Abstract
Edema is the abnormal accumulation of fluid in the interstitial compartment of tissues within the body. In nephrotic syndrome, edema is often seen in dependent areas such as the legs, but it can progress to cause significant accumulation in other areas leading to pulmonary edema, ascites, and/or anasarca. In this review, we focus on mechanisms and management of edema in children with nephrotic syndrome. We review the common mechanisms of edema, its burden in pediatric patients, and then present our approach and algorithm for management of edema in pediatric patients. The extensive body of experience accumulated over the last 5 decades means that there are many options, and clinicians may choose among these options based on their experience and careful monitoring of responses in individual patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Edema is a major component of nephrotic syndrome (NS) (defined by Kidney Disease Improving Global Outcomes (KDIGO) guidelines as having edema, urine protein/creatinine ratio ≥ 2 mg/mg, and hypoalbuminemia ≤ 2.5 g/dl). Edema is a late Middle English (modern Latin) word that originated from the Greek word: oidēma, from oidein, which means “to swell.” Most of the early writings on this subject in the Sumerian, Babylonian, Egyptian and Greek cultures centered on dropsy, likely cardiac-induced edema. The concept of “dropsy of the chest” began to attract attention sometime by the end of the seventeenth century and it was well appreciated by the eighteenth century.
By the beginning of the nineteenth century, reports by John Blackall and Richard Bright provided new insights by differentiating dropsy into that of cardiac and renal origins [1, 2].
The role of salt, initially thought of in terms of its anion chloride, in the development of edema began to be appreciated by the middle to the late nineteenth century. Its mobilization or removal, however, remained problematic for physicians at that time.
The “cure de dechloruration,” which gained widespread acceptance by the end of the nineteenth century, was not always a successful undertaking. This treatment of dropsy, which centered on augmenting secretions (diaphoretics, purgatives) or mechanical removal of body fluids (bleeding, leeching, lancing), remained a frustrating and chancy undertaking while it was pursued for much of the eighteenth century and into the nineteenth century.
The discovery of sulfanilamide-induced sodium bicarbonate diuresis in the late 1940s provided the first step in the new age of clinically effective diuretics. This new age began in earnest in the 1950s with the introduction of chlorothiazide, the first orally effective agent to mobilize sodium chloride, followed by other effective diuretic agents. The subsequent introduction of the list of potent diuretics we have today was made possible by concurrent advances in defining normal kidney physiology and specific understanding of the mechanisms of sodium handling by the kidney.
Burden and complications of edema
Edema is often the presenting feature of this disorder. Children often present with facial and periorbital edema that is frequently attributed to allergy and treated with allergy medications or diagnosed as sinus infection. When NS is not recognized or not sensitive to treatment, edema can progress to cause leg edema, abdominal distention, and significant weight gain. Uncontrolled edema can cause serious complications such as pulmonary edema or pleural effusion. Significant edema can also result in recurrent and/or prolonged admissions to the hospital.
Edema can also affect the quality of life of pediatric patients with NS, even when it is only mild to moderate in severity, and this is especially seen in patients with steroid-resistant NS (SRNS). The recent work of Gipson et al. looking at quality of life in children with NS highlighted the burden of edema during active disease [3]. This study included 151 children and showed that edema was associated with worsening anxiety, pain, fatigue, and decreased mobility in affected children.
Selewski et al. subsequently studied quality of life measures in 127 children with NS between ages 8 and 17 years in a prospective cohort study [4]. Patients completed a baseline assessment while they were edematous with active NS, a follow-up assessment at the time of their first complete proteinuria remission or study month 3 if no remission occurred, and a final assessment at study month 12. This study showed that the major self-identified problem for children who continued to have NS disease activity was the pain and discomfort of their edema.
One of the main concerns in managing edema with diuretics is the possibility of inducing or worsening acute kidney injury (AKI). Rheault et al. (in a retrospective and multicenter study) studied the incidence of AKI in 336 children with NS and found that the highest risk factors for developing AKI were infection (odds ratio, 2.24; 95% confidence interval, 1.37 to 3.65; P = 0.001) and nephrotoxic medication exposure (odds ratio, 1.35; 95% confidence interval, 1.11 to 1.64; P = 0.002). Interestingly, using loop diuretics at home was not associated with increased risk of AKI on admission [5], suggesting that home use of diuretics for management of edema may not be that problematic, at least in terms of AKI. Other investigators however reported increased risk of AKI with using diuretics, so caution is still important with diuretic use in children with NS [6, 7].
Mechanisms of edema
There are multiple mechanisms that have been proposed to drive edema formation within various disorders, including:
-
Reduced oncotic pressure within vessels (hypoalbuminemia as in NS, other causes of low serum albumin and other proteins)
-
Increased hydrostatic pressure (kidney insufficiency, heart failure)
-
Increased blood vessel wall permeability with increased tissue oncotic pressure (capillary leak syndrome such as inflammation, anaphylaxis)
-
Obstruction of the lymphatic system (masses, lymphangiomatosis)
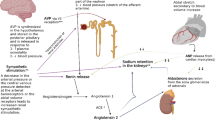
In NS, there have been two major hypotheses postulated to explain the pathophysiology of edema (see Fig. 1):
The “Underfill” hypothesis [8,9,10] suggests that an initial decrease in oncotic pressure (induced by significant proteinuria and resultant hypoalbuminemia) leads to excess loss of fluid from intravascular space to interstitial space, causing edema. This also results in intravascular hypovolemia and kidney hypoperfusion (which may present as hypotension, tachycardia, postural hypotension, and/or oliguria), and this ultimately activates the renin–angiotensin–aldosterone system and arginine vasopressin (AVP) causing secondary water and sodium retention. It is believed that most children with idiopathic NS fall into this category [8] .
The “Overfill” hypothesis, [8, 11] on the other hand, proposes that edema in NS is caused by primary renal sodium retention resulting in an “intravascular overfill” state and a rise in capillary hydrostatic pressure. As a result, fluid filters across vessels to accumulate in the interstitial compartment and cause edema. It is believed that this is the main mechanism of edema formation in most adults with active NS [8] and this may manifest clinically with hypertension and/or pulmonary edema.
It is not always straightforward to identify which pathway is the major process for edema formation in an individual patient, especially since accurately defining the subject’s volume status can be challenging. Clearly, some patients have significant elements of both processes, and the compensations in effect to maintain effective circulating blood volume and perfusion of vital organs tend to bring patients in from one extreme to a balance of both mechanisms. Edema mechanisms can vary between patients and also vary at different times in the same patient [12, 13]. Previous studies showed that adults with minimal change disease demonstrated one of three primary patterns: the majority had normal vascular volume, about 1/3 had low vascular volume and 1/6 had expanded vascular volume [14, 15]. Figure 1 summarizes the two major mechanisms for edema formation in NS.
Early studies showed that the collecting ducts are the main segment of the kidney tubules involved in primary Na retention in NS [16], which was initially thought to be mainly induced by increased activity of the basolateral Na+/K+-ATPase pump [17]. A major advance in our understanding of primary Na retention in NS was the discovery that proteinuria can activate the ENaC channels on the principal cells of the collecting ducts. This mechanism is likely present in all patients with active NS, to some degree, regardless of their intravascular volume status, since most studies show that the majority of patients with NS have urine FeNA < 1% [18,19,20]. ENaC activation occurs via the effects of plasminogen that is filtered by nephrotic glomeruli into the urinary filtrate. This plasminogen is eventually converted to plasmin by urokinase-type plasminogen activator in cortical collecting duct cells. Plasmin then proteolytically removes the C inhibitory domain of ENaC, resulting in its activation and subsequent Na retention through an aldosterone-independent process [21]. This mechanism suggests that ENaC suppression through amiloride can be an effective method to treat edema in NS and this was proven in a NS rat model when Na retention was prevented by amiloride [22]. Studies have also demonstrated an increased number and activity of the cortical collecting duct Na+/K+-ATPase pump in subjects with NS, which in turn can augment Na retention [23].
Further insights into the physiology of edema formation can be gained from briefly reviewing fluid movement in tissues based on the action of Starling Forces, first proposed by Ernest Starling in 1896 in the Journal of Physiology, and subsequently termed the Starling Hypothesis. As discussed later, this original equation has since been modified to more accurately describe our current understanding of the balance of forces that affect movement of fluid from tissues into the bloodstream. According to our current understanding, basic fluid movement is dictated by the difference in hydrostatic pressure (Pc–Pi) and oncotic pressure (πc–πi), the coefficient factor to protein (σ), and the overall filtration permeability constant to volume flow (Kf).
-
Jv net fluid movement between compartments
-
Kf overfall filtration permeability constant to volume flow
-
[Pc-Pi] capillary–interstitial hydrostatic pressure
-
𝜎 reflection coefficient to proteins (capillary permeability)
-
[𝜋c-𝜋i] oncotic pressure (c capillary, I interstitial)
However, looking at a micro or cellular level, oncotic forces around an individual cell are further defined by local differences in protein concentration across the glycocalyx and by small compartments beneath the glycocalyx, between cells, restricted by the basement membrane and pericytes [24]. This rich and complex system that we are just now beginning to appreciate can significantly alter fluid movement and may explain some of the variability of edema in patients with NS, similar to how this system can explain differences in response to fluid resuscitation in individuals with severe illness [25]. Furthermore, strategies that promote the health of the glycocalyx at the cellular level appear to minimize tissue edema formation in several animal models [26].
Other factors that may alter fluid movement on a cellular level include reduced interstitial oncotic pressure (that tends to parallel reduced plasma oncotic pressure) [27] and perturbations in hormonal systems that can ultimately result in salt retention (including renin, angiotensin II, aldosterone, sympathetic nervous system, vasopressin, and atrial natriuretic peptide ANP), as explored in a variety of different animal models and human studies [28,29,30]. For example. Angiotensin II is known to have a direct effect on sodium retention independent of glomerular filtration rate [31], while vasopressin results in insertion of ENaC into the apical membrane of the collecting duct cells, thus promoting Na retention. Both Angiotensin II and vasopressin are elevated in patients with active NS, especially when underfill pathophysiology dominates.
ANP on the other hand stimulates sodium and water excretion at the level of the collecting duct thus alleviating interstitial edema [32]; however, its natriuresis effect is blunted in NS [33]. This dampening could be caused by multiple mechanisms such as increased activity of the sympathetic system of kidneys [13] or enhanced cyclic GMP–phosphodiesterase [34].
In summary, at a macro level for all subjects with edema in NS, multiple pathophysiologic forces contribute to a variety of different phenotypes of edema through generation and maintenance of excess fluid in interstitial compartments, plasma volume alterations, and Na retention. At a micro level, the cellular factors that lead to localized edema and that modify these different phenotypes of edema are just now being explored. Future work will likely add further complexity to our understanding of edema formation and its evolution in subjects with NS.
General aspects of edema management
Overall, the most effective method of managing edema is the use of specific medical therapy directed towards inducing remission of NS. Until achieving remission or when managing children with SRNS, there are general measures to control edema that are appropriate for all children with NS:
-
1.
Reduce dietary salt intake.
-
2.
Fluid restriction is not usually recommended as it may worsen hypovolemia- underfill and cause hypotension and/or AKI. However, this can be used with caution in selected cases (such as massive anasarca or significant hyponatremia).
-
3.
Regular monitoring of the individual’s volume status (urine output, blood pressure, heart rate, and capillary refill), electrolytes, and kidney function, especially when children are sick and/or are in the hospital.
-
4.
Providing adequate nutrition with normal protein intake (RDI) for age, as lower protein intake is a risk for a negative nitrogen balance, malnutrition, and poor growth. High-protein diet should be avoided as this may cause progression of the kidney disease [35].
-
5.
Elevation of extremities and the use of compression socks can reduce discomfort and leg pain.
-
6.
Avoidance of nephrotoxic medications including NSAIDs.
-
7.
Avoidance of central catheter insertion (if possible) as this increases risk of thrombotic events.
-
8.
Use of angiotensinogen convertase enzyme inhibitors (ACEis) and/or angiotensin-receptor blockers (ARBs) can be pursued/considered in patients with NS and edema (these should not be viewed as contraindicated), but should be done with caution and regular safety monitoring.
-
9.
Active lifestyle as tolerated, as this can help edema mobilization and minimize risk of thrombosis.
Indications for adding specific medical interventions (diuretics, diuretic + albumin, other medical therapies) in management of edema in children with NS are (mostly opinion based, and influenced by center and case considerations):
-
1.
Pulmonary edema, pleural infusions, and/or hypoxia
-
2.
Congestive heart failure
-
3.
Volume-related hypertension
-
4.
Oliguria—concern for evolving AKI
-
5.
Skin infection/cellulitis
-
6.
Significant ascites with discomfort
-
7.
Severe scrotal/labial edema
-
8.
Sleep difficulties related to edema
-
9.
Generalized discomfort (body ache and /or feeling unwell because of significant body edema)
While there are limited well-defined studies on clinical and urine indices in directing treatment approaches to children with significant edema in NS, a reasonable approach in considering its treatment options is to assess for the likely major pathophysiologic mechanism (underfill vs. overfill) and to use this to guide an initial treatment for the individual patient. For patients with underfill as their main mechanism, they may respond better to intravenous albumin to restore the intravascular volume before using diuretics since using diuretics alone may worsen intravascular volume depletion and increase risk of AKI and/or thrombosis. Patients with overfill on the other hand may respond well to diuretics only, and using a large dose of intravenous albumin alone may increase risk of pulmonary edema and hypertension.
Presence of pulmonary edema, modest reduction in serum albumin (> 2 g/dl), and/or hypertension point towards having overfill physiology as the primary drive for edema. On the other hand, presence of reduced urine output, significant hypoalbuminemia (< 2 g/dl), higher hemoglobin/hematocrit from baseline, and/or tachycardia and postural hypotension all support underfill physiology as the main drive for edema in an individual patient. Other findings that can help differentiate between the two mechanisms are presented in Table 1 [8, 36].
Checking urine indices may be helpful to differentiate between the two major mechanisms. Most children with NS have low urinary sodium with low FeNa and there have been multiple studies that have attempted to assess the value of urine Na threshold values to direct therapy for significant edema in children with NS. In a study published by Kapur et al. in 2009, 20 pediatric patients with NS had their urine indices checked at time of admission [37]. Based on results of urine FeNa, patients with FeNa < 0.2% were treated with IV albumin and furosemide. Patients with FeNa ≥ 0.2% received IV furosemide and oral spironolactone. This study showed that the children with FeNa < 0.2% had, on average, higher serum BUN, BUN/creatinine ratio, serum renin, aldosterone, and AVP compared with patients with FeNa ≥ 0.2%. They also showed equally good clinical results between both treatment arms and concluded that diuretic therapy alone can be safe and effective in pediatric patients with NS presenting with more evidence of ‘overfill’ physiology and severe edema. It is important to recognize that this study did not prove that children with NS and edema who have urine FeNa < 0.2% must have underfill (in fact they selected this value based on previous experience and the value has never been independently determined), but that this value makes therapy directed towards volume expansion and diuretics a reasonable consideration, although one that must be qualified by other clinical and laboratory observations and one that ultimately requires close monitoring when any therapy is initiated.
Patients with underfill physiology are expected to have elevated renin–aldosterone levels and while they can help differentiate from patients with overfill, these levels are not usually readily available in most hospitals. Elevated urinary potassium excretion (U K/U Na + K) can point to increased aldosterone activity and thus underfill physiology [38, 39].
It is important in considering whether a child with significant edema and NS has primarily underfill or overfill physiology to not rely on a single or limited number of observations/lab findings. The pattern of findings is of greatest value; none of these findings are specific for either pathophysiologic mechanism. For example, a child with NS and some element of chronic kidney disease may have elevated urine FeNa even with dominant underfill, due to tubular Na wasting. Conversely, low FeNa may be seen in early acute glomerulonephritis with signs of NS as part of the forces that lead to overfill.
The dominant pathophysiology may change in an individual patient quickly as clinical issues (such as change in GFR) alter the driving forces. Moreover, in treating any child with NS and significant edema with fluids, albumin, and/or diuretics, it is most important to monitor clinical and laboratory status of the child as part of the response to therapy and to adjust treatment based on these serial observations. Further detailed clinical studies are needed to define better measures and more evidence-based guidelines for clinicians to be able to effectively and safely manage complicated children with NS and significant edema.
Diuretics
Diuretics are the main medical therapy used to treat edema in patients with NS. The most frequently used diuretics in NS are loop diuretics such as furosemide. This medication is highly protein-bound and it induces diuresis through vascular delivery to proximal tubular cells, where it exits the vascular capillary to enter the tubular epithelial cells, and then is secreted into the tubular lumen where it is able to block the Na+, K+, and Cl− Co-transporter (NKCC2) in the thick ascending loop of Henle (which is responsible for absorption of about 30% of Na and water) [40].
Furosemide is usually started at a dose of 1–2 mg/kg/dose in children up to 20 kg and at a 20–40-mg dose in older children, and can be given every 6–12 h orally. Intravenous infusion can increase loop diuretic efficacy [41] as furosemide has variable bioavailability, even in healthy individuals [42]. Although most patients display a good response to furosemide, up to 10–40% of patients may not respond well. The etiology of such poor response is not always well understood, and in individual subjects this may be related to high salt intake [43], decreased kidney function, severe abdominal distension and bowel edema (leading to decreased intestinal absorption) [44], and/or abnormal glycocalyx and other proteins at the micro or cellular level.
Another possible mechanism for resistance to furosemide in NS is that reduced binding of furosemide to serum albumin, secondary to hypoalbuminemia, can result in a larger extravascular volume of distribution and diminished rate of delivery to the kidney [45]. Moreover, the furosemide excreted into the urinary filtrate may bind to albumin within the tubular lumen in subjects with active NS and thus render it unavailable to interact with the NKCC2 transporter on the apical membrane of cells of the thick ascending limb of the loop of Henle [45]. It is uncertain if this mechanism is important in humans. In a study of seven patients with NS, attempts to block intratubular furosemide binding to albumin by administration of sulfisoxazole had no effect on the diuretic response [46].
When encountering poor response to furosemide, a higher dose and / or more frequent administration can be considered. The use of other loop diuretics can also be considered, such as bumetanide, which is more potent that furosemide and has greater bioavailability [47], making it more effective than furosemide for some subjects [48]. This drug is used less often in children due to difficulties in dose titration, particularly in young children. Ethacrynic acid, another loop diuretic, is a good alternative for children with sulfa allergy.
Thiazides are another diuretic class than can be used to treat edema. These agents inhibit the NaCl co-transporter in the distal convoluted tubule, which is responsible for absorption of 5–10% of Na and water [49]. In children with severe edema, thiazides can be used in addition to loop diuretics to thereby block sodium and water reabsorption at two different parts of the tubule. This can augment diuresis while using smaller doses of loop diuretics.
Another good option for diuresis (and sometimes added to furosemide) is metolazone, which has thiazide-like effects but also can inhibit proximal tubular Na reabsorption [50], making it more potent (up to 10 times) than hydrochlorthiazide. It also has a long half-life so it can be used once daily at dose 2.5–10 mg/day; it is limited to oral dosing only and can be difficult to titrate in younger children.
Acetazolamide is another diuretic that is occasionally used in NS patients. It works by inhibiting the carbonic anhydrase enzyme in proximal tubular cells to inhibit absorption of Na and bicarbonate, leading to increased urinary excretion of these ions and water [51]. It is not frequently used to manage edema in children with NS, however it can be effective when administered in small doses to patients with severe edema associated with metabolic alkalosis that results from use of potent loop and thiazide diuretics. Patients on acetazolamide require close monitoring to avoid severe metabolic acidosis.
Finally, amiloride is a diuretic that can provide some benefits in NS patients. It blocks ENaC channels on the principal cells of the collecting ducts to induce naturesis while preserving potassium [52]. In animal models of NS, amiloride successfully abolished the abnormally high sodium reabsorption from the cortical collecting duct and prevented Na retention [22]. A recent case report in an adult patient with heavy proteinuria and drug-resistant hypertension and edema, demonstrated significant improvement in the control of the edema once amiloride was added [53]. However, there are no strong controlled studies that support its role in NS, especially in children. Since ENaC channels are particularly activated during active NS, amiloride has a strong theoretical basis and this should be tested in a prospective way to confirm its expected efficacy. Since amiloride is a potassium sparing diuretic, it is often used in combination with loop diuretics to augment diuresis and decrease the risk of hypokalemia. Table 2 lists the clinically available diuretics used in children with NS with their typical doses and duration of action.
Albumin infusion
Albumin infusions can be a highly effective method to treat edema in patients with NS. Intravenous infusions of albumin increase oncotic pressure in the intravascular space to augment fluid mobilization from the interstitial space to the intravascular space and ultimately improve fluid delivery to the kidneys. By increasing the serum albumin, these infusions may also improve delivery of protein-bound diuretics (such as furosemide and metolazone) to tubular cells; however, the effect of these infusions is short-lived as most infused albumin will be lost quickly in the urine (“expensive urine”).
When the goal of the infusion is to replenish serum albumin, albumin 25% is usually recommended at 0.5–1 g/kg per dose. This can be considered in cases of significant body edema, severe hypoalbuminemia and / or significant underfill. On the other hand, albumin 5% is often used in settings when volume expansion is needed during active NS. Duffy et al. reviewed several well-defined studies and trials of the use of albumin/furosemide in NS. This included one randomized trial in children (16 patients) and five randomized trials in adults (total of 58 patients) [45] and found that giving albumin and furosemide together was more effective than using furosemide alone in terms of fluid excretion. However, there was significant variability in the doses employed and timing for each of the agents in these trials. Albumin infusion should also be conducted under close monitoring as it can cause significant complications, including pulmonary edema. Albumin infusion is obviously a temporary supportive therapy to help in management of edema, as most of the infused dose is lost in the urine in the next few hours during active NS phase. Table 3 summarizes the present consensus as to albumin and furosemide infusions and the most common complications in children with NS.
Angiotensin-converting enzyme inhibitors and angiotensin-receptor blockers
Angiotensin-converting enzyme inhibitors (ACEis) or Angiotensin-receptor blockers (ARBs) are often used, along with immunosuppressive agents, to control proteinuria and hypertension in patients with complicated forms of NS. By lowering proteinuria and boosting serum albumin in steroid-resistant and/or hypertensive NS patients, these agents can help minimize edema, but they are not used for acute mobilization and excretion of edema. The main mechanism for ACEis and ARBs to reduce proteinuria is their effect on relaxing the efferent arteriole of the glomerulus, thus reducing intra-glomerular pressure and ultimately reducing proteinuria by limiting movement of serum proteins from the glomerular capillary into the urinary space. ACEis and ARBs have also been demonstrated to impact fibrosis through inhibition of transforming growth factor-beta (TGF-beta 1) in the kidney [54, 55]. Thus, chronic use of ACEis or ARBs may decrease long-term glomerular and interstitial fibrosis in patients with SRNS and thereby reduce glomerular proteinuria and edema formation through that mechanism as well.
Water immersion
Water immersion is not a recent development in management of edema in NS. This intervention was first reported in 1981 when five adults suffering from NS were immersed up to the neck in 1.3-m water pressure of warm water for 4 h [56]. These subjects had, on average, a mean weight loss of 2 kg; 1 kg was lost through sweat and 1 kg was lost through urine. On average, 35 mmol of sodium was excreted in the urine in 4 h, 15 times more than what was documented on non-intervention control days. The mechanism of action is not entirely clear; in this study, aldosterone levels did not change on immersion in three patients but fell from elevated levels in two others.
This method is not widely used for edema management and it is not a current standard of treatment of edema in NS; however, it may be considered in children with less than desired response to standard diuretics.
Aquaretics (tolvaptan)
Tolvaptan is a selective competitive vasopressin receptor 2 (V2) antagonist that acts on the principal cells at the distal convoluted tubule and collecting duct to block the effects of vasopressin on water reabsorption. It is used to treat hyponatremia associated with congestive heart failure [57], cirrhosis [58], and the syndrome of inappropriate antidiuretic hormone (SIADH) [59, 60].
There is now limited evidence of benefits from aquaretics in adults with NS. Several case reports showed good results with the use of tolvaptan in subjects with NS whose edema was resistant to standard diuretics [61,62,63,64]. This approach deserves further investigation in children although these agents should be used carefully since they can cause significant hypernatremia, and may worsen intravascular underfill with subsequent risk of AKI and/or thrombosis.
Urea channel inhibitors
These agents are recently discovered molecules that block urea channels. The main urea transporter, urea transporter A1, is located on the apical surface of the inner medullary collecting duct cells. Activation of these channels through AVP leads to passive transport of urea through these cells to reabsorb up to 70% of the original filtered load of urea and maintain the urine countercurrent concentrating mechanism. In rats, these agents decrease water reabsorption and augment diuresis by weakening the countercurrent gradient [65]. There are limited reports of their use in NS patients to date [66], and these agents also deserve further exploration in children with edema in NS.
Novel agents
-
1.
Particulate-guanylyl-cyclase A receptor activator (trial name ZD100): this agent activates cGMP to promote natriuresis, inhibit aldosterone and reduce BP [67]. There are no reports on its use for management of edema in NS.
-
2.
Relaxin (an endogenous neurohormone): this agent increases expression of epithelial and endothelial endothelin B receptors (ETB) to indirectly stimulate ETB to inhibit Na+/K+-ATPase and vasopressin effects. This promotes natriuresis and diuresis without changes in levels of aldosterone [68]. There is some experience with this agent in adults with heart failure [69], but no reports in management of edema in NS.
-
3.
Synthetic human ANP (carperitide): atrial natriuretic peptide promotes natriuresis through increasing cardiac output but more directly through effects on medullary collecting duct cells, in particular sodium channels on both the apical (ENaC) and basolateral Na+/K+-ATPase sides [70]. ANP also increases glomerular filtration rate and glomerular permeability through direct dilation of the afferent arteriole and blocking norepinephrine-induced vasoconstriction of the afferent arteriole. Combined, these effects result in reducing edema in subjects with NS while preserving GFR. There are small series of their effectiveness in adult patients with NS reported by Ueda [71] (Japan) but no studies reported yet in children.
-
4.
Luteolin: this phenolic compound with anti-inflammatory and anti-allergic effects also stimulates muscarinic acetylcholine receptors to increase natriuresis and diuresis in rats [72]. There are no human studies reported to date.
-
5.
Epicatechin: these flavonoids in food and plant extracts induce diuresis and K, Na, and Cl excretion in rats [73]. Again, there are no human studies to date.
Key summary points
-
Edema can cause significant morbidity in children with active NS and decrease their quality of life.
-
The pathophysiology of edema in NS is complex and considering underfill vs. overfill mechanisms in affected children can help guide appropriate medical therapy.
-
Many diuretics are now available for management of edema in children with NS but few high-quality clinical trials and reports are available to help guide us. Even our present insights into the best methods to employ albumin infusions in management of NS in children are based on little carefully collected evidence.
-
Some exciting new agents to manage edema are now in development.
-
We propose in Fig. 2 a current opinion-based algorithm to guide medical decision making for management of edema in pediatric patients with NS.
References
Eknoyan G (1997) A history of edema and its management. Kidney Int Suppl 59:S118–S126
Pal A, Kaskel F (2016) History of nephrotic syndrome and evolution of its treatment. Front Pediatr 4:56. https://doi.org/10.3389/fped.2016.00056
Gipson DS, Selewski DT, Massengill SF, Wickman L, Messer KL, Herreshoff E, Bowers C, Ferris ME, Mahan JD, Greenbaum LA, MacHardy J, Kapur G, Chand DH, Goebel J, Barletta GM, Geary D, Kershaw DB, Pan CG, Gbadegesin R, Hidalgo G, Lane JC, Leiser JD, Plattner BW, Song PX, Thissen D, Liu Y, Gross HE, DeWalt DA (2013) Gaining the PROMIS perspective from children with nephrotic syndrome: a Midwest pediatric nephrology consortium study. Health Qual Life Outcomes 11:30. https://doi.org/10.1186/1477-7525-11-30
Selewski DT, Troost JP, Massengill SF, Gbadegesin RA, Greenbaum LA, Shatat IF, Cai Y, Kapur G, Hebert D, Somers MJ, Trachtman H, Pais P, Seifert ME, Goebel J, Sethna CB, Mahan JD, Gross HE, Herreshoff E, Liu Y, Song PX, Reeve BB, DeWalt DA, Gipson DS (2015) The impact of disease duration on quality of life in children with nephrotic syndrome: a Midwest Pediatric Nephrology Consortium study. Pediatr Nephrol 30:1467–1476. https://doi.org/10.1007/s00467-015-3074-x
Rheault MN, Zhang L, Selewski DT, Kallash M, Tran CL, Seamon M, Katsoufis C, Ashoor I, Hernandez J, Supe-Markovina K, D'Alessandri-Silva C, De Jesus-Gonzalez N, Vasylyeva TL, Formeck C, Woll C, Gbadegesin R, Geier P, Devarajan P, Carpenter SL, Kerlin BA, Smoyer WE, Midwest Pediatric Nephrology Consortium (2015) AKI in children hospitalized with nephrotic syndrome. Clin J Am Soc Nephrol 10:2110–2118. https://doi.org/10.2215/CJN.06620615
Meyrier A, Niaudet P (2018) Acute kidney injury complicating Nephrotic syndrome of minimal change disease. Kidney Int 94:861–869
Prasad BS, Kumar M, Dabas A, Mishra K (2019) Profile of acute kidney injury in hospitalized children with idiopathic nephrotic syndrome. Indian Pediatr 56:119–122
Ellis D (2015) Pathophysiology, evaluation, and management of edema in childhood nephrotic syndrome. Front Pediatr 3:111. https://doi.org/10.3389/fped.2015.00111
Humphreys MH (1994) Mechanisms and management of nephrotic edema. Kidney Int 45:266–281. https://doi.org/10.1038/ki.1994.33
Noddeland H, Riisnes SM, Fadnes HO (1982) Interstitial fluid colloid osmotic and hydrostatic pressures in subcutaneous tissue of patients with nephrotic syndrome. Scand J Clin Lab Invest 42:139–146
Bockenhauer D (2013) Over- or underfill: not all nephrotic states are created equal. Pediatr Nephrol 28:1153–1156. https://doi.org/10.1007/s00467-013-2435-6
Siddall EC, Radhakrishnan J (2012) The pathophysiology of edema formation in the nephrotic syndrome. Kidney Int 82:635–642. https://doi.org/10.1038/ki.2012.180
Gupta S, Pepper RJ, Ashman N, Walsh SB (2019) Nephrotic syndrome: oedema formation and its treatment with diuretics. Front Physiol 9:1868
Dorhout EJ, Roos JC, Boer P, Yoe OH, Simatupang TA (1979) Observations on edema formation in the nephrotic syndrome in adults with minimal lesions. Am J Med 67:378–384. https://doi.org/10.1016/0002-9343(79)90782-4
Meltzer JI, Keim HJ, Laragh JH, Sealey JE, Jan KM, Chien S (1979) Nephrotic syndrome: vasoconstriction and hypervolemic types indicated by renin-sodium profiling. Ann Intern Med 91:688–696. https://doi.org/10.7326/0003-4819-91-5-688
Ichikawa I, Rennke HG, Hoyer JR, Badr KF, Schor N, Troy JL, Lechene CP, Brenner BM (1983) Role for intrarenal mechanisms in the impaired salt excretion of experimental nephrotic syndrome. J Clin Invest 71:91–103
Vogt B, Favre H (1991) Na+,K(+)-ATPase activity and hormones in single nephron segments from nephrotic rats. Clin Sci (Lond) 80:599–604
Buyukavci MA, Civilibal M, Elevli M, Selcuk Duru HN (2015) Hypo- and hypervolemic edema in children with steroid sensitive nephrotic syndrome. Turk J Med Sci 45:178–183
Iyengar AA, Kamath N, Vasudevan A, Phadke KD (2011) Urinary indices during relapse of childhood nephrotic syndrome. Indian J Nephrol 21:172–176. https://doi.org/10.4103/0971-4065.83030
Lu H, Kapur G, Mattoo TK, Lyman WD (2012) Hypoxia decreases podocyte expression of slit diaphragm proteins. Int J Nephrol Renov Dis 5:101–107. https://doi.org/10.2147/IJNRD.S27332
Svenningsen P, Bistrup C, Friis UG, Bertog M, Haerteis S, Krueger B, Stubbe J, Jensen ON, Thiesson HC, Uhrenholt TR, Jespersen B, Jensen BL, Korbmacher C, Skott O (2009) Plasmin in nephrotic urine activates the epithelial sodium channel. J Am Soc Nephrol 20:299–310. https://doi.org/10.1681/ASN.2008040364
Deschênes G, Wittner M, Stefano A, Jounier S, Doucet A (2001) Collecting duct is a site of sodium retention in PAN nephrosis: a rationale for amiloride therapy. J Am Soc Nephrol 12:598–601
Deschenes G, Doucet A (2000) Collecting duct (Na+/K+)-ATPase activity is correlated with urinary sodium excretion in rat nephrotic syndromes. J Am Soc Nephrol 11:604–615
Kottke MA, Walters TJ (2016) Where's the leak in vascular barriers? A review. Shock 46(3 Suppl 1):20–36. https://doi.org/10.1097/SHK.0000000000000666
Myburgh JA, Mythen MG (2013) Resuscitation fluids. N Engl J Med 369:1243–1251
Chignalia AZ, Yetimakman F, Christiaans SC, Unal S, Bayrakci B, Wagener BM, Russell RT, Kerby JD, Pittet JF, Dull RO (2016) The glycocalyx and trauma: a review. Shock 45:338–348
Fiorotto M, Coward WA (1979) Pathogenesis of oedema in protein-energy malnutrition: the significance of plasma colloid osmotic pressure. Br J Nutr 42:21–31
Geers AB, Koomans HA, Roos JC, Boer P, Dorhout Mees EJ (1984) Functional relationships in the nephrotic syndrome. Kidney Int 26:324–330. https://doi.org/10.1038/ki.1984.176
Siddall EC, Radhakrishnan J (2012) The pathophysiology of edema formation in the nephrotic syndrome. Kidney Int 82:635–642. https://doi.org/10.1038/ki.2012.180
Vande Walle JG, Donckerwolcke RA, Koomans HA (1999) Pathophysiology of edema formation in children with nephrotic syndrome not due to minimal change disease. J Am Soc Nephrol 10:323–331
Johnson MD, Malvin RL (1977) Stimulation of renal sodium reabsorption by angiotensin II. Am J Phys 232:F298–F306
Light DB, Schwiebert EM, Karlson KH, Stanton BA (1989) Atrial natriuretic peptide inhibits a cation channel in renal inner medullary collecting duct cells. Science 243:383–385
Perico N, Delaini F, Lupini C, Benigni A, Galbusera M, Boccardo P, Remuzzi G (1989) Blunted excretory response to atrial natriuretic peptide in experimental nephrosis. Kidney Int 36:57–64. https://doi.org/10.1038/ki.1989.161
Valentin JP, Qiu C, Muldowney WP, Ying WZ, Gardner DG, Humphreys MH (1992) Cellular basis for blunted volume expansion natriuresis in experimental nephrotic syndrome. J Clin Invest 90:1302–1312. https://doi.org/10.1172/JCI115995
Mansy H, Goodship TH, Tapson JS, Hartley GH, Keavey P, Wilkinson R (1989) Effect of a high protein diet in patients with the nephrotic syndrome. Clin Sci (Lond) 77:445–451
Cadnapaphornchai MA, Tkachenko O, Shchekochikhin D, Schrier RW (2014) The nephrotic syndrome: pathogenesis and treatment of edema formation and secondary complications. Pediatr Nephrol 29:1159–1167
Kapur G, Valentini RP, Imam AA, Mattoo TK (2009) Treatment of severe edema in children with nephrotic syndrome with diuretics alone--a prospective study. Clin J Am Soc Nephrol 4:907–913
Matsumoto H, Miyaoka Y, Okada T, Nagaoka Y, Wada T, Gondo A, Esaki S, Hayashi A, Nakao T (2011) Ratio of urinary potassium to urinary sodium and the potassium and edema status in nephrotic syndrome. Intern Med 50:551–555
Donckerwolcke RAMG, France A, Raes A, Vande Walle J (2003) Distal nephron sodium-potassium exchange in children with nephrotic syndrome. Clin Nephrol 59:259–266
Brater DC (1991) Clinical pharmacology of loop diuretics. Drugs 41:14–22. https://doi.org/10.2165/00003495-199100413-00004
Hammarlund MM, Paalzow LK, Odlind B (1984) Pharmacokinetics of furosemide in man after intravenous and oral administration. Application of moment analysis. Eur J Clin Pharmacol 26:197–207
Waller ES, Hamilton SF, Massarella JW, Sharanevych MA, Smith RV, Yakatan GJ, Doluisio JT (1981) Disposition and absolute bioavailability of furosemide in healthy males. J Pharm Sci 71:1105–1108
Hoorn EJ, Ellison DH (2017) Diuretic resistance. Am J Kidney Dis 69:136–142
Sica DA (2003) Drug absorption in the management of congestive heart failure: loop diuretics. Congest Heart Fail 9:287–292
Duffy M, Jain S, Harrell N, Kothari N, Reddi AS (2015) Albumin and furosemide combination for management of edema in nephrotic syndrome: a review of clinical studies. Cells 4:622–630
Agarwal R, Gorski JC, Sundblad K, Brater DC (2000) Urinary protein binding does not affect response to furosemide in patients with nephrotic syndrome. J Am Soc Nephrol 11:1100–1105
Brater DC, Day B, Burdette A, Anderson S (1984) Bumetanide and furosemide in heart failure. Kidney Int 26:183–189
Lau K, DeFronzo R, Morrison G, Rascoff J, Goldberg M, Agus ZS (1976) Effectiveness of bumetanide in nephrotic syndrome: a double-blind crossover study with furosemide. J Clin Pharmacol 16:489–497
Hropot M, Fowler N, Karlmark B, Giebisch G (1985) Tubular action of diuretics: distal effects on electrolyte transport and acidification. Kidney Int 28:477–489
Suki WN, Dawoud F, Eknoyan G, Martinez-Maldonado M (1972) Effects of metolazone on renal function in normal man. J Pharmacol Exp Ther 180:6–12
Preisig PA, Toto RD, Alpern RJ (1987) Carbonic anhydrase inhibitors. Renal Physiol 10:136–159
Vidt DG (1981) Mechanism of action, pharmacokinetics, adverse effects, and therapeutic uses of amiloride hydrochloride, a new potassium-sparing diuretic. Pharmacotherapy 1:179–187
Hinrichs GR, Mortensen LA, Jensen BL, Bistrup C (2018) Amiloride resolves resistant edema and hypertension in a patient with nephrotic syndrome; a case report. Phys Rep 6:e13743
Peters H, Noble NA (1997) Angiotensin II and L-arginine in tissue fibrosis: more than blood pressure. Kidney Int 51:1481–1486. https://doi.org/10.1038/ki.1997.203
Shin GT, Kim SJ, Ma KA, Kim HS, Kim D (2000) ACE inhibitors attenuate expression of renal transforming growth factor-beta1 in humans. Am J Kidney Dis 36:894–902
Berlyne GM, Brown C, Adler A, Feinroth MV, Feinroth M, Hirsch S, Friedman EA (1981) Water immersion in nephrotic syndrome. Arch Intern Med 141:1275–1278
Gheorghiade M, Orlandi C, Burnett JC, Demets D, Grinfeld L, Maggioni A, Swedberg K, Udelson JE, Zannad F, Zimmer C, Konstam MA (2005) Rationale and design of the multicenter, randomized, double-blind, placebo-controlled study to evaluate the efficacy of vasopressin antagonism in heart failure: outcome study with tolvaptan (EVEREST). J Card Fail 11:260–269
Okita K, Sakaida I, Okada M, Kaneko A, Chayama K, Kato M, Sata M, Yoshihara H, Ono N, Murawaki Y (2010) A multicenter, open-label, dose-ranging study to exploratively evaluate the efficacy, safety, and dose-response of tolvaptan in patients with decompensated liver cirrhosis. J Gastroenterol 45:979–987
Chen S, Zhao JJ, Tong NW, Guo XH, Qiu MC, Yang GY, Liu ZM, Ma JH, Zhang ZW, Gu F (2014) Randomized, double blinded, placebo-controlled trial to evaluate the efficacy and safety of tolvaptan in Chinese patients with hyponatremia caused by SIADH. J Clin Pharmacol 54:1362–1367
Petereit C, Zaba O, Teber I, Luders H, Grohe C (2013) A rapid and efficient way to manage hyponatremia in patients with SIADH and small cell lung cancer: treatment with tolvaptan. BMC Pulm Med 13:55
Park ES, Huh YS, Kim GH (2015) Is tolvaptan indicated for refractory oedema in nephrotic syndrome? Nephrology (Carlton) 20:103–106. https://doi.org/10.1111/nep.12348
Shimizu M, Ishikawa S, Yachi Y, Muraoka M, Tasaki Y, Iwasaki H, Kuroda M, Ohta K, Yachie A (2014) Tolvaptan therapy for massive edema in a patient with nephrotic syndrome. Pediatr Nephrol 29:915–917
Takada T, Masaki T, Hoshiyama A, Toki T, Kamata Y, Shichiri M (2018) Tolvaptan alleviates excessive fluid retention of nephrotic diabetic renal failure unresponsive to furosemide. Nephrology (Carlton) 23:883–886
Tanaka A, Nakamura T, Sato E, Ueda Y, Node K (2017) Different effects of tolvaptan in patients with idiopathic membranous nephropathy with nephrotic syndrome. Intern Med 56:191–196
Cil O, Esteva-Font C, Tas ST, Su T, Lee S, Anderson MO, Ertunc M, Verkman AS (2015) Salt-sparing diuretic action of a water-soluble urea analog inhibitor of urea transporters UT-A and UT-B in rats. Kidney Int 88:311–320
Knepper MA, Miranda CA (2013) Urea channel inhibitors: a new functional class of aquaretics. Kidney Int 83:991–993
Chen Y, Burnett JC (2018) Particulate guanylyl cyclase a/cGMP signaling pathway in the kidney: physiologic and therapeutic indications. Int J Mol Sci 19:1006. https://doi.org/10.3390/ijms19041006
Wilson SS, Ayaz SI, Levy PD (2015) Relaxin: a novel agent for the treatment of acute heart failure. Pharmacotherapy 35:315–327. https://doi.org/10.1002/phar.1548
Gimpelewicz C, Metra M, Cleland JGF, Szecsody P, Chang Wun CC, Boer-Martins L, Cotter G, Davison BA, Felker GM, Filippatos G, Greenberg BH, Pang P, Ponikowski P, Severin T, Voors AA, Teerlink JR (2017) Effects of serelaxin on the outcome of patients with or without substantial peripheral edema: a subgroup analysis from the RELAX-AHF trial. Am Heart J 190:113–122
Theilig F, Wu Q (2015) ANP-induced signaling cascade and its implications in renal pathophysiology. Am J Physiol Ren Physiol 308:F1047–F1055
Ueda K, Hirahashi J, Seki G, Tanaka M, Kushida N, Takeshima Y, Nishikawa Y, Fujita T, Nangaku M (2014) Successful treatment of acute kidney injury in patients with idiopathic nephrotic syndrome using human atrial natriuretic peptide. Intern Med 53:865–869
Boeing T, da Silva LM, Mariott M, Andrade SF, de Souza P (2017) Diuretic and natriuretic effect of luteolin in normotensive and hypertensive rats: role of muscarinic acetylcholine receptors. Pharmacol Rep 69:1121–1124. https://doi.org/10.1016/j.pharep.2017.05.010
Mariano LNB, Boeing T, da Silva R, Cechinel-Filho V, Niero R, da Silva LM, de Souza P, Andrade SF (2018) Preclinical evaluation of the diuretic and saluretic effects of (-)-epicatechin and the result of its combination with standard diuretics. Biomed Pharmacother 107:520–525
Availability of data and material
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Additional information
Answers:
1. c; 2. a; 3. b; 4. a
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Multiple-choice questions (answers follow reference list)
Multiple-choice questions (answers follow reference list)
-
1-
A 12-year-old male with SRNS presented to the ER with worsening edema. Exam showed facial and leg edema with ascites and he looked uncomfortable. Vital signs: heart rate 75/min, respiratory rate 24/min, BP 138/76 with no orthostatic hypotension. Work up showed: serum albumin 2.2 g/dl, serum Cr 0.5 mg/dl, urine protein > 300 mg/dl and negative for blood.
Of the following, the most likely pathophysiologic mechanism of edema in this child:
-
a)
Lack of hematuria makes overfill more likely than underfill as the dominant mechanism
-
b)
Underfill is the most likely mechanism with his serum albumin > 2 g/dl
-
c)
The absence of hypotension makes overfill the more likely dominant mechanism
-
d)
We cannot determine his most likely mechanism since we do not have the urine Na measured.
-
a)
-
2-
In the previous case, the best initial plan to treat his edema is:
-
a)
Furosemide
-
b)
Albumin 25% followed by Furosemide
-
c)
Amiloride
-
d)
Hydrochlorothiazide.
-
a)
-
3-
A 9-year-old male with SRNS presented for a routine clinic visit. You noticed that he gained 12 lbs. since his last clinic visit six weeks ago and he complains of discomfort with his feet. He appeared comfortable otherwise and denied any pain. His physical examination showed pitting edema in his feet and legs and mild abdominal distention. His BP was 90/55, heart rate 90 /min. When approaching edema management in this child, of the following options, the best one to pursue is to:
-
a)
Avoid the outpatient use of loop diuretics as these agents are associated with increased risk of acute kidney injury
-
b)
Initiate diuretics since reducing edema has been shown to improve quality of life in subjects with NS
-
c)
Initiate a small dose of Lisinopril and follow up his weight in 1 week
-
d)
There is no indication to initiate diuretics since he has minimal symptoms.
-
a)
-
4-
A 7-year-old female with NS was admitted to the hospital for management of severe edema. Physical examination showed significant edema overall, abdominal distention and mild abdominal pain with palpation. Her BP was 132/85 and heart rate 90/min. Serum albumin was 2.3 g/dl. After 36 h of diuresis with furosemide, her fluid balance was negative for 560 ml and she continued to complain of abdominal discomfort. Of the following factors, this poor response to diuretics may best be explained by:
-
a)
Decreased kidney function
-
b)
Decreased distribution of furosemide in extracellular space with edema
-
c)
Increased excretion of furosemide into the urinary filtrate in NS
-
d)
Lack of binding of furosemide to albumin in the intratubular space
-
a)
Rights and permissions
About this article
Cite this article
Kallash, M., Mahan, J.D. Mechanisms and management of edema in pediatric nephrotic syndrome. Pediatr Nephrol 36, 1719–1730 (2021). https://doi.org/10.1007/s00467-020-04779-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-020-04779-x