Abstract
Background
Infants with a solitary functioning kidney (SFK) are at risk for chronic kidney injury (CKI). Lack of compensatory kidney growth (CKG) is associated with CKI, but measuring CKG is challenging since it is typically reported relative to normal kidneys. This study aims to (1) standardize SFK growth in infants, (2) investigate the relationship between standardized kidney length and clinical outcomes, and (3) use these results to develop a risk-based prediction model and local clinical pathway for SFK care.
Methods
This was a quality improvement study of 166 infants with an SFK. Linear regression was used to assess kidney growth from 0 to 180 days of life. Univariate binary regression analysis was used to identify kidney length to body length thresholds associated with the development of CKI, defined as the composite outcome of chronic kidney disease (eGFR < 60 mL/min/1.73 m2), hypertension, or proteinuria.
Results
Kidneys grew in length from 0 to 180 days, and growth was constant when standardized to body length. Over follow-up, infants with a baseline kidney length to body length ≤ 0.088 were more likely to experience CKI than the rest of the cohort (27 vs. 8%, p = 0.04). Kidney length to body length ≤ 0.088 was also significantly associated with CKI development (OR 4.17, 95% CI 1.14–15.28, p = 0.04).
Conclusions
In this study, kidney length to body length ratio was a stable CKG metric over 0–180 days, and a baseline ratio ≤ 0.088 was a risk factor for CKI. Results will aid in developing a practical, point-of-care risk assessment tool, and overarching risk-stratified clinical pathway for infants with an SFK.
Graphical abstract
A higher resolution version of the Graphical abstract is available as Supplementary information

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Infants born with a congenital anomaly of the kidney and urinary tract (CAKUT) are at risk of progressive loss of kidney function over time [1]. In particular, those with a solitary functioning kidney (SFK) can develop hypertension, proteinuria, and chronic kidney disease (CKD) over long-term follow-up [2, 3]. This loss of kidney function is likely due to a congenital deficit in nephron number, influenced by genetic background. In children with renal hypodysplasia (RHD), for example, renal parenchymal mass has been shown to predict the likelihood of developing kidney failure [4].
A number of risk factors are associated with chronic kidney injury in infants with an SFK. These include structural and/or functional anomalies in the SFK, associated genetic syndromes, recurrent urinary tract infections, and the absence of compensatory growth in the solitary kidney [2, 5,6,7]. “Normal” compensatory kidney growth in the SFK is attributed primarily to hyperfiltration resulting in glomerular and tubular hypertrophy [8], although an increase in glomerular number has also been reported in select models of fetal SFK [9, 10]. Appropriate compensatory growth in the SFK has been used as evidence of integrity of existing nephrons [11]. In the absence of a direct measure of glomerular number, kidney length in particular has been identified as an important surrogate measure of nephron mass and as a predictor of outcome in children with various congenital kidney anomalies such as RHD, posterior urethral valves (PUV), and an SFK [4, 7, 12,13,14]. In most reports, kidney length is expressed as a percentile of normative age-related kidneys [7]. However, SFK grow larger than normal kidneys and therefore warrant their own unique nomograms to assess “normative” growth.
The objectives of this work are as follows: (1) to standardize “normal” kidney growth in infants with an SFK, (2) to use these data to study the relationship of kidney size at early diagnosis with kidney outcomes, and (3) to incorporate these results into a risk prediction model and local clinical pathway.
Methods
This was a quality improvement (QI) study conducted to help develop a standardized clinical pathway for the management of infants with an SFK at our center. The local Data Collections and Solutions Steering Committee vetted our study objectives and data management plan prior to study initiation. Ethics approval was not sought, as QI initiatives are exempt from Research Ethics Board review at our center, in accordance with article 2.5 of the Tri Council Policy Statement [15].
Patient selection
We identified all patients born from 2000 to 2017 with a congenital SFK due to either multicystic dysplastic kidney (MCDK) disease or unilateral renal agenesis (URA), as previously described [2]. Patients whose SFK was acquired secondary to another condition were excluded from this study, as early-acquired SFK (as seen with unilateral nephrectomy for cancer and urologic abnormalities) have been shown to have differing long-term outcomes from congenital SFK [3]. Relevant clinical and ultrasound data from all patients meeting study inclusion criteria were subsequently entered into a QI REDCap data collection tool [16]. Given that the aim of the proposed clinical pathway is to identify infants at risk for developing chronic kidney injury, to intervene when necessary and in a timely fashion, and to triage those not needing longer-term follow-up, we included infants 180 days or younger at initial assessment, as this will be our recommended period of initial referral and assessment for our final clinical pathway. For the purpose of this analysis, as in our previous retrospective review [2], this age of inclusion also allows for an appropriate length of follow-up.
Clinical outcomes
Clinical outcomes studied included CKD, hypertension, and proteinuria over follow-up, as well as the composite of all three outcomes (hereafter referred to as chronic kidney injury). A detailed description of the definitions and measurement techniques for each outcome has been previously summarized [2]. Briefly, CKD was defined as an estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2 on two consecutive outpatient visits separated by at least 3 months, as calculated using the appropriate Schwartz equations. Hypertension was defined as a systolic and/or diastolic blood pressure (sBP/dBP) ≥ 95th percentile for age, sex, and height on two consecutive visits occurring at least 3 months apart. Proteinuria was defined as a urinalysis with ≥ 0.3 g/L protein, urine dipstick > 1 + , urine protein to creatinine ratio (PCR) > 25 mg/mmol, or urine albumin to creatinine ratio (ACR) ≥ 3.4 mg/mmol on two consecutive visits at least 3 months apart. The time to development of the clinical outcome, as defined, was determined to be the time at which it was first documented.
Kidney ultrasounds
Ultrasounds were performed almost exclusively at our tertiary referral center by trained expert pediatric radiologists. Examinations were performed using a GE LOGIQ E9 unit. Kidney measurements were obtained using a C1-6 MHz or C2-9 convex transducer and standard gray-scale B-mode imaging. Kidney length was measured in the sagittal plane using the image depicting the greatest longitudinal dimension of the kidney. This was repeated twice and the longest measurement was recorded. The patients were scanned either in the supine or slightly left or right lateral decubitus positions.
Body lengths
Recumbent infant body lengths were measured at each clinic visit by a trained nephrology nurse and/or nurse’s assistant using a length board on a flat table.
Kidney length to body height ratio
To standardize for overall size differences among infants, we used the ratio of kidney length to body length from the first postnatal assessment with measured body lengths taken ± 30 days from the date of ultrasound, or imputed lengths based on age, sex, and the closest height available, as previously described [2]. For the analysis of outcomes, we stratified patients according to whether their SFK kidney length to body length ratio was above or below the 5th, 10th, and 25th percentiles for the whole cohort.
Statistical analyses
All analyses were conducted using SPSS version 27 software (IBM Corp., Armonk, NY) with a threshold of p < 0.05 for statistical significance. Various summary measures were used to characterize patients and first kidney ultrasounds occurring within the first 180 days of life. Parametric and non-parametric group data were expressed as means ± standard deviation (SD) and medians (interquartile range) (IQR), respectively, based on visual inspection and normality testing. Categorical data were expressed as proportions (percent). Between group comparisons were made using Student’s t test, Mann–Whitney U test, and Pearson’s chi square or Fisher’s exact test, as indicated. Linear regression analysis was used to determine kidney growth over time. Univariate binary logistic regression was used to identify kidney length to body length ratio thresholds that were associated with the development of chronic kidney injury, reported as odds ratios (OR) and 95% confidence intervals (CI). Kaplan–Meier analysis was used to determine outcome-free survival according to age and stratified for kidney length to body length ratio. Univariate Cox regression was utilized to obtain hazard ratios (HR) and their corresponding 95% confidence intervals comparing patients above and below the thresholds. Sensitivity, specificity and positive and negative predictive values were calculated for kidney length to body length ratio as a test for the various outcomes.
Results
We identified a total of 230 cases with an SFK, 70 (30%) with URA and 160 (70%) with MCDK (Table 1). Of those, 166/230 (72%) were identified in infants at 180 days of life or younger, with a median age at first postnatal ultrasound of 9 (IQR 38) days, and a median of 1 ultrasound (range 1 to 8) done prior to 180 days. The 0–180-day cohort did not differ significantly from the full cohort with regards to clinical features such as sex, primary diagnosis, kidney sidedness, being born preterm (< 36 weeks’ gestation), or associated genetic syndromes, non-renal anomalies, and CAKUT (Table 1). At first postnatal assessment, the mean kidney length per patient was 6.13 ± 1.73 cm and 5.42 ± 0.90 cm (p < 0.001) for the full cohort and 0–180-day cohort, respectively, while the mean kidney length to body length ratio was 0.101 ± 0.015 for the full cohort and 0.104 ± 0.013 for the 0–180 day cohort (p = 0.08) (Table 1). There was no significant difference in the median age at last clinic visit between the full and 0–180-day cohorts (5.05 vs. 4.79 years, p = 0.10). The follow-up period, however, was variable, with the age at last clinic visit for the patients in the 0–180 day cohort ranging from 0.01 to 16.27 years.
Similar to the outcomes previously reported for the full cohort [2], of the 166 patients in the 0–180 day cohort, 8 (5%) developed CKD, 9 (5%) developed hypertension, 9 (5%) developed proteinuria, and 16 (10%) developed the composite outcome over follow-up (Table 2). The time to developing the outcome was also variable, with a median (IQR) of 1.07 (1.36) years for CKD, 5.67 (5.41) years for hypertension, 5.35 (7.02) years for proteinuria, and 2.47 (5.88) years for the composite outcome (Table 2).
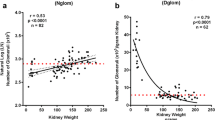
To develop a kidney length metric to predict outcome, we required that the initial kidney assessment occur at the earliest point-of-care contact, for that to be in the first 180 days of life, and that the metric be easy to measure and incorporate into a risk model. We considered using absolute kidney length; however, kidney length, as expected, changed over this short time period making it an unreliable metric that was dependent on age at first assessment (Fig. 1A). We have previously used the ratio of kidney length to body length as a reliable and standardized metric of kidney length to predict outcome in cases of children with RHD [4]. When we applied this same metric to our cases of SFK in the first 180 days, the ratio remained constant with a slope over time approximating zero (Fig. 1B).
A–B Scatterplots depicting kidney size as a function of age at first ultrasound for infants aged 0–180 days. Kidney growth was approximated based on the slope of the linear regression line of best fit (R2). Distinguishing features of each scatterplot are as follows: A growth based on kidney length (solid red line represents regression line, n = 157) of first ultrasounds done from 0 to 180 days, with associated 95% CI (dotted black lines). B Growth based on kidney length to body length ratio (solid red line represents regression line, n = 157) of first ultrasounds done from 0 to 180 days, with associated 95% CI (dotted black lines) and 0.088 threshold (dash-dotted blue line), below which there is an increased likelihood of developing chronic kidney injury
Given that the ratio of kidney length to body length did not change regardless of age within the first 180 days, we used it to predict outcome. At the first postnatal ultrasound assessment, infants 180 days and younger with a kidney length to body length ratio ≤ 0.088 (below the 10th percentile for the cohort) were more likely to have CKD (21 vs. 6%, p = 0.09), hypertension (33 vs. 6%, p = 0.03), and proteinuria (21 vs. 4%, p = 0.03) over follow-up compared to those with a kidney length to body length ratio > 0.088 (greater than the 10th percentile), respectively (Table 3). Infants with a baseline kidney length to body length ratio ≤ 0.088 were also more likely to develop the composite outcome of chronic kidney injury when compared to those with a baseline kidney length to body length ratio > 0.088 (27 vs. 8%, p = 0.04, respectively). Univariate logistic regression showed that a kidney length to body length ratio ≤ 0.088 was associated with chronic kidney injury (OR 4.17, 95% CI 1.14–15.28, p = 0.04) (Table 4). By Kaplan–Meier survival analysis, a kidney length to body length ratio of ≤ 0.088 was also associated with a significantly worse outcome-free survival for all the outcomes studied (Figs. 2A–D). Patients with a kidney length to body length ≤ 0.088 had significantly higher outcome rates over the study period with a HR of 4.54 (95% CI 1.01–20.29) for CKD, a HR of 5.40 (95% CI 1.29–22.67) for hypertension, a HR of 6.46 (95% CI 1.44–28.96) for proteinuria, and a HR of 4.73 (95% CI 1.45–15.41) for the composite outcome of chronic kidney injury, as compared to patients with a kidney length to body length > 0.088 (Figs. 2A–D). As a test, a kidney length to body length ratio ≤ 0.088 had a sensitivity of 27% and specificity of 92% to predict chronic kidney injury, with a sensitivity of 38% and specificity of 87% to predict CKD.
A–D Outcome-free survival by kidney length to body length ratio. Kaplan–Meier curves stratified by kidney length to body length ratio (KL:BL) of ≤ 0.088 and > 0.088 for A chronic kidney disease (CKD)-free survival, B hypertension-free survival, C proteinuria-free survival, and D the composite outcome of chronic kidney injury-free survival. Red line designates kidney length to body length ratio ≤ 0.088 and black line designates a ratio > 0.088. Vertical hatched lines designate right-censored cases. HR = hazard ratio, as defined by univariate Cox regression and corresponding 95% confidence intervals and p-values. The remaining cases for analysis over the time of follow-up are designated below the x-axis
Discussion
In this report, we have demonstrated the value of determining kidney length at the initial assessment of infants with an SFK. This is the first report where SFK size compared to “normal” SFK growth has been used to evaluate the risk of chronic kidney injury. Previous reports defining factors that predict a poor outcome in this population have highlighted the importance of kidney length. These reports have used various kidney length metrics, including kidney length standard deviation score [3, 5, 17], kidney length percentile [7], and kidney length insufficiency, calculated as percent deviation from expected [18]. However, these reports often use a percentile comparison of the SFK with the growth of normal kidneys. This can be problematic as there is an assortment of varying nomograms for normal kidney growth, with kidney growth compared to body length, body weight, sex, age, and kidney sidedness [19,20,21,22,23,24,25]. These normative growth data are also sometimes hampered by being outdated, having small sample sizes, and not always being easy to use at point-of-care.
Healthy SFK undergo compensatory kidney growth due to the congenital nephron deficit. As has been highlighted and emphasized by others, lack of “normal” growth in these SFK suggests an intrinsic abnormality, the severity of which impacts on long-term kidney outcome.
Recognizing the shortcomings of comparing SFK growth with the growth of normal kidneys, in this report, we developed a kidney growth nomogram for infants with an SFK from our local population. We chose to focus on kidney size and growth in the first 180 days of life. This time period corresponds with our recommended time during which an infant with an SFK should be referred and evaluated for kidney health, risk of progression or loss of kidney function, and subsequent stratification of care based on this risk.
For the purpose of being able to use these data at the first point-of-care contact and for a quick and accurate assessment of risk, we opted instead to standardize kidney length to body length. As expected, kidneys grew over the first 180 days of life and therefore kidney length could not be used by itself as an assessment of risk given the variability in the age at which infants were first referred for care and assessed. However, when we standardized kidney length to body length at the time of assessment, whether this was at 1 day or 180 days of life, this metric remained constant. We suggest then that an estimate of kidney length to body length ratio at first assessment in the first 180 days of life in infants with an SFK can be used to assess risk of longer-term kidney injury. Since kidney length approximates nephron number, a lower kidney length to body length ratio would indicate a greater nephron deficit and worse outcome.
In our cohort, infants with an SFK kidney length to body length ratio at or below 0.082 (5th percentile), 0.088 (10th percentile), and 0.096 (25th percentile) were more likely to develop chronic kidney injury over follow-up. In particular, 43% of infants with a baseline kidney length to body length ratio below the 5th, 27% of those below the 10th, and 16% below the 25th percentile for kidney length to body length ratio developed chronic kidney injury. As a test for chronic kidney injury, a kidney length to body length ratio ≤ 0.088 had a low false-positive rate but a high false-negative rate. For clinical pathway development and risk stratification, it would therefore be useful and safe, with a low likelihood of excluding patients who would develop an unfavorable outcome. However, the performance of this metric in predicting outcomes and enabling stratification of risk would likely be enhanced by the incorporation of other relevant clinical risk factors.
There were a number of limitations of this study. Due to its retrospective design, ultrasound data within the first 180 days of life were missing for some patients in the full cohort, and, as a result, our sample size was reduced from 230 to 166, thereby possibly affecting the robustness of our findings, including the possibility of committing type 2 errors in the analyses—where significant differences between groups are not identified. Also, while the majority of initial assessments were done in the first 180 days, the variation in age at first assessment in this time period may have affected the precision of the kidney length cut offs associated with chronic kidney injury. Developing a standardized referral and assessment approach as part of our proposed pathway will mitigate this variability. Furthermore, as this was a retrospective study of prevalent cases of SFK, there was variability in the length of follow-up. This likely caused an underestimate of the risk of developing the outcomes of interest, with patients with a shorter follow-up being less likely to have developed the outcome. Another drawback is the inability to comment on the generalizability of the results, given the limited diversity data available as part of the retrospective chart review. Finally, although assessment of kidney length by ultrasound is relatively simple and inexpensive, it is nonetheless a coarse estimate of absolute nephron number in that kidney. Newer methods of estimating nephron number, such as with cationized ferritin enhanced-magnetic resonance imaging, will further help identify patients at risk for developing chronic kidney injury [26].
In summary, this study represents an important step towards our goal of developing a locally relevant clinical pathway for the management of infants with an SFK at our center. We have shown that the ratio of kidney length to body length within the first 180 days of life is a useful metric of SFK growth, and that a value of 0.088 is an easy threshold to implement below which there is an increased likelihood of developing chronic kidney injury over follow-up. However, suboptimal standardized kidney length is just one of several potential risk factors. We have also previously identified URA and CAKUT as independent risk factors for chronic kidney injury [2]. The next steps in our pathway development process will be to develop a predictive model that best balances the strengths of existing models [7] with our relevant local findings and the results of this study. We also aim to fill an existing gap in the availability of point-of-care tools that can be easily used for stratifying care in infants with an SFK according to clinical variables identified early on.
References
Murugapoopathy V, Gupta IR (2020) A primer on congenital anomalies of the kidneys and urinary tracts (CAKUT). Clin J Am Soc Nephrol 15:723–731
Matsell DG, Bao C, Po White T, Chan E, Matsell E, Cojocaru D, Catapang M, Pediatric Nephrology Clinical Pathway Development Team (2021) Outcomes of solitary functioning kidneys-renal agenesis is different than multicystic dysplastic kidney disease. Pediatr Nephrol 36:3673–3680
Westland R, Schreuder MF, Bokenkamp A, Spreeuwenberg MD, van Wijk JA (2011) Renal injury in children with a solitary functioning kidney–the KIMONO study. Nephrol Dial Transplant 26:1533–1541
Matsell DG, Cojocaru D, Matsell EW, Eddy AA (2015) The impact of small kidneys. Pediatr Nephrol 30:1501–1509
Westland R, Kurvers RA, van Wijk JA, Schreuder MF (2013) Risk factors for renal injury in children with a solitary functioning kidney. Pediatrics 131:e478-485
Urisarri A, Gil M, Mandia N, Aldamiz-Echevarria L, Iria R, Gonzalez-Lamuno D, Couce ML (2018) Retrospective study to identify risk factors for chronic kidney disease in children with congenital solitary functioning kidney detected by neonatal renal ultrasound screening. Medicine 97:e11819
Poggiali IV, Simoes ESAC, Vasconcelos MA, Dias CS, Gomes IR, Carvalho RA, Oliveira MCL, Pinheiro SV, Mak RH, Oliveira EA (2019) A clinical predictive model of renal injury in children with congenital solitary functioning kidney. Pediatr Nephrol 34:465–474
McArdle Z, Schreuder MF, Moritz KM, Denton KM, Singh RR (2020) Physiology and pathophysiology of compensatory adaptations of a solitary functioning kidney. Front Physiol 11:725
Douglas-Denton R, Moritz KM, Bertram JF, Wintour EM (2002) Compensatory renal growth after unilateral nephrectomy in the ovine fetus. J Am Soc Nephrol 13:406–410
van Vuuren SH, Sol CM, Broekhuizen R, Lilien MR, Oosterveld MJ, Nguyen TQ, Goldschmeding R, de Jong TP (2012) Compensatory growth of congenital solitary kidneys in pigs reflects increased nephron numbers rather than hypertrophy. PLoS One 7:e49735
Gilad N, Weissmann-Brenner A, Gilboa Y, Dekel B, Achiron R, Perlman S (2020) Multicystic dysplastic kidney: prenatal compensatory renal growth pattern. J Ultrasound Med 40:2165–2171
Pulido JE, Furth SL, Zderic SA, Canning DA, Tasian GE (2014) Renal parenchymal area and risk of ESRD in boys with posterior urethral valves. Clin J Am Soc Nephrol 9:499–505
Westland R, Schreuder MF, van Goudoever JB, Sanna-Cherchi S, van Wijk JA (2014) Clinical implications of the solitary functioning kidney. Clin J Am Soc Nephrol 9:978–986
Krill A, Salami S, Rosen L, Friedman SC, Gitlin J, Palmer LS (2012) Evaluating compensatory hypertrophy: a growth curve specific for solitary functioning kidneys. J Urol 188:1613–1617
https://ethics.gc.ca/eng/tcps2-eptc2_2018_chapter2-chapitre2.html#a Last accessed Feb 28, 2022
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG (2009) Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42:377–381
Marzuillo P, Guarino S, Grandone A, Di Somma A, Diplomatico M, Rambaldi PF, Decimo F, Miraglia Del Giudice E, La Manna A, Polito C (2019) Congenital solitary kidney size at birth could predict reduced eGFR levels later in life. J Perinatol 39:129–134
La Scola C, Ammenti A, Puccio G, Lega MV, De Mutiis C, Guiducci C, De Petris L, Perretta R, Venturoli V, Vergine G, Zucchini A, Montini G (2016) Congenital solitary kidney in children: size matters. J Urol 196:1250–1256
Currarino G, Williams B, Dana K (1984) Kidney length correlated with age: normal values in children. Radiology 150:703–704
Dinkel E, Ertel M, Dittrich M, Peters H, Berres M, Schulte-Wissermann H (1985) Kidney size in childhood. Sonographical growth charts for kidney length and volume. Pediatr Radiol 15:38–43
Rosenbaum DM, Korngold E, Teele RL (1984) Sonographic assessment of renal length in normal children. AJR Am J Roentgenol 142:467–469
Loftus WK, Gent RJ, LeQuesne GW, Metreweli C (1998) Renal length in Chinese children: sonographic measurement and comparison with western data. J Clin Ultrasound 26:349–352
Capaccioli L, Stecco A, Vanzi E, Brizzi E (2000) Ultrasonographic study on the growth and dimensions of healthy children and adults organs. Ital J Anat Embryol 105:1–50
Chen JJ, Zhi J, Mao W, Steinhardt GF (2006) MrNomogram: a web-based multivariable pediatric renal nomogram. J Pediatr Urol 2:436–438
Obrycki L, Sarnecki J, Lichosik M, Sopinska M, Placzynska M, Stanczyk M, Mirecka J, Wasilewska A, Michalski M, Lewandowska W, Derezinski T, Pac M, Szwarc N, Annusewicz K, Rekuta V, Azukaitis K, Cekuolis A, Wierzbicka-Rucinska A, Jankauskiene A, Kalicki B, Jobs K, Tkaczyk M, Feber J, Litwin M (2021) Kidney length normative values in children aged 0–19 years - a multicenter study. Pediatr Nephrol. https://doi.org/10.1007/s00467-021-05303-5
Morozov D, Parvin N, Conaway M, Oxley G, Baldelomar EJ, Cwiek A, deRonde K, Beeman SC, Charlton JR, Bennett KM (2022) Estimating nephron number from biopsies: impact on clinical studies. J Am Soc Nephrol 33:39–48. https://doi.org/10.1681/ASN.2021070998
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Matsell, D.G., Bao, C., White, T.P. et al. Kidney length standardized to body length predicts outcome in infants with a solitary functioning kidney. Pediatr Nephrol 38, 173–180 (2023). https://doi.org/10.1007/s00467-022-05544-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-022-05544-y