Abstract
Purpose of review
To explore the mechanisms underlying edema formation in nephrotic syndrome. We highlight new methods of volume assessment and a stepwise approach to the management of edema.
Recent findings
New tools are available for intravascular volume assessment. New medications and modalities have been developed for the management of edema.
Summary
Edema is a major feature of nephrotic syndrome in children. It can be secondary to multiple mechanisms. Regardless of the causing mechanism, targeted management of edema should be guided by an adequate assessment of the intravascular volume. Urine studies e.g. urine sodium and potassium, as well as echocardiogram, can prove to be valuable tools. Besides diuretics, new agents and modalities are being developed for edema management including Aquaretics and isolated ultrafiltration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Edema is a presenting feature of nephrotic syndrome (NS) in children and is first noticed in the periorbital area. Kidney Disease Improving Global Outcomes (KDIGO) guidelines define NS as having edema, urine protein/creatinine ratio ≥2 mg/mg, and hypoalbuminemia ≤ 2.5 g/dl. Edema manifests in the form of weight gain and is worse in the lower limbs. It can be local or generalized and may progress to pleural and pericardial effusions, ascites, scrotal or labial edema, anasarca, and bowel wall edema. Edema can significantly affect the quality of life of children by causing pain, fatigue, anxiety, and discomfort which adds a psychological burden [1, 2].
The main treatment for NS is steroids. Known complications of NS include increased risk of thrombosis, infections, and possibly acute kidney injury in case of severe volume depletion. However, addressing edema and the complications associated with it is paramount especially when severe and causing significant discomfort. Severe persistent edema is accentuated in children who fail to respond to steroids.
Understanding the mechanisms involved in the development and worsening of edema in children with NS is essential in making management decisions. Therefore, we designed this review to: (I) describe the currently known mechanisms of edema formation in NS, (II) define the factors involved in sodium retention in NS, and (III) identify the management options for children with edema and NS
Mechanisms of edema formation in nephrotic syndrome
Proposed mechanisms
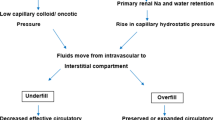
There are two proposed hypotheses to explain edema formation in patients with NS. The “underfill hypothesis” has been the prevailing one for decades. It suggests that a decrease in capillary oncotic pressure (secondary to hypoalbuminemia due to albuminuria) leads to leakage of fluids to the interstitium. The resultant hypovolemia reduces the effective renal blood flow and, therefore, activates the renin-angiotensin-aldosterone system and arginine vasopressin. The result is salt and water retention. It is believed that this is the main mechanism in patients who have massive proteinuria, marked hypoalbuminemia, minimal or no renal inflammation, and normal kidney function as is the case in children with minimal change NS [3].
On the other hand, the “overfill hypothesis” proposes that the main drive for edema formation is salt and water retention occurring secondary to a reduced filtered load of sodium. Though this is understandable in the setting of reduced GFR or renal inflammation, it has also been demonstrated to occur as a primary mechanism in patients with NS due to increased activation of epithelial sodium channels (ENAC) in the collecting ducts, which results in sodium retention. This occurs despite initial hypoalbuminemia. The intravascular volume is preserved or expanded in this hypothesis [4].
Body fluid compartments and fluid transport
Total body water (TBW) is distributed into intracellular and extracellular compartments. The latter forms around one-third of TBW and is divided into interstitial fluid and intravascular plasma. The role of the interplay between hydrostatic and oncotic pressures of the capillary and interstitium in determining the movement of fluids between the different compartments in the body was described by Starling in 1896 after an experiment that he performed on dogs [5].
The following equation has served as the basis of our understanding of fluid transport for several decades:
Where Jν is the net fluid transport, Kf is the hydraulic permeability coefficient, Pc the capillary hydrostatic pressure, Pi the interstitial hydrostatic pressure, σ the reflection coefficient to proteins, πc the capillary oncotic pressure, and πi the interstitial oncotic pressure.
For decades, the prevailing theory to explain molecular transport exchange across the capillary wall was the “pore theory” [6]. In 1980, Curry and Michel proposed the “fiber matrix theory” as an alternative to the “pore theory” as they highlighted the role that the matrix of glycoprotein chains has in determining capillary permeability [7, 8]. Further studies laid the basis for the revised Starling equation which differs from the original one in that the oncotic pressures involved are those of the endothelial surface layer (πesl) and the area beneath it which is the sub-glycocalyx (πb) rather than those of the capillary and the interstitium [9,10,11].
The reflection coefficient (σ) of a substance reflects its permeability through the capillary membrane. A σ of 1 means that the substance would not pass, while a σ of 0 means that it is completely permeable [13]. Studies have shown that the σ of albumin decreases in response to inflammation or injury [14, 15] and is mediated by inflammatory mediators e.g. histamine and bradykinin [16]. Kf is the product of the capillary surface area and the hydraulic conductance. Kf has been shown to increase in cases of hyperglycemia or high intravascular pressure [17, 18]. Both Kf and σ reflect vascular permeability. The current understanding of the role of the glycocalyx in fluid transport explains the changes in vascular permeability occurring as a result of glycocalyx injury after ischemia-reperfusion [19], TNF-alpha release as is the case in sepsis [20], hypervolemia secondary to ANP release [21] and hyperglycemia [22]. More importantly, is the impact that this has on our understanding of the fluid distribution in different tissues in different disease states and on the varied response to fluid therapy. Fluid distribution depends on the type of capillaries present in a tissue (continuous, fenestrated, or sinusoidal) [12].
Applications of these principles in nephrotic syndrome
Changes in the aforementioned factors that comprise the Starling equation explain the edema state occurring in patients with NS.
Changes in the capillary oncotic pressure (πc)
The basis of the “underfill hypothesis” is that a decrement in capillary oncotic pressure (πc) relative to the interstitial oncotic pressure (πi) results in an exodus of fluids from the intravascular compartment to the interstitium. However, studies performed in the 1980s using the wick technique to measure the interstitial fluid oncotic pressure have shown a parallel decrease in this pressure compared to the capillary pressure in NS patients [23, 24]. It is possible that fluid released in the interstitial space reduces the concentration of the proteins and then increases their clearance via lymphatics [25,26,27]. This is a protective mechanism occurring in slowly developing hypoalbuminemia and serves to preserve the intravascular compartment and reduce edema formation. No studies were done after the release of the revised Starling equation.
Changes in hydrostatic pressure gradient between the capillary and interstitium
Researchers have tried to estimate the blood volume in NS patients as this may help support one of the two hypotheses for edema formation. Geers et al. attempted to measure blood volume (BV) and extracellular fluid volume (ECFV) using 131I-albumin and 82Br, respectively, in 20 adult NS patients before, during, and after treatment of edema with diuretics. Blood volume remained intact despite the decrease in ECFV as the edema resolved. Plasma renin activity (PRA) increased as ECVF decreased, however, PRA at baseline was higher in NS patients compared to controls [28]. A similar study in adults showed no difference in BV in NS patients compared to controls [29]. These studies argue against the underfill hypothesis as the BV was maintained though the patients had hypoalbuminemia. Using a similar technique, a third study in 43 children with NS who were divided into 4 groups: remission, incipient nephrotic (normal serum albumin), symptomatic active nephrotic, and active nephrotic without hypovolemic symptoms. Patients with active NS had only slightly lower BV compared to patients in remission. Blood volume was similar in both groups and the study concluded that the development of these symptoms reflects the rate of edema formation rather than the actual BV. The mean levels of plasma norepinephrine, renin, and aldosterone were higher in the symptomatic nephrotic group compared to the asymptomatic one [30, 31]. Similarly, at an experimental level, rapid severe hypoproteinemia -induced by plasmapheresis with isotonic fluids in dogs- resulted in a decrease in BV and a rise in plasma renin and aldosterone [32]. The blood volume is decreased either in case of rapidly developing edema or possibly if there are acute fluid losses e.g. aggressive diuretic use, diarrhea, or vomiting.
Mechanisms for sodium retention in NS (Fig. 1)
Renin-angiotensin-aldosterone system (RAAS) activation
The RAAS activation regulates blood pressure and ECFV. The Underfill hypothesis suggests that the activation of the renin-angiotensin-aldosterone system is secondary to hypovolemia. The previously described study done by Vande Walle et al showed elevated levels of renin-aldosterone and norepinephrine in patients with active nephrotic syndrome with hypoalbuminemia compared to patients with incipient NS or in remission. However, the levels were only significantly higher in those with symptoms of hypovolemia [30]. An early study done in 12 NS patients of ages ranging between 12-60 years with idiopathic NS showed that the PRA and aldosterone were elevated in the NS patients, while BV -as measured by chromium tagged RBCs- was only mildly elevated compared to controls [33]. A more recent study compared the results of aldosterone levels at debut and remission in 20 patients with childhood nephrotic syndrome. The group that had significantly higher aldosterone levels at debut did not show symptoms of hypovolemia, but the estimated GFR was significantly lower possibly due to hypovolemia [34]. Another study confirmed the finding of elevated aldosterone, renin, and norepinephrine in children with non-MCD but only those presenting with symptoms of hypovolemia [35]. It can be concluded that the levels of these hormones -PRA and aldosterone- are elevated in cases of “symptomatic’ hypovolemia but not in stable patients with hypoalbuminemia.
Mechanisms of sodium retention and natriuresis. ACE Angiotensin converting enzyme. ** Sodium retention mechanism: Angiotensin 2 acts on the proximal tubules and the thiazide-sensitive NaCl channels in the distal tubules [92]. Aldosterone acts on the mineralocorticoid receptors within the principal cells of the distal tubules and collecting ducts. Sodium reabsorption is then stimulated secondary to upregulation of the basolateral Na/K pump as well as the ENaC channels in the collecting ducts. Aldosterone also increases sodium absorption from the gut. Sympathetic stimulation acts on the proximal tubules and thick ascending limb of Henle [36]. *** AVP action on the kidney is mediated by the V2 receptors located in the basolateral membrane of the principal cells in the late distal tubule and collecting duct [36]. Activation of V2 receptors leads to insertion of aquaporin2 (AQP2) channel into the apical membrane and consequently water reabsorption. ^^ Sympathetic stimulation causes vasoconstriction of afferent and to a lesser extent efferent arteriole. @ ANP suppresses Aldosterone release, sodium retention in the kidneys and renin release
Renal sympathetic stimulation
Sympathetic stimulation causes salt retention both directly and through stimulation of renin release (See Fig.1). The above-mentioned studies demonstrate evidence of sympathetic stimulation in some patients with active NS especially when symptoms of hypovolemia are present [30, 35].
Atrial natriuretic peptide (ANP)
ANP is released in response to volume overload manifested in the form of atrial stretch. It then acts as a counter-regulatory hormone that promotes natriuresis. The prevailing understanding is that in NS there is a blunted response to the effect of ANP even when it is elevated. In a study that was done on 31 adult patients with NS and primary GN, the ANP levels were elevated at baseline compared to controls [36]. It has been suggested that the blunted response is due to enhanced cGMP-phosho-di-esterase activity [37]. In a study of childhood NS, ANP levels were the highest in the active NS patients followed by those in remission and then controls. Urinary sodium levels were significantly lower in those with active NS. Certain polymorphisms for the genes controlling ANP: ANP gene (A2843G), the natriuretic peptide clearance receptor (NPRC) gene polymorphism C (-55) A, and ScaI polymorphism of ANP gene were studied in both active NS patients and those in remission but there were no significant differences in their frequencies [38].
The role of Corin
Corin is a transmembrane protease that cleaves pro ANP and pro Brain natriuretic peptide (pro-BNP) to the active forms. A decrease in corin expression has been shown in puromycin aminonucleoside (PAN)-induced nephrosis rat models [39]. A reduction in corin would lead to salt and water retention. Data in humans suggest that corin defects may be linked to hypertension and heart failure [90, 93].
Arginine vasopressin (AVP)
Earlier studies have shown that AVP levels were higher in NS patients compared to controls both at baseline state [40] and after water loading [41]. AVP levels in NS patients remained high after water loading but decreased after albumin 20% infusion [42]. A more recent study confirmed the AVP activation in adults with NS as the levels of copeptin, which is the glycosylated peptide of the C-terminal area of the AVP precursor, were found to be elevated. Copeptin is more stable than AVP and is considered a marker of AVP release [43].
Aquaporin
The action of AVP is mediated by the V2 receptors whose activation leads to the insertion of Aquaporin 2 (AQP2) into the apical membrane of principal cells resulting in increased water reabsorption. In addition to AQP2, other aquaporins are found in the kidney (AQP1, AQP3, AQP4). AQP1 is responsible for permanent high water permeability and is present in apical and basolateral membranes of proximal tubules and the descending limb of the loop of Henle [33]. Studies done in rats have shown decreased expression of AQP2, 3, and 4 in the collecting ducts in response to Adriamycin injections [44]. Urinary AQP2 was found to be elevated along with copeptin in adults with NS [43]. However, further studies are needed to ascertain whether the increase in AQP2 is a direct result of high AVP or “an escape mechanism” to counteract the elevated levels and avoid excessive water reabsorption.
Primary sodium retention
Ichikawa et al demonstrated in PAN-induced NS rats persistent sodium retention despite normal GFR suggesting that the mechanism for sodium retention was related to renal handling of sodium rather than to reduced GFR [45]. Kim et al showed increased abundance and apical targeting of the γ-subunit of ENaC in the same rat model, however, the aldosterone level was elevated in this experiment [46]. Other studies in the same rat model showed increased activity and expression of Na/K ATPase in the cortical collecting ducts [47] and that it is independent of aldosterone [48]. ENaC channels undergo activation by serine proteases mainly plasmin [49]. In rats, cleavage of the γ-subunit of ENaC is increased in pathological states with proteinuria. Plasminogen filtered due to proteinuria is activated by urokinase released from the tubular epithelium [50, 51]. In patients with type 1 diabetes, a high level of urinary plasminogen was found to be a risk factor for hypertension supposedly because of salt retention and volume expansion [52]. This suggests that sodium retention may be linked to albuminuria itself rather than hypovolemia. Increased urinary plasminogen-plasmin was found to be a risk factor for edema in adult patients with NS [53]. In another study, ACEi did not promote natriuresis despite the reduced level of aldosterone [54].
Approach to a child with edema and nephrotic syndrome (Table 1)
The possible mechanisms of edema that we have discussed so far show that the underfill and overfill hypotheses can be seen as “dynamic states” rather than a sole mechanism for each patient. A patient can have “underfill”, in other words, low intravascular volume initially if edema develops too fast or if subjected to volume losses (e.g. diarrhea). Blood volume can be stable despite hypoalbuminemia and the presence of edema. Patients can shift into an “overfill” i.e., volume expansion state if renal impairment is superimposed. Thus, when approaching a child with NS, it is important to view this as a quest to accurately estimate intravascular volume. The appropriate management relies on accurate determination of the intravascular volume.
Several tools can be used to assess intravascular volume. The first is the clinical assessment. Whenever there are signs of hypovolemia (volume contraction), it is advisable to use other complementary tools to assess the volume status of children (as outlined in Table 1).
Non-invasive techniques for volume assessment
-
Point of care echocardiography has been increasingly used to assess volume status both in the emergency room and ICU settings. Estimation of changes in cardiac output and stroke volume occurring 1 to 2 minutes after passive leg raising (PLR) is used to check for fluid responsiveness [55]. Several studies have applied this tool to childhood NS. An early study showed no difference in inferior vena cava (IVC) collapsibility index and left atrial diameter (LAD) in children with NS and different grades of edema despite the presence of elevated levels of aldosterone and renin initially [56]. LAD was found to be significantly lower in hypovolemic children with active INS in a more recent study [57]. In a third study, IVC diameter and LAD along with plasma renin and aldosterone were used to assess volume status in 19 patients with NS before treatment and while in remission. The results of the hormones and the echo parameters were not statistically significant [57]. More studies are needed in patients with NS to determine if echocardiography can be used in this population for intravascular volume assessment.
Bioelectrical impedance
Water in the extra and intracellular compartments contains ions. The conductance or resistance to different frequencies will depend on the composition of the various tissues. Single-frequency bioelectrical impedance analysis and Bioimpedance spectroscopy (BIS) have been used to estimate total body water and intracellular body water using mathematical models [58]. They were validated against the standard radio-isotope dilution methods [59]. BIS showed increased ECW in active NS compared to those in remission [60]. It should be noted that the provided data do not discern the different compartments of extracellular water.
Management of edema in nephrotic syndrome
Edema management in many cases is a temporizing measure to relieve symptoms. Steroids and other immunomodulators -which are utilized to induce remission and reduce proteinuria- are the most effective ways to control edema.
1- General measures when managing edema in children with NS
-
Maintaining activity and exercise as able will decrease the risk of thrombosis and help mobilize fluids.
-
Family education
Initial and ongoing family education on NS is critical. Regular monitoring of weight, blood pressure, and urine output at home is recommended. The managing physician should be notified if symptoms of palpitation or orthostatic dizziness develop. Children are at risk of infections, so fevers and fluid losses e.g., diarrhea or vomiting should also be reported.
-
Adjunctive therapies
In some conditions with persistent proteinuria despite immunosuppressive treatment or with persistent low-grade proteinuria, ACEi or ARBs are used to reduce proteinuria and so preserve the renal function in the long term. Regular safety monitoring after starting these drugs is recommended (urine output, creatinine, K). Though not directed specifically at edema management, they can help minimize edema by reducing proteinuria [57].
-
Avoiding nephrotoxic medications including NSAIDs as these medications not only predispose patients to the development of AKI (especially during intravascular depletion) but can also worsen fluid overload.
2- Diet
The first dietary modification recommended is salt restriction. The recommended salt intake for age is: 1–2 mEq/day for infants up to 6 months, 370 mg/day 6–12 months, 1 gm /day for children 1–3 years of age, 1.2 gm/day for 4–8 years and 1.5 gm/day for 9–18 years [62]. Children with NS are advised to eat 130–140% of the recommended daily protein intake for their age to make up for the urinary losses. Protein intake of 3-4gm/kg/day had been recommended in the past, however, it was noted to accelerate glomerular injury [63••]. The intake of foods rich in saturated fats and trans-fats should be reduced as they have been associated with inflammation. Carbohydrates should be in the form of starch or dextrin maltose but not sucrose as it increases lipid abnormalities [64]. Water restriction may only be needed in case of severe confirmed hyponatremia (< 125 meq/L) [64]. For those with persistent proteinuria, in addition to the initial dietary assessment, ongoing assessment should be done monthly in infants then every 3 months [65••]. For infants with congenital nephrosis, a high intake of calories (130 Kcal/kg/day) and proteins (4 g/kg/day) is recommended [65••]. In the presence of mild edema (< 7% weight gain), salt restriction might be enough [66••].
3- Medications
Medications targeted at edema or fluid management are only indicated in certain situations (see Table 1):
-
1-
Symptoms of significant hypovolemia (Intravascular volume depletion) e.g. tachycardia, hypotension, oliguria (which may denote developing AKI) especially if occurring after fluid losses or excessive use of diuretics.
-
2-
Symptoms of significant hypervolemia e.g. respiratory distress with physical findings of pulmonary edema, heart failure, and hypertension.
-
3-
Distressing edema: ascites impairing daily activities, severe scrotal or labial edema, edema interfering with mobility or sleep.
Diuretics (Table 2)
Diuretics are indicated in the presence of symptoms or evidence of hypervolemia. They can also be used for symptomatic management of edema if there is significant (>7%) weight gain. Before deciding to use a diuretic, providers should exclude intravascular depletion using the methods described above. The use of diuretics alone in patients with increased intravascular volume is safe, effective, can prevent readmissions and improve the quality of life [67]. Oral Furosemide may initially be used at a dose of 1–4 mg/kg/day along with salt restriction. Spironolactone 2–3 mg/kg/day or amiloride 0.2–0.5 mg/kg/day may be added if there is poor response to furosemide. If refractory edema is present, one alternative is adding another diuretic e.g., metolazone 0.2–0.4 mg/kg/day or hydrochlorothiazide 1–2 mg/kg/day or IV Furosemide 1–2 mg/kg/Q12 hours may be used. The other alternative, or if there is a poor response, is to administer IV albumin 20% (or 25%) 0.5–1 g/kg with IV furosemide 1–2 mg/kg/dose at the end of the infusion [66••].
For infants with congenital nephrosis, diuretics should be given with caution and stopped in case of anuria. In most cases, they can improve diuresis and allow the provision of adequate nutrition. Furosemide 2–5 mg/kg/day PO (max 10 mg/kg/day) is usually given in combination with a thiazide or a potassium-sparing diuretic (preferably amiloride) [65••].
Furosemide resistance is defined as an absence of diuresis 2-4 hours after an oral dose. This may be due to poor adherence to salt restriction, decreased bioavailability due to gut edema, severe hypoalbuminemia, hypovolemia, or salt reabsorption in the distal tubule. Furosemide is >90% albumin-bound, so in cases of severe hypoalbuminemia (< 2 g/dl) it diffuses into the tissues and fewer amounts are filtered through the glomerulus to reach their binding sites in the kidney tubules [68]. Furosemide is also bound to the albumin that is filtered which may decrease its activity, however, inhibition of this binding with sulfisoxazole did not show any benefit [69].
Adding a thiazide provides the benefit of “double block” given that their action is on the distal tubules.
Given the evidence that ENaC channels are activated during active NS, the use of amiloride may be beneficial. In mouse models, amiloride was found to decrease the sodium absorption from the collecting tubules regardless of the level of aldosterone [70]. Amiloride was noted to have an added benefit as an antiproteinuric. Adding Amiloride to ACEi and ARBs in a group of 14 children with SRNS reduced proteinuria [71]. However, another study in adults concluded that there was a reduction in proteinuria regardless of the class of diuretic added (Spironolactone vs HCTZ vs HCTZ + Amiloride) suggesting that the mechanism is probably related to a decrease in GFR [72].
The use of all diuretics- separately and more commonly in combination- carries the risks of causing hypovolemia, AKI, and electrolyte disturbances. (See Table 2)
Albumin
Patients with evidence of intravascular depletion and hypotension are managed with IV albumin 5% (10-15 ml/kg over 30-60 minutes). Another alternative if Albumin 5% is not available is to give IV saline. Albumin 20% or 25% 0.5-1 g/kg over 4 hours can be used after initial resuscitation [66••].
Patients with edema and weight gain >7% who are refractory to escalating therapy with diuretics are recommended to receive albumin 20% with furosemide as detailed above. A meta-analysis of 4 adult and pediatric studies comparing the use of albumin + furosemide vs furosemide alone revealed that more effective diuresis was achieved following the combination treatment. However, there was no change in natriuresis suggesting that albumin did not increase the efficacy of furosemide [73]. In another small adult study, there was a modest improvement in urine output when albumin was given alone [74]. This supports the revised Starling equation and the “no-absorption” rule where fluids escaping to the interstitium of tissues having continuous capillaries (muscles, lungs, connective tissues) are not pulled back to the intravascular compartment after the restoration of the oncotic pressure in that compartment. Diuretics are needed to achieve this mobilization [12].
In infants with congenital nephrosis, the practice varies between centers. While some centers only administer albumin when deemed clinically necessary, others use regular infusions (1-4 g/kg/day) to support growth, and psychomotor development and to maintain the intravascular volume [65••].
Adverse reactions to albumin infusions are usually mild and transient (e.g. fever, nausea). However, there is the risk of precipitating pulmonary edema, heart failure, or hypertension. This risk is higher if large doses are used, no diuretic is given or if there is renal impairment. Albumin should be given as a slow infusion over a few hours and under strict monitoring [72].
4- Isolated ultrafiltration
Patients with refractory edema may have fluid overload in the setting of kidney function impairment. In these cases, patients may require isolated ultrafiltration (UF) or dialysis [66••]. In infants and younger children with severe fluid overload with or without kidney function impairment, newer therapies for ultrafiltration can be considered. Such therapies including aquapheresis via the AquadexTM can allow gentle fluid removal in patients with severe edema, even in smaller infants [76]. The use of these techniques requires the placement of a central IV access. The possible risks of infections and clotting may need to be put into consideration. The utilization, effectiveness, and safety of such therapies and ultrafiltration devices in children with NS need further attention.
5- Others
Head-out water immersion was tried in the past to promote diuresis and natriuresis in patients with refractory edema. It is no longer used in clinical practice. Aquaretics have been studied to promote water diuresis in NS. Diuretics as well as other medications and investigational therapies are listed in Table 2.
Conclusions
Edema is one of the main symptoms of NS and it may be the primary concern for children and their parents. Regardless of the mechanism underlying edema formation in children with NS, assessment of the intravascular volume is essential. New investigational tools have been developed to help the clinician in this task. Besides treatment with steroids and/or other immunosuppressives, management directed at the edema per se may be needed in certain cases. The currently available options for targeted- management of edema include diuretics and albumin. Novel ultrafiltration therapies to treat fluid overload in children with NS are understudied.
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Selewski DT, Troost JP, Massengill SF, et al. The impact of disease duration on quality of life in children with nephrotic syndrome: a Midwest Pediatric Nephrology Consortium study. Pediatr Nephrol. 2015;30(9):1467–76. https://doi.org/10.1007/s00467-015-3074-x.
Gipson DS, Selewski DT, Massengill SF, Wickman L, Messer KL, Herreshoff E, Bowers C, Ferris ME, Mahan JD, Greenbaum LA, MacHardy J, Kapur G, Chand DH, Goebel J, Barletta GM, Geary D, Kershaw DB, Pan CG, Gbadegesin R, et al. Gaining the PROMIS perspective from children with nephrotic syndrome: a Midwest pediatric nephrology consortium study. Health Qual Life Outcomes. 2013 Mar;4(11):30. https://doi.org/10.1186/1477-7525-11-30.
Ellis D. Pathophysiology, evaluation, and management of edema in childhood nephrotic syndrome. Front Pediatr. 2015;3:111. https://doi.org/10.3389/fped.2015.00111.
Bockenhauer D. Over- or underfill: not all nephrotic states are created equal. Pediatr Nephrol. 2013;28:1153–6. https://doi.org/10.1007/s00467-013-2435-6.
Starling EH. On the absorption of fluids from the connective tissue spaces. J Physiol. 1896;19:312–26. https://doi.org/10.1113/jphysiol.1896.sp000596.
Deen WM, Bohrer MP, Brenner BM. Macromolecule transport across glomerular capillaries: application of pore theory. Kidney Int. 1979;16(3):353–65. https://doi.org/10.1038/ki.1979.138.
Curry FE, Michel CC. A fiber matrix model of capillary permeability. Microvasc Res. 1980;20:96–9. https://doi.org/10.1016/0026-2862(80)90024-2.
Michel CC. Filtration coefficients and osmotic reflexion coefficients of the walls of single frog mesenteric capillaries. J Physiol. 1980;309:341–55. https://doi.org/10.1113/jphysiol.1980.sp013512.
Alphonsus CS, Rodseth RN. The endothelial glycocalyx: a review of the vascular barrier. Anaesthesia. 2014;69:777–84. https://doi.org/10.1111/anae.12661.
Levick JR, Michel CC. Microvascular fluid exchange and the revised Starling principle. Cardiovasc Res. 2010;87:198–210.
Woodcock TE, Woodcock TM. Revised Starling equation and the glycocalyx model of transvascular fluid exchange: an improved paradigm for prescribing intravenous fluid therapy. British Journal of Anaesthesia. 2012;108(3):384–94. ISSN 0007-0912. https://doi.org/10.1093/bja/aer515.
Erstad BL. The revised starling equation: the debate of albumin versus crystalloids continues. Ann Pharmacother. 2020;54(9):921–7. https://doi.org/10.1177/1060028020907084.
Rippe B, Haraldsson B. Capillary permeability in rat hindquarters as determined by estimations of capillary reflection coefficients. Acta Physiol Scand. 1986 Jul;127(3):289–303. https://doi.org/10.1111/j.1748-1716.1986.tb07908.x.
Paguio VME, Kappel F, Kotanko P. A model of vascular refilling with inflammation. Mathematical Biosciences. 2018;303:101–14, ISSN 0025-5564. https://doi.org/10.1016/j.mbs.2018.06.007.
Kongstad L, Möller AD, Grände PO. Reflection coefficient for albumin and capillary fluid permeability in cat calf muscle after traumatic injury. Acta Physiol Scand. 1999;165(4):369–77. https://doi.org/10.1046/j.1365-201x.1999.00521.x.
Carter RD, Joyner WL, Renkin EM. Effects of histamine and some other substances on molecular selectivity of the capillary wall to plasma proteins and dextran. Microvasc Res. 1974;7:31–48.
Perrin RM, Harper SJ, Corrall R, et al. Hyperglycemia stimulates a sustained increase in hydraulic conductivity in vivo without any change in reflection coefficient. Microcirculation. 2007;14:683–96.
Baldwin AL, Wilson LM, Simon BR. Effect of pressure on aortic hydraulic conductance. Arterioscler Thromb. 1992;12:163–71.
Seal JB, Gewertz BL. Vascular dysfunction in ischemia reperfusion injury. Ann Vasc Surg. 2005;19:572–84.
Henry CB, Duling BR. TNF-alpha increases entry of macromolecules into luminal endothelial cell glycocalyx. Am J Physiol Heart Circ Physiol. 2000;279:2815–23.
Bruegger D, Jacob M, Rehm M, Loetsch M, Welsch U, Conzen P, Becker BF. Atrial natriuretic peptide induces shedding of endothelial glycocalyx in coronary vascular bed of guinea pig hearts. Am J Physiol Heart Circ Physiol. 2005;289:H1993–9.
Nieuwdorp M, Mooij HL, Kroon J, Atasever B, Spaan JA, Ince C, et al. Endothelial glycocalyx damage coincides with microalbuminuria in type 1 diabetes. Diabetes. 2006;55:1127–32.
Fauchald P, Noddeland H, J. Norseth Interstitial fluid volume, plasma-volume and colloid osmotic-pressure in patients with nephrotic syndrome. Scand J Clin Lab Invest. 1984;44:661–7.
Koomans HA, Geers AB, Dorhout Mees EJ, et al. Lowered tissue-fluid oncotic pressure protects the blood volume in the nephrotic syndrome. Nephron. 1986;42:317–22.
Aukland K, G. Nicolaysen Interstitial fluid volume: local regulatory mechanisms. Physiol Rev. 1981;61:556–643.
Aukland K, Reed RK. Interstitial-lymphatic mechanisms in the control of extracellular fluid volume. Physiol Rev. 1993 Jan;73(1):1–78. https://doi.org/10.1152/physrev.1993.73.1.1.
Ostgaard G, Reed RK. Increased lymphatic hyaluronan output and preserved hyaluronan content of the rat small intestine in prolonged hypoproteinaemia. Acta Physiol Scand. 1994 Sep;152(1):51–6. https://doi.org/10.1111/j.1748-1716.1994.tb09783.x.
Geers AB, Koomans HA, Roos JC, Evert J, Mees D. Preservation of blood volume during edema removal in nephrotic subjects. Kidney Int. 1985;28(4):652–7, ISSN 0085-2538. https://doi.org/10.1038/ki.1985.179.
Olmer M, Berland Y, Purgus R, Schultz G. Determination of blood volume in nephrotic patients. Am J Nephrol. 1989;9(3):211–4. https://doi.org/10.1159/000167967.
Vande Walle JG, Donckerwolcke RAMG, van Isselt JW, Joles JA, Koomans HA, Derkx FHM. Volume regulation in children with early relapse of minimal-change nephrosis with or without hypovolaemic symptoms. The Lancet. 1995;346(8968):148–52, ISSN 0140-6736. https://doi.org/10.1016/S0140-6736(95)91210-X.
Geers AB, Koomans HA, Boer P, Dorhout Mees EJ. Plasma and blood volumes in patients with the nephrotic syndrome. Nephron. 1984;38(3):170–3. https://doi.org/10.1159/000183302.
Manning RD Jr, A.C. Guyton Effects of hypoproteinemia on fluid volumes and arterial pressure. Am J Physiol. 1983;245:H284–93.
Yoo HD, Choi KS, Jung MH, et al. A study of the renin-angiotensin system and the blood volume in the nephrotic syndrome. Korean J Intern Med. 1986;1(1):72–7. https://doi.org/10.3904/kjim.1986.1.1.72.
Andersen RF, Nørgaard H, Hagstrøm S, Bjerre J, Jespersen B, Rittig S. High plasma aldosterone is associated with a risk of reversible decreased eGFR in childhood idiopathic nephrotic syndrome. Nephrol Dial Transplant. 2013 Apr;28(4):944–52. https://doi.org/10.1093/ndt/gfs527.
Vande Walle JG, Donckerwolcke RA, Koomans HA. Pathophysiology of edema formation in children with nephrotic syndrome not due to minimal change disease. .
J. Plum, Y. Mirzaian, B. Grabensee, Atrial natriuretic peptide, sodium retention, and proteinuria in nephrotic syndrome, Nephrol Dial Transplant, Volume 11, Issue 6, June 1996, Pages 1034–1042, https://doi.org/10.1093/ndt/11.6.1034.
Valentin JP, Qiu C, Muldowney WP, Ying WZ, Gardner DG, Humphreys MH. Cellular basis for blunted volume expansion natriuresis in experimental nephrotic syndrome. J Clin Invest. 1992;90:1302–12. https://doi.org/10.1172/JCI115995.
El-Halaby H, Bakr A, Eid R, Abdalla HA, Hamdy N, Shamekh N, Adel A, El-Husseiny A. Edema in childhood nephrotic syndrome: possible genes-hormones interplay. J Genet Eng Biotechnol. 2022;20(1):30. https://doi.org/10.1186/s43141-022-00310-x.
Polzin D, Kaminski HJ, Kastner C, Wang W, Krämer S, Gambaryan S, Russwurm M, Peters H, Wu Q, Vandewalle A, Bachmann S, Theilig F. Decreased renal corin expression contributes to sodium retention in proteinuric kidney diseases. Kidney Int. 2010;78:650–9.
Rascher W, Tulassay T. Hormonal regulation of water metabolism in children with nephrotic syndrome. Kidney Int Suppl. 1987 Aug;21:S83–9.
Pedersen EB, Danielsen H, Sørensen SS, Jespersen B. Renal water excretion before and after remission of nephrotic syndrome: relationship between free water clearance and kidney function, arginine vasopressin, angiotensin II and aldosterone in plasma before and after oral water loading. Clin Sci (Lond). 1986 Jul;71(1):97–104. https://doi.org/10.1042/cs0710097.
Usberti M, Federico S, Meccariello S, Cianciaruso B, Balletta M, Pecoraro C, Sacca L, Ungaro B, Pisanti N, Andreucci VE. Role of plasma vasopressin in the impairment of water excretion in nephrotic syndrome. Kidney Int. 1984 Feb;25(2):422–9. https://doi.org/10.1038/ki.1984.34.
Brovko M, Kozlovskaya L, Pulin A, et al. Low aquaporin-2 excretion in the nephrotic syndrome: an escape from the vasopressin regulating effect. Int J Nephrol Renovasc Dis. 2018;11:271–7. https://doi.org/10.2147/IJNRD.S177469.
Fernández-Llama P, Andrews P, Nielsen S, Ecelbarger CA, Knepper MA. Impaired aquaporin and urea transporter expression in rats with adriamycin-induced nephrotic syndrome. Kidney Int. 1998 May;53(5):1244–53. https://doi.org/10.1046/j.1523-1755.1998.00878.x.
Ichikawa I, Rennke HG, Hoyer JR, et al. Role for intrarenal mechanisms in the impaired salt excretion of experimental nephrotic syndrome. J Clin Invest. 1983;71:91–103.
Kim SW, Wang W, Nielsen J, Praetorius J, Kwon TH, Knepper MA, Frøkiaer J, Nielsen S. Increased expression and apical targeting of renal ENaC subunits in puromycin aminonucleoside-induced nephrotic syndrome in rats. Am J Physiol Renal Physiol. 2004 May;286(5):F922–35. https://doi.org/10.1152/ajprenal.00277.2003.
Deschenes G, Gonin S, Zolty E, et al. Increased synthesis and AVP unresponsiveness of Na, K-ATPase in collecting duct from nephrotic rats. J Am Soc Nephrol. 2001;12:2241–52.
Lourdel S, Loffing J, Favre G, Paulais M, Nissant A, Fakitsas P, Créminon C, Féraille E, Verrey F, Teulon J, Doucet A, Deschênes G. Hyperaldosteronemia and activation of the epithelial sodium channel are not required for sodium retention in puromycin-induced nephrosis. J Am Soc Nephrol. 2005;16(12):3642–50. https://doi.org/10.1681/ASN.2005040363.
Hughey RP, Mueller GM, Bruns JB, et al. Maturation of the epithelial Naþ channel involves proteolytic processing of the alpha- and gamma-subunits. J Biol Chem. 2003;278:37073–82.
Svenningsen P, Bistrup C, Friis UG, et al. Plasmin in nephrotic urine activates the epithelial sodium channel. J Am Soc Nephrol. 2009;20:299–310.
Passero CJ, Mueller GM, Rondon-Berrios H, et al. Plasmin activates epithelial Naþ channels by cleaving the gamma subunit. J Biol Chem. 2008;283:36586–91.
Ray EC, Miller RG, Demko JE, et al. Urinary plasmin(ogen) as a prognostic factor for hypertension. Kidney Int Rep. 2018;3(6):1434–42. https://doi.org/10.1016/j.ekir.2018.06.007.
Chen JL, Wang L, Yao XM, Zang YJ, Wang Y, Li ZJ, Pearce D, Wang H. Association of urinary plasminogen-plasmin with edema and epithelial sodium channel activation in patients with nephrotic syndrome. Am J Nephrol. 2019;50(2):92–104. https://doi.org/10.1159/000501059.
Brown EA, Markandu ND, Sagnella GA, Jones BE, MacGregor GA. Lack of effect of captopril on the sodium retention of the nephrotic syndrome. Nephron. 1984;37(1):43–8. https://doi.org/10.1159/000183206.
Boyd JH, Sirounis D, Maizel J, Slama M. Echocardiography as a guide for fluid management. Crit Care. 2016;20(1):274. https://doi.org/10.1186/s13054-016-1407-1.
Dönmez O, Mir S, Özyürek R, et al. Inferior vena cava indices determine volume load in minimal lesion nephrotic syndrome. Pediatr Nephrol. 2001;16:251–5. https://doi.org/10.1007/s004670000536.
Büyükavci MA, Çivilibal M, Elevli M, Selçuk Duru HN. Hypo- and hypervolemic edema in children with steroid sensitive nephrotic syndrome. Turk J Med Sci. 2015;45(1):178–83.
Earthman C, Traughber D, Dobratz J, Howell W. Bioimpedance spectroscopy for clinical assessment of fluid distribution and body cell mass. Nutr Clin Pract. 2007 Aug;22(4):389–405. https://doi.org/10.1177/0115426507022004389.
Jaffrin MY, Morel H. Body fluid volumes measurements by impedance: A review of bioimpedance spectroscopy (BIS) and bioimpedance analysis (BIA) methods. Med Eng Phys. 2008 Dec;30(10):1257–69. https://doi.org/10.1016/j.medengphy.2008.06.009.
Brantlov S, Jødal L, Frydensbjerg Andersen R, Lange A, Rittig S, Ward LC. Bioimpedance Resistance Indices and Cell Membrane Capacitance Used to Assess Disease Status and Cell Membrane Integrity in Children with Nephrotic Syndrome. ScientificWorldJournal. 2019;2019:4274856. https://doi.org/10.1155/2019/4274856.
Cadnapaphornchai MA, Tkachenko O, Shchekochikhin D, et al. The nephrotic syndrome: pathogenesis and treatment of edema formation and secondary complications. Pediatr Nephrol. 2014;29:1159–67. https://doi.org/10.1007/s00467-013-2567-8.
National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Food and Nutrition Board; Committee to Review the Dietary Reference Intakes. Appendix J. Dietary reference intakes summary tables. In: Oria M, Harrison M, Stallings VA, editors. Dietary reference intakes for sodium and potassium. Washington, DC: National Academies Press (US); 2019.
•• Hampson KJ, Gay ML, Band ME. Pediatric nephrotic syndrome: pharmacologic and nutrition management. Nutr Clin Pract. 2021;36(2):331–43. https://doi.org/10.1002/ncp.10622An updated review article that focuses on the nutritional requirements and pharmacological management in nephrotic syndrome patients.
Patrick Niaudet and Olivia Boyer. Avner ED et al. (eds.), Idiopathic childhood nephrotic syndrome. Pediatric nephrology, Springer-Verlag Berlin Heidelberg 2016. https://doi.org/10.1007/978-3-662-43596-0_24.
•• Boyer O, Schaefer F, Haffner D, Bockenhauer D, Hölttä T, Bérody S, Webb H, Heselden M, Lipska-Zie Tkiewicz BS, Ozaltin F, Levtchenko E, Vivarelli M. Management of congenital nephrotic syndrome: consensus recommendations of the ERKNet-ESPN Working Group. Nat Rev Nephrol. 2021;17(4):277–89. https://doi.org/10.1038/s41581-020-00384-1 Erratum in: Nat Rev Nephrol. 2021 Jun;17(6):434. These are the consensus recommendations of the ERKNet-ESPN Working Group that were Published in the Nat Rev Neph in 2021. These provide detailed up-to-date recommendations about the management of patients with congenital NS.
•• Sinha A, Bagga A, Banerjee S, Mishra K, Mehta A, Agarwal I, Uthup S, Saha A, Mishra OP. Expert Group of Indian Society of Pediatric Nephrology. Steroid sensitive nephrotic syndrome: revised guidelines. Indian Pediatr. 2021;58(5):461–81. https://doi.org/10.1007/s13312-021-2217-3These are the guidelines of the Indian society of pediatric nephrology. They provide a stepwise approach to the management of childhood NS including the targeted management of edema.
Kapur G, Valentini RP, Imam AA, Mattoo TK. Treatment of severe edema in children with nephrotic syndrome with diuretics alone--a prospective study. Clin J Am Soc Nephrol. 2009;4(5):907–913. https://doi.org/10.2215/CJN.04390808.
Duffy M, Jain S, Harrell N, Kothari N, Reddi AS. Albumin and furosemide combination for management of edema in nephrotic syndrome: a review of clinical studies. Cells. 2015;4(4):622–30. https://doi.org/10.3390/cells4040622.
Agarwal R, Gorski JC, Sundblad K, Brater DC. Urinary protein binding does not affect response to furosemide in patients with nephrotic syndrome. J Am Soc Nephrol. 2000 Jun;11(6):1100–5. https://doi.org/10.1681/ASN.V1161100.
Deschênes G, Wittner M, Stefano AD, Jounier S, Doucet A. Collecting duct is a site of sodium retention in PAN nephrosis: a rationale for amiloride therapy. J Am Soc Nephrol. 2001b;12:598–601.
Liern M, Colazo A, Vallejo G, Zotta E. Antiproteinuric action of amiloride in paediatric patient with corticoresistant nephrotic syndrome. Nefrologia (Engl Ed). 2021;41(3):304-310. English, Spanish. https://doi.org/10.1016/j.nefro.2020.11.014.
Morales E, Caro J, Gutierrez E, Sevillano A, Auñón P, Fernandez C, Praga M. Diverse diuretics regimens differentially enhance the antialbuminuric effect of renin-angiotensin blockers in patients with chronic kidney disease. Kidney Int. 2015 Dec;88(6):1434–41. https://doi.org/10.1038/ki.2015.249.
Hedin E, Bijelić V, Barrowman N, Geier P. Furosemide and albumin for the treatment of nephrotic edema: a systematic review. Pediatr Nephrol. 2022. https://doi.org/10.1007/s00467-021-05358-4.
Ghafari A, Mehdizadeh A, Alavi-Darazam I, Rahimi E, Kargar C, Sepehrvand N. Co-administration of albumin-furosemide in patients with the nephrotic syndrome. Saudi J Kidney Dis Transpl. 2011 May;22(3):471–5.
•• Kallash M, Mahan JD. Mechanisms and management of edema in pediatric nephrotic syndrome. Pediatr Nephrol. 2021;36(7):1719–30. https://doi.org/10.1007/s00467-020-04779-xA review article that explores the mechanisms of edema formation in children with NS. It gives recommendations about the approach and specific management of edema. It compiles a list of all known management strategies and therapies.
Menon S, Broderick J, Munshi R, Dill L, DePaoli B, Fathallah-Shaykh S, Claes D, Goldstein SL, Askenazi DJ. Kidney support in children using an ultrafiltration device: a multicenter, retrospective study. Clin J Am Soc Nephrol. 2019;14(10):1432–40. https://doi.org/10.2215/CJN.03240319.
Matsumoto H, Miyaoka Y, Okada T, Nagaoka Y, Wada T, Gondo A, Esaki S, Hayashi A, Nakao T. Ratio of urinary potassium to urinary sodium and the potassium and edema status in nephrotic syndrome. Intern Med. 2011;50(6):551–5. https://doi.org/10.2169/internalmedicine.50.4537.
Pharmacologic Treatment of Hypertension. Bryan Williams, Megan Borkum. Comprehensive clinical nephrology. Feehally J et al. Elsevier 2019.
Costello JM, Almodovar MC. Emergency care for infants and children with acute cardiac disease. Clin Pediatr Emerg Med. 2007;8(3):145–55.
Carpenter RJ, Kouyoumjian S, Moromisato DY, Lieu P, Amirnovin R. Lower-dose, intravenous chlorothiazide is an effective adjunct diuretic to furosemide following pediatric cardiac surgery. J Pediatr Pharmacol Ther. 2020;25(1):31–8. https://doi.org/10.5863/1551-6776-25.1.31.
Giefer MJ, Murray KF, Colletti RB. Pathophysiology, diagnosis, and management of pediatric ascites. J Pediatr Gastroenterol Nutr. 2011;52(5):503–13.
Van der Vorst MM, Kist JE, van der Heijden AJ, et al. Diuretics in pediatrics: current knowledge and future prospects. Paediatr Drugs. 2006;8(4):245–64.
Shimizu M, Ishikawa S, Yachi Y, Muraoka M, Tasaki Y, Iwasaki H, Kuroda M, Ohta K, Yachie A. Tolvaptan therapy for massive edema in a patient with nephrotic syndrome. Pediatr Nephrol. 2014 May;29(5):915–7. https://doi.org/10.1007/s00467-013-2687-1.
Saimiya M, Kaku Y, Nishimura M. Efficacy of oral tolvaptan for severe edema and hyponatremia in a patient with refractory nephrotic syndrome. CEN Case Rep. 2021;10(4):523–6. https://doi.org/10.1007/s13730-021-00601-1.
Meena J, Hari P, Sinha A, Bagga A. Efficacy and safety of combination therapy with tolvaptan and furosemide in children with nephrotic syndrome and refractory edema: a prospective interventional study. Indian J Pediatr. 2022. https://doi.org/10.1007/s12098-021-03988-y.
Kamiya M, Sato N, Matsuda J, Nozaki A, Akiya M, Sato T, Okazaki H, Takahashi Y, Shimizu W. Predictors of responders for low-dose carperitide monotherapy in patients with acute heart failure. Heart Vessels. 2020 Jan;35(1):59–68. https://doi.org/10.1007/s00380-019-01450-w.
Docherty KF, Vaduganathan M, Solomon SD, McMurray JJV. Sacubitril/Valsartan: Neprilysin Inhibition 5 Years After PARADIGM-HF. JACC Heart Fail. 2020;8(10):800–10. https://doi.org/10.1016/j.jchf.2020.06.020 Erratum in: JACC Heart Fail. 2020 Dec;8(12):1057.
Boeing T, da Silva LM, Mariott M, Andrade SF, de Souza P. Diuretic and natriuretic effect of luteolin in normotensive and hypertensive rats: Role of muscarinic acetylcholine receptors. Pharmacol Rep. 2017 Dec;69(6):1121–4. https://doi.org/10.1016/j.pharep.2017.05.010.
Yao C, Anderson MO, Zhang J, Yang B, Phuan PW, Verkman AS. Triazolothienopyrimidine inhibitors of urea transporter UT-B reduce urine concentration. J Am Soc Nephrol. 2012;23:1210–20.
Teoh CW, Robinson LA, Noone D. Perspectives on edema in childhood nephrotic syndrome. Am J Physiol Renal Physiol. 2015;309(7):F575–82. https://doi.org/10.1152/ajprenal.00229.2015.
Liantonio A, Gramegna G, Camerino GM, Dinardo MM, Scaramuzzi A, Potenza MA, Montagnani M, Procino G, Lasorsa DR, Mastrofrancesco L, Laghezza A, Fracchiolla G, Loiodice F, Perrone MG, Lopedota A, Conte S, Penza R, Valenti G, Svelto M, Camerino DC. In-vivo administration of CLC-K kidney chloride channels inhibitors increases water diuresis in rats: a new drug target for hypertension? J Hypertens. 2012;30:153–67.
Ashek A, Menzies RI, Mullins LJ, et al. Activation of thiazide-sensitive co-transport by angiotensin II in the cyp1a1-Ren2 hypertensive rat. PLoS ONE. 2012;7:e36311.
Dong N, Chen S, Wang W, Zhou Y, Wu Q. Corin in clinical laboratory diagnostics. Clin Chim Acta. 2012;413(3-4):378–83. https://doi.org/10.1016/j.cca.2011.10.032.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zeid, A., Mohamed, T. & Kallash, M. Management of edema in pediatric nephrotic syndrome – Underfill or overfill?. Curr Pediatr Rep 10, 182–194 (2022). https://doi.org/10.1007/s40124-022-00270-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40124-022-00270-3