique, and on the institutional resources and local practice.
-
PD is the modality of choice in pediatric AKI following cardiovascular surgery. It is the preferred dialysis for newborns, even if new miniaturized machines make CRRT a feasible option. It could be performed in units with no HD expertise and with minimal infrastructural support.
-
Intermittent HD is still considered the preferred dialysis option in conditions requiring rapid and effective solute and fluid removal.
-
CRRT is a technically challenging dialysis option. It is considered the most appropriate modality for the management of the critically ill patient with AKI. CRRT is almost exclusively performed in an ICU setting.
Multiple choice questions (answers are provided in Backmaterial following the Reference List
-
1.
Which of the following statements about peritoneal dialysis is true:
- a)
PD is usually performed in the intensive care unit (ICU)
- b)
The use of appropriate catheter and technique allows a predictable UF in critically ill children
- c)
PD cannot be performed without a well-functioning vascular access
- d)
Providing PD does not require expertise and individual nurses
- a)
-
2.
Between the following dialytic indications, in which case would CRRT be the most appropriate treatment approach?
- a)
A 15-year-old female trauma victim with significant myoglobinemia, hyperkalemia, and hyperphosphatemia
- b)
A 3-year-old patient with HUS with oliguria and acute kidney failure
- c)
A 16-year-old oncologic patient with sepsis, a fluid overload of 9%, and hemodynamic instability
- d)
A neonate with hyperammonemia secondary to an inborn error of metabolism without anuria and acute kidney injury
- a)
-
3.
When starting an acute HD:
- a)
Slow reduction of uremic toxins is required to avoid the disequilibrium syndrome
- b)
The UF rate may exceed 1–2% of patient’s body weight reduction hourly
- c)
An anticoagulation protocol is always recommended
- d)
Set the blood pump rate at 5–8 ml/kg/min for small infants
- a)
-
4.
Which of the following statements about the regional anticoagulation with citrate is true:
- a)
A patient’s iCa level monitoring should be performed after 30 min initially, and later on after 12 h
- b)
Calcium gluconate is the recommended supplemental calcium solution for the correction of a patient’s calcium levels
- c)
LMWH is the preferred anticoagulant when the presence of liver failure contraindicates regional citrate
- d)
None
- a)
References
Susantitaphong P, Cruz DN, Cerda J, Abulfaraj M, Alqahtani F, Koulouridis I, Jaber BL (2013) World incidence of AKI: a meta-analysis. Clin J Am Soc Nephrol 8:1482–1493
Rewa O, Bagshaw SM (2014) Acute kidney injury-epidemiology, outcomes and economics. Nat Rev Nephrol 10:193–207
Kaddourah A, Basu RK, Bagshaw SM, Goldstein SL, AWARE Investigators (2017) Epidemiology of acute kidney injury in critically ill children and young adults. N Engl J Med 376:11–20
Hsu RK, McCulloch CE, Dudley RA, Lo LJ, Hsu CY (2013) Temporal changes in incidence of dialysis-requiring AKI. J Am Soc Nephrol 24:37–42
Sutherland SM, Ji J, Sheikhi FH, Widen E, Tian L, Alexander SR, Ling XB (2013) AKI in hospitalized children: epidemiology and clinical associations in a national cohort. Clin J Am Soc Nephrol 8:1661–1669
Symons JM, Chua AN, Somers MJ, Baum MA, Bunchman TE, Benfield MR, Brophy PD, Blowey D, Fortenberry JD, Chand D, Flores FX, Hackbarth R, Alexander SR, Mahan J, McBryde KD, Goldstein SL (2007) Demographic characteristics of pediatric continuous renal replacement therapy: a report of the prospective pediatric continuous renal replacement therapy registry. Clin J Am Soc Nephrol 2:732–738
Akcan-Arikan A, Zappitelli M, Loftis LL, Washburn KK, Jefferson LS, Goldstein SL (2007) Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int 71:1028–1035
Zappitelli M, Bernier PL, Saczkowski RS, Tchervenkov CI, Gottesman R, Dancea A, Hyder A, Alkandari O (2009) A small postoperative rise in serum creatinine predicts acute kidney injury in children undergoing cardiac surgery. Kidney Int 76:885–892
Askenazi DJ, Feig DI, Graham NM, Hui-Stickle S, Goldstein SL (2006) 3-5 year longitudinal follow-up of pediatric patients after acute renal failure. Kidney Int 69:184–189
Mammen C, Al Abbas A, Skippen P, Nadel H, Levine D, Collet JP, Matsell DG (2012) Long-term risk of CKD in children surviving episodes of acute kidney injury in the intensive care unit: a prospective cohort study. Am J Kidney Dis 59:523–530
Bunchman TE, McBryde KD, Mottes TE, Gardner JJ, Maxvold NJ, Brophy PD (2001) Pediatric acute renal failure: outcome by modality and disease. Pediatr Nephrol 16:1067–1071
Bagshaw SM, Darmon M, Ostermann M, Finkelstein FO, Wald R, Tolwani AJ, Goldstein SL, Gattas DJ, Uchino S, Hoste EA, Gaudry S (2017) Current state of the art for renal replacement therapy in critically ill patients with acute kidney injury. Intensive Care Med 43:841–854
Neri M, Villa G, Garzotto F, Bagshaw S, Bellomo R, Cerda J, Ferrari F, Guggia S, Joannidis M, Kellum J, Kim JC, Mehta RL, Ricci Z, Trevisani A, Marafon S, Clark WR, Vincent JL, Ronco C, Nomenclature Standardization Initiative (NSI) alliance (2016) Nomenclature for renal replacement therapy in acute kidney injury: basic principles. Crit Care 20:318
Walters S, Porter C, Brophy PD (2009) Dialysis and pediatric acute kidney injury: choice of renal support modality. Pediatr Nephrol 24:37–48
Ponce D, Gobo-Oliveira M, Balbi AL (2017) Peritoneal dialysis treatment modality option in acute kidney injury. Blood Purif 43:173–178
Chionh CY, Soni SS, Finkelstein FO, Ronco C, Cruz DN (2013) Use of peritoneal dialysis in AKI: a systematic review. Clin J Am Soc Nephrol 8:1649–1660
Cullis B, Abdelraheem M, Abraham G, Balbi A, Cruz DN, Frishberg Y, Koch V, McCulloch M, Numanoglu A, Nourse P, Pecoits-Filho R, Ponce D, Warady B, Yeates K, Finkelstein FO (2014) Peritoneal dialysis for acute kidney injury. Perit Dial Int 34:494–517
Stojanović VD, Bukarica SS, Antić JB, Doronjski AD (2017) Peritoneal dialysis in very low birth weight neonates. Perit Dial Int 37:389–396
Bojan M, Gioanni S, Vouhé PR, Journois D, Pouard P (2012) Early initiation of peritoneal dialysis in neonates and infants with acute kidney injury following cardiac surgery is associated with a significant decrease in mortality. Kidney Int 82:474–481
Barhight MF, Soranno D, Faubel S, Gist KM (2018) Fluid management with peritoneal dialysis after pediatric cardiac surgery. World J Pediatr Congenit Heart Surg 9:696–704
Vasudevan A, Phadke K, Yap HK (2017) Peritoneal dialysis for the management of pediatric patients with acute kidney injury. Pediatr Nephrol 32:1145–1156
Haubitz M, Brunkhorst R, Wrenger E, Froese P, Schulze M, Koch KM (1996) Chronic induction of C-reactive protein by hemodialysis, but not by peritoneal dialysis therapy. Perit Dial Int 16:158–162
Podel J, Hodelin-Wetzel R, Saha DC, Burns G (2000) Glucose absorption in acute peritoneal dialysis. J Ren Nutr 10:93–97
Raina R, Chauvin AM, Bunchman T, Askenazi D, Deep A, Ensley MJ, Krishnappa V, Sethi SK (2017) Treatment of AKI in developing and developed countries: an international survey of pediatric dialysis modalities. PLoS One 12:e0178233
Bonilla-Félix M (2013) Peritoneal dialysis in the pediatric intensive care unit setting: techniques, quantitations and outcomes. Blood Purif 35:77–80
Strazdins V, Watson AR, Harvey B, European Pediatric Peritoneal Dialysis Working Group (2004) Renal replacement therapy for acute renal failure in children: European guidelines. Pediatr Nephrol 19:199–207
Reznik VM, Randolph G, Collins CM, Peterson BM, Lemire JM, Mendoza SA (1993) Cost analysis of dialysis modalities for pediatric acute renal failure. Perit Dial Int 13:311–313
Picca S, Dionisi-Vici C, Abeni D, Pastore A, Rizzo C, Orzalesi M, Sabetta G, Rizzoni G, Bartuli A (2001) Extracorporeal dialysis in neonatal hyperammonemia: modalities and prognostic indicators. Pediatr Nephrol 16:862–867
Bunchman TE, Meldrum MK, Meliones JE, Sedman AB, Walters MB, Kershaw DB (1992) Pulmonary function variation in ventilator dependent critically ill infants on peritoneal dialysis. Adv Perit Dial 8:75–78
Auron A, Warady BA, Simon S, Blowey DL, Srivastava T, Musharaf G, Alon US (2007) Use of the multipurpose drainage catheter for the provision of acute peritoneal dialysis in infants and children. Am J Kidney Dis 49:650–655
White CT, Gowrishankar M, Feber J, Yiu V, Canadian Association of Pediatric Nephrologists (CAPN), Peritoneal Dialysis Working Group (2006) Clinical practice guidelines for pediatric peritoneal dialysis. Pediatr Nephrol 21:1059–1066
Ladd AP, Breckler FD, Novotny NM (2011) Impact of primary omentectomy on longevity of peritoneal dialysis catheters in children. Am J Surg 201:401–404
Radtke J, Schild R, Reismann M, Ridelski RR, Kempf C, Nashan B, Rothe K, Koch M (2018) Obstruction of peritoneal dialysis catheter is associated with catheter type and independent of omentectomy: a comparative data analysis from a transplant surgical and pediatric surgical department. J Pediatr Surg 53:640–643
KDIGO AKI Work Group (2012) KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 17:1–138
Picca S, Dionisi-Vici C, Bartuli A, De Palo T, Papadia F, Montini G, Materassi M, Donati MA, Verrina E, Schiaffino MC, Pecoraro C, Iaccarino E, Vidal E, Burlina A, Emma F (2015) Short-term survival of hyperammonemic neonates treated with dialysis. Pediatr Nephrol 30:839–847
Davenport A (2012) Alternatives to standard unfractionated heparin for pediatric hemodialysis treatments. Pediatr Nephrol 27:1869–1879
Raina R, Vijayaraghavan P, Kapur G, Sethi SK, Krishnappa V, Kumar D, Bunchman TE, Bolen SD, Chand D (2017) Hemodialysis in neonates and infants: a systematic review. Semin Dial 31:289–299
Pollack S, Eisenstein I, Tarabeih M, Shasha-Lavski H, Magen D, Zelikovic I (2016) Long-term hemodialysis therapy in neonates and infants with end-stage renal disease: a 16-year experience and outcome. Pediatr Nephrol 31:305–313
Fischbach M, Edefonti A, Schröder C, Watson A, European Pediatric Dialysis Working Group (2005) Hemodialysis in children: general practical guidelines. Pediatr Nephrol 20:1054–1066
Marshall MR, Golper TA (2011) Low-efficiency acute renal replacement therapy: role in acute kidney injury. Semin Dial 24:142–148
Misra M (2005) The basics of hemodialysis equipment. Hemodial Int 9:30–36
Schiffl H, Lang SM, Haider M (1998) Bioincompatibility of dialyzer membranes may have a negative impact on outcome of acute renal failure, independent of the dose of dialysis delivered: a retrospective multicenter analysis. ASAIO J 44:M418–M422
Hakim RM, Wingard RL, Parker RA (1994) Effect of the dialysis membrane in the treatment of patients with acute renal failure. N Engl J Med 331:1338–1342
Alonso A, Lau J, Jaber BL (2008) Biocompatible hemodialysis membranes for acute renal failure. Cochrane Database Syst Rev 1:CD005283
Ponikvar JB, Rus RR, Kenda RB, Bren AF, Ponikvar RR (2001) Low-flux versus high-flux synthetic dialysis membrane in acute renal failure: prospective randomized study. Artif Organs 25:946–950
Ricci Z, Romagnoli S, Ronco C (2017) High cut-off membranes in acute kidney injury and continuous renal replacement therapy. Int J Artif Organs 40:657–664
Haase M, Bellomo R, Baldwin I, Haase-Fielitz A, Fealy N, Davenport P, Morgera S, Goehl H, Storr M, Boyce N, Neumayer HH (2007) Hemodialysis membrane with a high-molecular-weight cutoff and cytokine levels in sepsis complicated by acute renal failure: a phase 1 randomized trial. Am J Kidney Dis 50:296–304
Davenport A (2003) Anticoagulation options for pediatric hemodialysis. Hemodial Int 7:168–176
Dutt T, Schulz M (2013) Heparin-induced thrombocytopaenia (HIT)—an overview: what does the nephrologist need to know and do? Clin Kidney J 6:563–567
Chanard J, Lavaud S, Maheut H, Kazes I, Vitry F, Rieu P (2008) The clinical evaluation of low-dose heparin in haemodialysis: a prospective study using the heparin-coated AN69 ST membrane. Nephrol Dial Transplant 23:2003–2009
Kreuzer M, Bonzel KE, Büscher R, Offner G, Ehrich JH, Pape L (2010) Regional citrate anticoagulation is safe in intermittent high-flux haemodialysis treatment of children and adolescents with an increased risk of bleeding. Nephrol Dial Transplant 25:3337–3342
Bunchman TE, Brophy PD, Goldstein SL (2008) Technical considerations for renal replacement therapy in children. Semin Nephrol 28:488–492
Hackbarth R, Bunchman TE, Chua AN, Somers MJ, Baum M, Symons JM, Brophy PD, Blowey D, Fortenberry JD, Chand D, Flores FX, Alexander SR, Mahan JD, McBryde KD, Benfield MR, Goldstein SL (2007) The effect of vascular access location and size on circuit survival in pediatric continuous renal replacement therapy: a report from the PPCRRT registry. Int J Artif Organs 30:1116–1121
de Galasso L, Emma F, Picca S, Di Nardo M, Rossetti E, Guzzo I (2016) Continuous renal replacement therapy in children: fluid overload does not always predict mortality. Pediatr Nephrol 31:651–659
Goldstein SL (2011) Advances in pediatric renal replacement therapy for acute kidney injury. Semin Dial 24:187–191
Zhao Y, Li Z, Zhang L, Yang J, Yang Y, Tang Y, Fu P (2014) Citrate versus heparin lock for hemodialysis catheters: a systematic review and meta-analysis of randomized controlled trials. Am J Kidney Dis 63:479–490
Arechabala MC, Catoni MI, Claro JC, Rojas NP, Rubio ME, Calvo MA, Letelier LM (2018) Antimicrobial lock solutions for preventing catheter-related infections in haemodialysis. Cochrane Database Syst Rev 4:CD010597
Sutherland SM, Alexander SR (2012) Continuous renal replacement therapy in children. Pediatr Nephrol 27:2007–2016
Ronco C, Garzotto F, Ricci Z (2012) CA.R.PE.DI.E.M. (cardio–renal pediatric dialysis emergency machine): evolution of continuous renal replacement therapies in infants. A personal journey. Pediatr Nephrol 27:1203–1211
Coulthard MG, Crosier J, Griffiths C, Smith J, Drinnan M, Whitaker M, Beckwith R, Matthews JN, Flecknell P, Lambert HJ (2014) Haemodialysing babies weighing < 8 kg with the Newcastle infant dialysis and ultrafiltration system (Nidus): comparison with peritoneal and conventional haemodialysis. Pediatr Nephrol 29:1873–1881
Askenazi D, Ingram D, White S, Cramer M, Borasino S, Coghill C, Dill L, Tenney F, Feig D, Fathallah-Shaykh S (2016) Smaller circuits for smaller patients: improving renal support therapy with Aquadex™. Pediatr Nephrol 31:853–860
Godaly G, Carlsson O, Broman M (2016) Phoxilium® reduces hypophosphataemia and magnesium supplementation during continuous renal replacement therapy. Clin Kidney J 9:205–210
Ricci Z, Goldstein SL (2016) Pediatric continuous renal replacement therapy. Contrib Nephrol 187:121–130
VA/NIH Acute Renal Failure Trial Network, Palevsky PM, Zhang JH, O’Connor TZ, Chertow GM, Crowley ST, Choudhury D, Finkel K, Kellum JA, Paganini E, Schein RM, Smith MW, Swanson KM, Thompson BT, Vijayan A, Watnick S, Star RA, Peduzzi P (2008) Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med 359:7–20
RENAL Replacement Study Investigators, Bellomo R, Cass A, Cole L, Finfer S, Gallagher M, Lo S, McArthur C, McGuinness S, Myburgh J, Norton R, Scheinkestel C, Su S (2009) Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med 361:1627–1638
Jun M, Heerspink HJ, Ninomiya T, Gallagher M, Bellomo R, Myburgh J, Finfer S, Palevsky PM, Kellum JA, Perkovic V, Cass A (2010) Intensities of renal replacement therapy in acute kidney injury: a systematic review and meta-analysis. Clin J Am Soc Nephrol 5:956–963
Romagnoli S, Lark WR, Ricci Z, Ronco C (2017) Renal replacement therapy for AKI: when? How much? When to stop? Best Pract Res Clin Anaesthesiol 31:371–385
Wierstra BT, Kadri S, Alomar S, Burbano X, Barrisford GW, Kao RL (2016) The impact of “early” versus “late” initiation of renal replacement therapy in critical care patients with acute kidney injury: a systematic review and evidence synthesis. Crit Care 20:122
Sutherland SM, Zappitelli M, Alexander SR, Chua AN, Brophy PD, Bunchman TE, Hackbarth R, Somers MJ, Baum M, Symons JM, Flores FX, Benfield M, Askenazi D, Chand D, Fortenberry JD, Mahan JD, McBryde K, Blowey D, Goldstein SL (2010) Fluid overload and mortality in children receiving continuous renal replacement therapy: the prospective pediatric continuous renal replacement therapy registry. Am J Kidney Dis 55:316–325
Garzotto F, Ostermann M, Martin-Langerwerf D, Sánchez-Sánchez M, Teng J, Robert R, Marinho A, Herrera-Gutierrez ME, Mao HJ, Benavente D, Kipnis E, Lorenzin A, Marcelli D, Tetta C, Ronco C, DoReMIFA study group (2016) The dose response multicentre investigation on fluid assessment (DoReMIFA) in critically ill patients. Crit Care 20:196
Atan R, Peck L, Prowle J, Licari E, Eastwood GM, Storr M, Goehl H, Bellomo R (2018) A double-blind randomized controlled trial of high cutoff versus standard hemofiltration in critically ill patients with acute kidney injury. Crit Care Med 46:e988–e994
Bai M, Zhou M, He L, Ma F, Li Y, Yu Y, Wang P, Li L, Jing R, Zhao L, Sun S (2015) Citrate versus heparin anticoagulation for continuous renal replacement therapy: an updated meta-analysis of RCTs. Intensive Care Med 41:2098–2110
Bunchman TE, Maxvold NJ, Barnett J, Hutchings A, Benfield MR (2002) Pediatric hemofiltration: normocarb dialysate solution with citrate anticoagulation. Pediatr Nephrol 17:150–154
Soltysiak J, Warzywoda A, Kocinski B, Ostalska-Nowicka D, Benedyk A, Silska-Dittmar M, Zachwieja J (2014) Citrate anticoagulation for continuous renal replacement therapy in small children. Pediatr Nephrol 29:469–475
Rico MP, Fernández Sarmiento J, Rojas Velasquez AM, González Chaparro LS, Gastelbondo Amaya R, Mulett Hoyos H, Tibaduiza D, Quintero Gómez AM (2016) Regional citrate anticoagulation for continuous renal replacement therapy in children. Pediatr Nephrol 32:703–711
Zaoral T, Hladík M, Zapletalová J, Trávníček B, Gelnarová E (2016) Circuit lifetime with citrate versus heparin in pediatric continuous venovenous hemodialysis. Pediatr Crit Care Med 17:e399–e405
Davis TK, Neumayr T, Geile K, Doctor A, Hmeil P (2014) Citrate anticoagulation during continuous renal replacement therapy in pediatric critical care. Pediatr Crit Care Med 15:471–785
Morabito S, Pistolesi V, Tritapepe L, Fiaccadori E (2014) Regional citrate anticoagulation for RRTs in critically ill patients with AKI. Clin J Am Soc Nephrol 9:2173–2188
Slowinski T, Morgera S, Joannidis M, Henneberg T, Stocker R, Helset E, Andersson K, Wehner M, Kozik-Jaromin J, Brett S, Hasslacher J, Stover JF, Peters H, Neumayer HH, Kindgen-Milles D (2015) Safety and efficacy of regional citrate anticoagulation in continuous venovenous hemodialysis in the presence of liver failure: the liver citrate anticoagulation threshold (L-CAT) observational study. Crit Care 19:349
Sponholz C, Settmacher U, Bauer M, Kortgen A (2015) Regional citrate anticoagulation for continuous renal replacement therapy in the perioperative care of liver transplant recipients: a single center experience. Ther Apher Dial 19:8–15
Ronco C, Garzotto F, Brendolan A, Zanella M, Bellettato M, Vedovato S, Chiarenza F, Ricci Z, Goldstein SL (2014) Continuous renal replacement therapy in neonates and small infants: development and first-in-human use of a miniaturised machine (CARPEDIEM). Lancet 383:1807–1813
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Answers: 1. d; 2. c; 3. a; 4. d
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Galasso, L., Picca, S. & Guzzo, I. Dialysis modalities for the management of pediatric acute kidney injury. Pediatr Nephrol 35, 753–765 (2020). https://doi.org/10.1007/s00467-019-04213-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-019-04213-x