Abstract
Studies of human nephron number have been conducted for well over a century and have uncovered a large variability in nephron number. However, the mechanisms influencing nephron endowment and loss, along with the etiology for the wide range among individuals are largely unknown. Advances in imaging technology have allowed investigators to revisit the principles of renal structure and physiology and their roles in the progression of kidney disease. Here, we will review the latest data on the influences impacting nephron number, innovations made over the last 6 years to understand and integrate renal structure and function, and new developments in the tools used to count nephrons in vivo.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
“The human kidney manufactures the kind of urine that it does, and it maintains the blood in the composition which that fluid has because this kidney has a certain functional architecture; and it owes that architecture not to design or foresight or to any plan, but to the fact that the earth is an unstable sphere with a fragile crust, to the geologic revolutions that for six hundred million years have raised and lowered continents and seas, to the predaceous enemies, and heat and cold, and storms and droughts; to the unending succession of vicissitudes that have driven the mutant vertebrates from seas into fresh water, into desiccated swamps, out upon the dry land, from one habitation to another, perpetually in search of the free and independent life, perpetually failing, for one reason or another, to find it.” - Homer W. Smith [1].
The structure and function of the human kidney are interdependent, each adapting to endogenous and environmental stresses to maintain homeostasis over a lifetime. Advances in imaging technology allow us to revisit the principles of renal structure and physiology and their roles in the progression of kidney disease. In 2014, a review described the perinatal factors that influence nephron endowment and emerging techniques to count nephrons [2]. Since then, these technologies have rapidly advanced. Here, we will review the innovations made over the last 6 years to understand variability in human nephron number and new developments in the tools used to count nephrons in vivo.
Variability in nephron number
An individual’s nephron endowment has a significant impact on their future lifetime risk for kidney disease. Autopsy studies in humans have consistently revealed a large range in nephron number [3,4,5] from 210,000 to 2,700,000. Table 1 provides a summary of historic and contemporary studies using several techniques to evaluate nephron number. Figure 1 shows glomerular number across the life course of humans, using available data from the studies in Table 1.
Nephron number in healthy humans (a) and stratified by sex (b) and race (c). Using individual data available from the cross-sectional autopsy studies in Table 1, glomerular number is variable at birth and throughout life but appears to decline over time (a). For panel a, data were included from the following studies: Moore [78], Hayman [79], Moritz [80], Nyengaard [3], Keller [4], Dunnill [81], and Lenihan [86] were included. When stratified by sex (b), the reduced glomerular number may be more prominent in aging females as compared with males. Data from the studies conducted by Moore [78], Hayman [79], Moritz [79], Nyengaard [3], Keller [4], and Lenihan [86] were included. A limited subset of data was available in the above studies to stratify by race, but the trend of lower glomerular number in aging appears to apply across races. Data were included from Moore [78] and Lenihan [86]. As the techniques to detect glomerular number in vivo improve, the influence of aging, sex, and ethnicity on glomerular number will be answered
Nephron endowment is fixed in humans at full-term birth [6]. Nephron endowment is influenced by perinatal, genetic, and environmental factors [2, 7]. Although variability in human nephron number has been observed for well over a century, the reasons for the wide range in number between individuals are largely unknown. In a recent review [8], Chevalier argues that the variability in nephron number and the prevalence of CKD may be due to adaptation to natural selective pressure [9]. In response to intrauterine stress, the fetus adapts to reduced energy supplies by poor somatic growth, favoring early survival. In the kidney, the trade-off is reduced nephron number, leading to a higher risk for chronic kidney disease (CKD) at a post-reproductive age [10].
The duration of nephrogenesis may influence nephron endowment. This has been studied at autopsy [11,12,13]. Ryan and colleagues [14] investigated fetal human kidney samples from 71 infants aged 20–41 weeks. The investigators restricted their cohort to healthy fetuses, excluding those with intrauterine growth restriction and chorioamnionitis [15], conditions that disrupt or decrease the duration of nephrogenesis. Consistent with earlier literature [6, 12], the nephrogenic zone was present in all fetuses before 35 weeks of gestation. Interestingly, three subjects had nephrogenic zones at 35, 36, and 37 weeks. Mature glomeruli were also counted in glomerular generations—the number of layers of glomeruli from the capsule to the medulla. Number of generations ranged from 7 to 10 per kidney, consistent with previous studies [12]. Small differences in number of glomerular generations may be clinically relevant. The duration of nephrogenesis, which varies between individuals, may also affect nephron number.
Nephrogenesis primarily occurs in utero in humans. Maternal protein restriction [16,17,18], iron deficiency [19], and vitamin A deficiency [20] have all been implicated in altered fetal nephrogenesis. A recent report maternal fasting for 16 h per day demonstrated the importance of fetal programming in nephrogenesis [21]. Rat dams were restricted from food and water for 16 h; the majority occurred overnight during the animal’s active period. The animals received food and water ad libitum for the remainder of the day. The kidneys of the offspring of the fasting maternal rats had more connective tissue, fewer medullary rays, and one third fewer glomeruli. The timing and connection between fasting and reduced nephron number require further investigation [21].
Although human nephrogenesis is usually complete prior to birth, modern neonatal intensive care units have significantly reduced the mortality of the extremely preterm population but consequently subjected these neonates to ex utero organogenesis. The effects of preterm birth on kidney development and risk for future CKD have not been sufficiently studied. It is clear from limited human autopsy studies that glomerulogenesis can occur in a postnatal environment [12, 22]. Yet the large normal variation in human nephron endowment, cumulative perinatal ex utero exposures, and later comorbidities such as obesity, diabetes, along with hypertension, all play a role in the development of CKD in the preterm population. Large epidemiologic studies [23, 24] and smaller single center studies [25,26,27,28] have demonstrated the association between preterm birth and CKD, but no studies to date have examined nephron number past the perinatal period. The relationships between preterm birth, acute kidney injury, and the risk for CKD have been recently reviewed [29].
Acute kidney injury represents a condition in which nephrons can be lost, but AKI may also influence ongoing nephron development, important in preterm neonates [12]. Several notable reports have originated from a large, retrospective, multi-institutional study, the Assessment of Worldwide Acute Kidney injury Epidemiology in Neonates (AWAKEN) [30]. The AWAKEN dataset has confirmed many of the smaller, single center studies, which demonstrate that neonatal acute kidney injury (AKI) is common and is associated with poor short-term outcomes [31]. The AWAKEN study showed that AKI was particularly common in the most preterm cohort (48% in gestational ages of 22–28 weeks) and was associated with intraventricular hemorrhage [32] and bronchopulmonary dysplasia [33]. However, some limitations of the AWAKEN study include its retrospective nature, widely variable data collection of creatinine measurements, and a lack of follow-up. In preclinical work, Menendez-Castro and colleagues [34] hypothesized that nephron loss during nephrogenesis would lead to more severe renal consequences than loss after nephrogenesis. They described early morphologic changes, such as larger glomerular area and cell number, in animal models of uninephrectomy. Neonatal nephron loss during nephrogenesis led to more severe histologic damage in the glomeruli and tubules in aged animals. This study provides evidence that preterm infants with a lower nephron endowment may be at higher risk for kidney damage.
Other animal models have been studied to understand the short-term effects of preterm birth and associated life-sustaining therapies on the kidney. The preterm baboon model [35] is particularly suited for kidney research due to its long gestational period, completion of nephrogenesis prior to birth, and a body size that allows for clinically relevant therapies. Callaway et al. [36] recently evaluated the influence of hyperglycemia on nephrogenesis in preterm baboons. Preterm baboons were exposed to hyperglycemia or maintained normoglycemic for the first 5 days after birth, and kidneys were evaluated at 2–4 weeks. The width of the nephrogenic zone of the hyperglycemic baboons exhibited a reduced nephrogenic zone, more mature glomeruli, and evidence of increased oxidative stress compared with normoglycemic baboons. Research needs to be focused on the effect of therapies on nephron development in the preterm neonate to preserve the short window of nephrogenesis.
Nephron loss
The number of functional glomeruli decreases with age. This was first described by Arataki in 1926 [37], who observed a 66% reduction in nephron number by 500 days of age when compared with the average adult rat. Nyengaard et al. [3] observed a similar reduction in glomerular number in humans over 60 years of age. For 19 of the 37 Danish subjects who were > 60 years of age, the median glomerular number was 554,000 (IQR 428,000–608,000) compared with 680,000 (IQR 610,800–827,800) for subjects < 60 years of age. Kanzaki and colleagues [38] have reported a similar glomerular number (666,140 ± 159,775) in a cohort of normotensive Japanese males. Using individual published data, Fig. 1 shows glomerular number which is lower in older individuals and may decline with age; a finding was more significant in females (Fig. 1b). Further work is necessary to determine the role of sex in the relationship between glomerular number, sex, race (Fig. 1c), and aging.
The mechanisms of glomerular loss are poorly understood. Ischemic injury likely leads to glomerulosclerosis, tubular atrophy, and probably reabsorption of the nephron [39]. While nephron loss with aging has been well-documented, the focus on glomerulosclerosis may underestimate the magnitude of this overall glomerular loss. Denic et al. [40] estimated glomerular number using cortical volume acquired by CT scans, and glomerular density was estimated by renal biopsy (described below) in 1638 living kidney donor candidates of various ages. The average number of nonsclerotic glomeruli was significantly reduced in the older age group (70–75 years) compared with the youngest age group (18–29 years). The average number of globally sclerotic glomeruli was significantly increased in the older group compared with the younger group. These differences were proportional to a lower glomerular filtration rate (GFR) in the older group [40]. They concluded that almost 50% of nephrons are lost between young adulthood and old age [40]. The researchers in this group have cleverly used the transplant donor evaluation to estimate glomerular number in the largest cohort of healthy humans, and they will have the ability to answer important questions as this cohort is followed.
Nephron loss likely induces a cascade of structural and functional changes in the remaining nephrons. Most notably, decreased nephron number is associated with significant tubular hypertrophy and an increase in the volume of remaining glomeruli [41, 42]. While nephron number is inversely correlated with glomerular size, average glomerular size is also correlated with body size and can be larger in certain racial groups [43]. These anatomic changes reflect functional changes. As nephrons are lost, single-nephron GFR increases, a process termed glomerular hyperfiltration. Kanzaki et al. [38] concluded that an increase in glomerular size or glomerular hypertrophy may be a significant factor in maintaining GFR in those with low nephron number. Advancements in imaging may prompt further evaluation of glomerular hypertrophy. Cationic ferritin-enhanced-MRI (CFE-MRI, described below) was used to evaluate glomerular number and size in the oligosyndyctalism (Os/+) mouse [44], a model of oligomeganephronia with glomerular hypertrophy as early as 9 days after birth [45]. Unexpectedly, there was no difference in measured glomerular volumes between the Os/+ mice and controls in vivo. However, glomerular volumes measured ex vivo of the Os/+ kidneys were significantly larger than in controls. Although glomerular size may be an early marker of low nephron number, glomerular size and distensibility need to be further evaluated.
Obesity may be an independent risk factor for kidney disease [46]. In a cohort of living kidney donors, several indices of obesity correlated with increased glomerular volume on biopsy. These indices included visceral fat, waist circumference, subcutaneous fat, and pararenal and perihilar fat [47]. Body mass index (BMI) had the highest correlation with glomerular volume. Yim and colleagues [48] reported an association between early overnutrition and decreased glomerular number in male rats. At 12 months of age, rats that received overnutrition for the first 21 days of life had approximately one third fewer glomeruli compared with controls. These rats did not remain obese, suggesting that the effects of obesity on young kidneys may persist despite the normalization of BMI [48].
Several autopsy studies have described a relationship between low nephron number and hypertension [38]. In a cohort of hypertensive patients with mild to moderate glomerulosclerosis, glomerular hypertrophy was a primary component of hypertensive nephropathy [49]. However, glomerular number was not lower in hypertensive patients in general. This contradicts Keller et al. [4], who reported that German subjects with hypertension had an average of 700,000 glomeruli (530,000–950,000) compared with normotensive age-matched controls (1,400,000 (800,000-2,000,000)). The Kanzaki study [38] examined the kidney function of Japanese subjects separated into three age-matched elderly groups—normotensive subjects, hypertensive subjects, and subjects with CKD. The hypertensive subjects in the Japanese study had fewer nephrons than the normotensive group with significantly higher single-nephron GFR. Using several studies where individual data was available, Fig. 2 shows the relationship between glomerular number as compared with their age in both hypertensive patients and those with renal disease. The inability to repeatedly assess nephron number in the same patient or even in the same animal has made it impossible to determine the relationship of hypertension and nephron number. Does hypertension develop in those with low nephron number or does hypertension cause progressive glomerular loss? This question requires longitudinal in vivo tracking of individual glomeruli within the whole kidney.
Nephron number in humans with hypertension (a) and renal disease (b). Few studies provided individual data on subjects with hypertension (a) and those studies that did report had a narrow age range beginning near 40 years. From this data, there appears to be significantly fewer glomeruli in the older subjects with hypertension. Data from hypertensive subjects were included from studies conducted by Keller [4] and Lenihan [86]. In subjects with renal disease (mainly Bright’s disease with chronic glomerulonephritis and cardiac disease), there is no correlation with nephron number and age. Data from subjects with CKD were included from studies conducted by Hayman [79] and Moritz [80]
The impact of race and ethnicity on nephron number is difficult to assess in autopsy studies (Fig. 2c). African Americans exhibit higher rates of CKD and hypertension, but the mechanisms are still under investigation. While studies have also demonstrated increased glomerular volume in African Americans compared with Caucasian subjects, it is unclear whether this finding is associated with glomerulosclerosis or decreased nephron number [49]. One evident factor is the presence of genetic variants of APOL1 that may increase the risk for kidney disease in African Americans [50]. In a study of African American subjects without evidence of kidney disease at autopsy, there was a trend toward a higher glomerular number in early adulthood in males with two APOL1 high-risk alleles. Notably, the same high-risk genetic profile was associated with a lower glomerular number and larger glomerular volume over time. These changes were enhanced by increased BMI but did not appear to be associated with coexisting hypertension. This suggests that a high-risk APOL1 genotype could predispose patients to earlier or more rapid nephron loss [50]. Further work is necessary to determine if nephron “loss” is occurring on an individual level and what mechanisms may be involved.
Measuring form and function in the kidney: lessons and limitations
The first explorations of nephron function in isolated, perfused organs provided a fundamental understanding of kidney filtration, removed from systemic circulation. Studies involving micropuncture of individual nephrons [51] have further revealed factors that affect filtration in the single nephron. Optical microscopy has been used in specific animal models in vivo to view vascular and tubular flow and filtration in real time [52]. More recently, X-ray computed tomography (CT) has been used after injection of an intravascular contrast agent to provide high-resolution imaging of the renal vasculature and glomeruli ex vivo [53]. Optical clearing is another recently developed method that has made it possible to image every nephron in the intact kidney with superior optical microscopic tissue penetration depths compared with previously available methods [54, 55]. This can be used to image and detect nephron loss in the entire kidney of small animals ex vivo.
In the clinic, there are few diagnostic tools for early detection of kidney disease. Functional metrics of kidney health have been the standard method for the detection of CKD for decades. Metrics such as GFR often remain within a normal range as the kidney adapts to the progression of kidney disease. Currently accepted structural metrics of the kidney include total kidney volume and microstructural data acquired through a single, randomly acquired biopsy; neither of these are robust surrogates for nephron number [56, 57].
CKD is associated with having too few nephrons to maintain homeostasis. Low nephron number at birth and conditions that lead to nephron loss have been associated with hypertension, and evidence of CKD is detectable even in childhood [23, 58]. Despite the acceptance that nephron number is an important metric of kidney health, there are no direct, noninvasive methods to measure nephron number in humans. The majority of information about nephron number has been derived from two methodologies to measure glomerular number and size—the physical dissector-fractionator combination—and maceration where the kidney is digested to separate the glomeruli from the tubules [59,60,61]. The need for clinical translation motivates investigators to search for clinically applicable surrogates for nephron number.
New ways to count nephrons
Several groups have begun to establish nondestructive, whole-kidney assessments of nephron number and have made significant progress over the last 5 years. One such technique uses the product of glomerular density and cortical volume to estimate nephron number [40, 62]. Glomerular density is determined by kidney biopsy using the Weibel Gomez stereological formula, accounting for sclerotic and nonsclerotic glomeruli and cortex volume that is determined by CT or MRI scans. Investigators obtained biopsy and imaging data on a large number of patients. Several studies of the clinical risk factors and associated outcomes following transplant [63] have been conducted, and a great deal of knowledge has been derived using this method [64].
Cationic ferritin-enhanced MRI (CFE-MRI, Fig. 3) has been proposed to provide a direct measurement of nephron number [44, 65,66,67,68], and early studies indicated that CFE-MRI could also detect nephrons in vivo [69,70,71]. Recently, CFE-MRI was applied in vivo in rats and mice to detect and measure individual nephrons throughout the entire kidney [44, 65]. Importantly, CFE-MRI [44] was also used to longitudinally track nephrons, potentially facilitating a wide range of new investigations in disease models. To date, cationic ferritin appears to be nontoxic for use in vivo in MRI-detectable doses [72, 73], suggesting a broader role for CFE-MRI in preclinical, clinical, and translational studies.
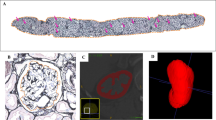
CFE-MRI in ex vivo human, rat, and mouse kidneys. Human kidneys deemed unsuitable for transplant were injected with CF and scanned ex vivo both sagittal (a–c) and axial views are provided (d–f) [66]. Each black dot is an individual glomerulus (column 1) and the glomeruli are highlighted using this red overlay (column 2). The glomeruli can be rendered in 3D (column 3). CFE-MRI has been applied for use in rats (panels g–i) [87] and mice (panels j–l) [67]
It is critical to detect pathology before progression to fibrosis or end-stage renal disease. There have been several demonstrations of pathology detected using CFE-MRI. These include early focal and segmental glomerular sclerosis (FSGS) in rats [69], glomerular hypertrophy and nephron loss in mice [67], and altered nephron development in a rabbit model of acute kidney injury [68]. Similar pathologies have been detected by CFE-MRI in human donor kidneys [66].
These first reports of direct measurement of glomerular number (Nglom) and apparent glomerular volume (aVglom) in vivo have highlighted several technical challenges. These include challenges that can affect precision and accuracy in the measurement of Nglom and aVglom and can vary based on the chosen disease models.
Image resolution, sensitivity, and acquisition time
In CFE-MRI, image resolution is established by the MRI hardware and software. It is typical to prescribe an image resolution that allows the distinction of individual glomeruli in the image. The required resolution in CFE-MRI is lower in kidneys that have larger glomeruli or lower glomerular density. The sensitivity of MRI is determined by the image resolution, magnetic field strength, radiofrequency (RF) coil sensitivity, and properties of the contrast agent, among other factors. For in vivo imaging, motion must be minimized and the RF coil must be built to ensure sufficient signal to noise ratio over the entire imaging volume.
In most in vivo experiments, it is important to minimize image acquisition time. Physiologic changes can occur over short time intervals. Acquisition time is determined by the imaging pulse sequence, the prescribed image resolution, and the required signal-to-noise ratio. Most studies using CFE-MRI have employed a 3D gradient-recalled echo pulse sequence. Full 3D images of mouse kidneys in vivo were acquired within ~ 2 h at a resolution of 55 × 55 × 100 μm. 3D images of rat kidneys were acquired within ~ 1.5 h at a resolution of 74 × 74 × 190 μm [65]. There are numerous ways to decrease image acquisition time, such as sparse acquisition of the MRI data and the use of faster pulse sequences, and modified contrast agents [71]. These strategies will likely be explored as the technique is further refined, but are critical to the general application of CFE-MRI to preclinical and clinical imaging, in vivo.
Quantitative image processing
Detection of individual CF-labeled glomeruli in 3D MR images has required the development of novel image processing strategies [74,75,76]. These strategies have focused on reducing the influence of noise in large datasets and the ability of software to identify true CF-labeled glomeruli against image background and measure glomerular number. While challenging, the detection of each CF-labeled glomerulus has been achieved and validated in many studies. Accurately, measuring glomerular volume is more challenging, to be addressed through emerging tools for image processing. Nonetheless, existing approaches can be used to detect relative differences between healthy and diseased cohorts, and they appear to accurately reflect the intrarenal distribution of glomerular volumes.
Outlook over the next 10 years
Over the past decade, a framework has been developed to enable a wide range of preclinical and clinical investigations of the kidney at the level of the single nephron. One exciting possibility is beginning to understand the relationship between nephron number and renal physiology, and their roles in disease progression. This new technology also provides the opportunity to understand the determinants of nephron endowment. Over the next decade, these tools will continue to be refined and applied to a number of new animal models and, potentially, in humans.
Key summary points
-
1.
Nephron number is highly variable in humans even as early as just after birth.
-
2.
There are unique factors that influence nephron endowment and loss, and those born preterm may be at high risk for chronic kidney disease due to altered nephrogenesis and nephron loss.
-
3.
Several new radiologic tools are being developed to count and measure the size of glomeruli with the aim of translation of this new technology to patients to diagnose and treat those with chronic kidney disease in an earlier phase.
Questions
-
1)
Which of the following statements regarding human nephrogenesis is true?
-
a)
Postnatal nephrogenesis is common and can occur for several weeks after full-term gestation.
-
b)
Nephrogenesis can be initiated after injury.
-
c)
There is a wide range of nephron number within and among various healthy populations.
-
d)
The population-wide variability in nephron number is only influenced by aging factors.
-
a)
-
2)
Nephron number inversely correlates with aging in which populations?
-
a)
Males
-
b)
Females
-
c)
Hypertensive
-
d)
Renal disease
-
e)
b and c
-
f)
a and d
-
a)
-
3)
Which of the following nephron number counting techniques has been used to estimate or whole count whole kidney nephron number in living patients?
-
a)
Acid maceration method
-
b)
Fractionator–disector technique
-
c)
Cationic ferritin-enhanced MRI
-
d)
Glomerular density by biopsy and cortical volume
-
e)
None of the above
-
a)
-
4)
Infants born at 24 weeks are at risk for decreased nephron number due to:
-
a)
Exposure to nephrotoxic medications
-
b)
Hyperglycemia
-
c)
Oxygen alterations
-
d)
All of the above
-
a)
-
5)
The parents of a 1-year-old child born with 2 copies of the high-risk APOL1 genes ask about preserving his nephrons and decreasing their son’s long-term risk of chronic kidney disease. Each of the following would be sound recommendations EXCEPT:
-
a)
Avoid nephrotoxic medications
-
b)
Begin vitamin A supplementation
-
c)
Encourage appropriate postnatal weight gain and avoidance of obesity
-
d)
Treat hypertension
-
a)
References
Smith HW (1953) From fish to philosopher. Little, Boston
Charlton JR, Springsteen CH, Carmody JB (2014) Nephron number and its determinants in early life: a primer. Pediatr Nephrol 29:2299–2308
Nyengaard JR, Bendtsen TF (1992) Glomerular number and size in relation to age, kidney weight, and body surface in normal man. Anat Rec 232:194–201
Keller G, Zimmer G, Mall G, Ritz E, Amann K (2003) Nephron number in patients with primary hypertension. N Engl J Med 348:101–108
Bertram JF, Douglas-Denton RN, Diouf B, Hughson MD, Hoy WE (2011) Human nephron number: implications for health and disease. Pediatr Nephrol 26:1529–1533
Osathanondh V, Potter E (1963) Development of human kidney as shown by microdissection. III Formation and interrelationship of collecting tubules and nephrons. Arch Pathol 76:290–302
Merlet-Benichou C, Gilbert T, Vilar J, Moreau E, Freund N, Lelievre-Pegorier M (1999) Nephron number: variability is the rule: causes and consequences. Lab Investig 79:515–527
Chevalier RL (2017) Evolutionary nephrology. Kidney Int Rep 2:302–317
Darwin C (1859) [Title page of on the origin of species]
Straub RH, Schradin C (2016) Chronic inflammatory systemic diseases: an evolutionary trade-off between acutely beneficial but chronically harmful programs. Evol Med Public Health 2016:37–51
Hinchliffe SA, Sargent PH, Howard CV, Chan YF, van Velzen D (1991) Human intrauterine renal growth expressed in absolute number of glomeruli assessed by the disector method and Cavalieri principle. Lab Invest 64:777–784
Rodriguez MM, Gomez AH, Abitbol CL, Chandar JJ, Duara S, Zilleruelo GE (2004) Histomorphometric analysis of postnatal glomerulogenesis in extremely preterm infants. Pediatr Devel Pathol 7:17–25
Faa G, Gerosa C, Fanni D, Nemolato S, Locci A, Cabras T, Marinelli V, Puddu M, Zaffanello M, Monga G, Fanos V (2010) Marked interindividual variability in renal maturation of preterm infants: lessons from autopsy. J Matern-Fetal Neo M 23(Suppl 3):129–133
Ryan D, Sutherland MR, Flores TJ, Kent AL, Dahlstrom JE, Puelles VG, Bertram JF, McMahon AP, Little MH, Moore L, Black MJ (2018) Development of the human fetal kidney from mid to late gestation in male and female infants. EBioMedicine 27:275–283
Galinsky R, Hooper SB, Polglase GR, Moss TJ (2013) Intrauterine inflammation alters fetal cardiopulmonary and cerebral haemodynamics in sheep. J Physiol 591:5061–5070
Welham SJ, Wade A, Woolf AS (2002) Protein restriction in pregnancy is associated with increased apoptosis of mesenchymal cells at the start of rat metanephrogenesis. Kidney Int 61:1231–1242
Woods LL, Weeks DA, Rasch R (2004) Programming of adult blood pressure by maternal protein restriction: role of nephrogenesis. Kidney Int 65:1339–1348
Hoppe CC, Evans RG, Bertram JF, Moritz KM (2007) Effects of dietary protein restriction on nephron number in the mouse. Am J Phys Regul Integr Comp Phys 292:R1768–R1774
Lisle SJ, Lewis RM, Petry CJ, Ozanne SE, Hales CN, Forhead AJ (2003) Effect of maternal iron restriction during pregnancy on renal morphology in the adult rat offspring. Br J Nutr 90:33–39
Lelievre-Pegorier M, Vilar J, Ferrier ML, Moreau E, Freund N, Gilbert T, Merlet-Benichou C (1998) Mild vitamin A deficiency leads to inborn nephron deficit in the rat. Kidney Int 54:1455–1462
Mohany M, Ashton N, Harrath AH, Nyengaard JR, Alomar SY, Alwasel S (2018) A new model for fetal programming: maternal Ramadan-type fasting programs nephrogenesis. J Dev Orig Health Dis 9:287–298
Sutherland MR, Gubhaju L, Moore L, Kent AL, Dahlstrom JE, Horne RS, Hoy WE, Bertram JF, Black MJ (2011) Accelerated maturation and abnormal morphology in the preterm neonatal kidney. J Am Soc Nephrol 22:1365–1374
Hsu CW, Yamamoto KT, Henry RK, De Roos AJ, Flynn JT (2014) Prenatal risk factors for childhood CKD. Journal of the American Society of Nephrology: Jasn 25(9):2105–2111. https://doi.org/10.1681/ASN.2013060582
Crump C, Sundquist J, Winkleby MA, Sundquist K (2019) Preterm birth and risk of chronic kidney disease from childhood into mid-adulthood: national cohort study. BMJ 365:l1346
Kwinta P, Klimek M, Drozdz D, Grudzien A, Jagla M, Zasada M, Pietrzyk JJ (2011) Assessment of long-term renal complications in extremely low birth weight children. Pediatr Nephrol 26:1095–1103
Nishizaki N, Hirano D, Nishizaki Y, Fujinaga S, Nagata S, Ohtomo Y, Kaneko K, Shimizu T (2014) Increased urinary angiotensinogen is an effective marker of chronic renal impairment in very low birth weight children. Clin Exp Nephrol 18:642–648
Harer MW, Pope CF, Conaway MR, Charlton JR (2017) Follow-up of acute kidney injury in neonates during childhood years (FANCY): a prospective cohort study. Pediatr Nephrol 32:1067–1076
Bruel A, Roze JC, Quere MP, Flamant C, Boivin M, Roussey-Kesler G, Allain-Launay E (2016) Renal outcome in children born preterm with neonatal acute renal failure: IRENEO-a prospective controlled study. Pediatr Nephrol 31:2365–2373
Selewski DT, Charlton JR, Jetton JG, Guillet R, Mhanna MJ, Askenazi DJ, Kent AL (2015) Neonatal acute kidney injury. Pediatrics 136:e463–e473
Jetton JG, Guillet R, Askenazi DJ, Dill L, Jacobs J, Kent AL, Selewski DT, Abitbol CL, Kaskel FJ, Mhanna MJ, Ambalavanan N, Charlton JR, Neonatal Kidney C (2016) Assessment of worldwide acute kidney injury epidemiology in neonates: design of a retrospective cohort study. Front Pediatr 4:68
Jetton JG, Boohaker LJ, Sethi SK, Wazir S, Rohatgi S, Soranno DE, Chishti AS, Woroniecki R, Mammen C, Swanson JR, Sridhar S, Wong CS, Kupferman JC, Griffin RL, Askenazi DJ (2017) Incidence and outcomes of neonatal acute kidney injury (AWAKEN): a multicentre, multinational, observational cohort study. Lancet Child Adolesc Health 1:184–194
Stoops C, Boohaker L, Sims B, Griffin R, Selewski DT, Askenazi D, on behalf of the National Kidney C (2019) The association of intraventricular hemorrhage and acute kidney injury in premature infants from the Assessment of the Worldwide Acute Kidney Injury Epidemiology in Neonates (AWAKEN) Study. Neonatology 116:321–330
Starr MC, Boohaker L, Eldredge LC, Menon S, Griffin R, Mayock DE, Li L, Askenazi D, Hingorani S, Neonatal Kidney C (2019) Acute kidney injury and bronchopulmonary dysplasia in premature neonates born less than 32 weeks’ gestation. Am J Perinatol
Menendez-Castro C, Nitz D, Cordasic N, Jordan J, Bauerle T, Fahlbusch FB, Rascher W, Hilgers KF, Hartner A (2018) Neonatal nephron loss during active nephrogenesis-detrimental impact with long-term renal consequences. Sci Rep 8:4542
Gubhaju L, Sutherland MR, Yoder BA, Zulli A, Bertram JF, Black MJ (2009) Is nephrogenesis affected by preterm birth? Studies in a non-human primate model. Am J Physiol Renal Physiol 297:F1668–F1677
Callaway DA, McGill-Vargas LL, Quinn A, Jordan JL, Winter LA, Anzueto D, Dick EJ Jr, Blanco CL (2018) Prematurity disrupts glomeruli development, whereas prematurity and hyperglycemia lead to altered nephron maturation and increased oxidative stress in newborn baboons. Pediatr Res 83:702–711
Arataki M (1926) On the postnatal growth of the kidney, with special reference to the number and size of glomeruli (albino rat). Am J Anat 36:399–463
Kanzaki G, Puelles VG, Cullen-McEwen LA, Hoy WE, Okabayashi Y, Tsuboi N, Shimizu A, Denton KM, Hughson MD, Yokoo T, Bertram JF (2017) New insights on glomerular hyperfiltration: a Japanese autopsy study. JCI insight 2. https://doi.org/10.1172/jci.insight.94334
Denic A, Glassock RJ, Rule AD (2016) Structural and functional changes with the aging kidney. Adv Chronic Kidney Dis 23:19–28
Denic A, Lieske JC, Chakkera HA, Poggio ED, Alexander MP, Singh P, Kremers WK, Lerman LO, Rule AD (2017) The substantial loss of nephrons in healthy human kidneys with aging. J Am Soc Nephrol 28:313–320
Pabico RC, McKenna BA, Freeman RB (1975) Renal function before and after unilateral nephrectomy in renal donors. Kidney Int 8:166–175
Hayslett JP, Kashgarian M, Epstein FH (1968) Functional correlates of compensatory renal hypertrophy. J Clin Invest 47:774–799
Hoy WE, Bertram JF, Denton RD, Zimanyi M, Samuel T, Hughson MD (2008) Nephron number, glomerular volume, renal disease and hypertension. Curr Opin Nephrol Hypertens 17:258–265
Baldelomar EJ, Charlton JR, deRonde KA, Bennett KM (2019) In vivo measurement of kidney glomerular number and size in healthy and Os(/+) mice using MRI. Am J Physiol Renal Physiol
Sergio M, Galarreta CI, Thornhill BA, Forbes MS, Chevalier RL (2015) The fate of nephrons in congenital obstructive nephropathy: adult recovery is limited by nephron number despite early release of obstruction. J Urol
Chagnac A, Zingerman B, Rozen-Zvi B, Herman-Edelstein M (2019) Consequences of glomerular hyperfiltration: the role of physical forces in the pathogenesis of chronic kidney disease in diabetes and obesity. Nephron 143:38–42
Chakkera HA, Chang YH, Thomas LF, Avula RT, Amer H, Lerman LO, Denic A, Rule AD (2015) Obesity correlates with glomerulomegaly but is not associated with kidney dysfunction early after donation. Transplant Direct 1:1–6
Yim HE, Yoo KH, Bae IS, Hong YS, Lee JW (2014) Postnatal early overnutrition causes long-term renal decline in aging male rats. Pediatr Res 75:259–265
Hughson MD, Puelles VG, Hoy WE, Douglas-Denton RN, Mott SA, Bertram JF (2014) Hypertension, glomerular hypertrophy and nephrosclerosis: the effect of race. Nephrol Dial Transplant 29:1399–1409
Hoy WE, Hughson MD, Kopp JB, Mott SA, Bertram JF, Winkler CA (2015) APOL1 risk alleles are associated with exaggerated age-related changes in glomerular number and volume in African-American adults: an autopsy study. J Am Soc Nephrol 26:3179–3189
Gottschalk CW, Mylle M (1956) Micropuncture study of pressures in proximal tubules and peritubular capillaries of the rat kidney and their relation to ureteral and renal venous pressures. Am J Phys 185:430–439
Yu W, Sandoval RM, Molitoris BA (2007) Rapid determination of renal filtration function using an optical ratiometric imaging approach. Am J Physiol Renal Physiol 292:F1873–F1880
Xie L, Layton AT, Wang N, Larson PE, Zhang JL, Lee VS, Liu C, Johnson GA (2016) Dynamic contrast-enhanced quantitative susceptibility mapping with ultrashort echo time MRI for evaluating renal function. Am J Physiol Renal Physiol 310:F174–F182
Chung K, Deisseroth K (2013) CLARITY for mapping the nervous system. Nat Methods 10:508–513
Klingberg A, Hasenberg A, Ludwig-Portugall I, Medyukhina A, Mann L, Brenzel A, Engel DR, Figge MT, Kurts C, Gunzer M (2017) Fully automated evaluation of total glomerular number and capillary tuft size in nephritic kidneys using lightsheet microscopy. J Am Soc Nephrol 28:452–459
Tsuboi N, Kanzaki G, Koike K, Kawamura T, Ogura M, Yokoo T (2014) Clinicopathological assessment of the nephron number. Clin Kidney J 7:107–114
Bueters RR, van de Kar NC, Schreuder MF (2013) Adult renal size is not a suitable marker for nephron numbers: an individual patient data meta-analysis. Kidney Blood Press Res 37:540–546
Calderon-Margalit R, Skorecki K, Vivante A (2018) History of childhood kidney disease and risk of adult end-stage renal disease. N Engl J Med 378:1751–1752
Bertram JF (2013) Estimating glomerular number: why we do it and how. Clin Exp Pharmacol Physiol 40:785–788
Bertram JF (2001) Counting in the kidney. Kidney Int 59:792–796
Bertram JF, Cullen-McEwen LA, Egan GF, Gretz N, Baldelomar E, Beeman SC, Bennett KM (2014) Why and how we determine nephron number. Pediatr Nephrol 29:575–580
Fulladosa X, Moreso F, Narvaez JA, Grinyo JM, Seron D (2003) Estimation of total glomerular number in stable renal transplants. J Am Soc Nephrol 14:2662–2668
Issa N, Vaughan LE, Denic A, Kremers WK, Chakkera HA, Park WD, Matas AJ, Taler SJ, Stegall MD, Augustine JJ, Rule AD (2019) Larger nephron size, low nephron number, and nephrosclerosis on biopsy as predictors of kidney function after donating a kidney. Am J Transplant 19:1989–1998
Sasaki T, Tsuboi N, Kanzaki G, Haruhara K, Okabayashi Y, Koike K, Kobayashi A, Yamamoto I, Ogura M, Hoy WE, Bertram JF, Shimizu A, Yokoo T (2019) Biopsy-based estimation of total nephron number in Japanese living kidney donors. Clin Exp Nephrol 23:629–637
Baldelomar EJ, Charlton JR, Beeman SC, Bennett KM (2017) Measuring rat kidney glomerular number and size in vivo with MRI. Am J Physiol Renal Physiol. https://doi.org/10.1152/ajprenal.00399.2017
Beeman SC, Cullen-McEwen LA, Puelles VG, Zhang M, Wu T, Baldelomar EJ, Dowling J, Charlton JR, Forbes MS, Ng A, Wu QZ, Armitage JA, Egan GF, Bertram JF, Bennett KM (2014) MRI-based glomerular morphology and pathology in whole human kidneys. Am J Physiol Renal Physiol 306:F1381–F1390
Baldelomar EJ, Charlton JR, Beeman SC, Hann BD, Cullen-McEwen L, Pearl VM, Bertram JF, Wu T, Zhang M, Bennett KM (2015) Phenotyping by magnetic resonance imaging nondestructively measures glomerular number and volume distribution in mice with and without nephron reduction. Kidney Int
Charlton JR, Baldelomar EJ, deRonde K, Cathro HP, Charlton NP, Criswell S, Hyatt D, Nam S, Pearl V, Bennett KM (2019) Nephron loss detected by MRI following neonatal acute kidney injury in rabbits. Pediatr Res
Bennett KM, Zhou H, Sumner JP, Dodd SJ, Bouraoud N, Doi K, Star RA, Koretsky AP (2008) MRI of the basement membrane using charged nanoparticles as contrast agents. Magn Reson Med 60:564–574
Qian C, Yu X, Chen DY, Dodd S, Bouraoud N, Pothayee N, Chen Y, Beeman S, Bennett K, Murphy-Boesch J, Koretsky A (2013) Wireless amplified nuclear MR detector (WAND) for high-spatial-resolution MR imaging of internal organs: preclinical demonstration in a rodent model. Radiology 268:228–236
Clavijo Jordan MV, Beeman SC, Baldelomar EJ, Bennett KM (2014) Disruptive chemical doping in a ferritin-based iron oxide nanoparticle to decrease r2 and enhance detection with T1-weighted MRI. Contrast Media Mol Imaging 9:323–332
Charlton JR, Pearl VM, Denotti AR, Lee JB, Swaminathan S, Scindia YM, Charlton NP, Baldelomar EJ, Beeman SC, Bennett KM (2016) Biocompatibility of ferritin-based nanoparticles as targeted MRI contrast agents. Nanomed-Nanotechnol 12(6):1735–45. https://doi.org/10.1016/j.nano.2016.03.007
Beeman SC, Georges JF, Bennett KM (2013) Toxicity, biodistribution, and ex vivo MRI detection of intravenously injected cationized ferritin. Magn Reson Med 69:853–861
Zhang M, Wu T, Beeman S, Cullen-McEwen L, Bertram J, Charlton J, Baldelomar E, Bennett K (2015) Efficient small blob detection based on local convexity, intensity and shape information. IEEE Trans Med Imaging
Zhang M, Wu T, Bennett K (2014) Small blob identification in medical images using regional features from optimum scale. IEEE Trans Biomed Eng
Xu Y, Wu T, Gao F, Charlton JR, Bennett KM (2020) Improved small blob detection in 3D images using jointly constrained deep learning and Hessian analysis. Sci Rep 10:326
Vimtrup BJ (1928) Of the number, shape, structure and surface are of the glomeruli in the kidneys of man and mammals. Am J Anat 41(1):123–151
Moore RA (1931) The total number of glomeruli in the normal human kidney. Anat Rec 48:153–168
Hayman JM, Johnston SM (1933) Experiments on the relation of creatinine and urea clearance tests of kidney function and the number of glomeruli in the human kidney obtained at autopsy. J Clin Invest 12:877–884
Moritz AR, Hayman JM (1934) The disappearance of glomeruli in chronic kidney disease. Am J Pathol 10:505–518.503
Dunnill MS, Halley W (1973) Some observations on the quantitative anatomy of the kidney. J Pathol 110:113–121
McLachlan MS, Guthrie JC, Anderson CK, Fulker MJ (1977) Vascular and glomerular changes in the ageing kidney. J Pathol 121:65–78
Zhang Z, Quinlan J, Hoy W, Hughson MD, Lemire M, Hudson T, Hueber PA, Benjamin A, Roy A, Pascuet E, Goodyer M, Raju C, Houghton F, Bertram J, Goodyer P (2008) A common RET variant is associated with reduced newborn kidney size and function. Am Soc Nephrol 19:2027–2034
Tan JC, Busque S, Workeneh B, Ho B, Derby G, Blouch KL, Sommer FG, Edwards B, Myers BD (2010) Effects of aging on glomerular function and number in living kidney donors. Kidney Int 78:686–692
Lenihan CR, Busque S, Derby G, Blouch K, Myers BD, Tan JC (2015) Longitudinal study of living kidney donor glomerular dynamics after nephrectomy. J Clin Invest 125:1311–1318
Lenihan CR, Busque S, Derby G, Blouch K, Myers BD, Tan JC (2015) The association of predonation hypertension with glomerular function and number in older living kidney donors. J Am Soc Nephrol 26:1261–1267
Beeman SC, Zhang M, Gubhaju L, Wu T, Bertram JF, Frakes DH, Cherry BR, Bennett KM (2011) Measuring glomerular number and size in perfused kidneys using MRI. Am J Physiol Renal Physiol 300:F1454–F1457
Acknowledgments
We thank Neda Parvin for creating Fig. 3.
Funding
JRC and KMB are funded by grants from the National Institutes of Health—National Institute of Diabetes and Digestive and Kidney Diseases (R01DK111861 (JRC and KMB), R01DK110622 (JRC and KMB), and U34DK117128 (JRC)).
Author information
Authors and Affiliations
Contributions
All authors have participated in the concept, design, drafting, and revising of this manuscript. All authors have approved the manuscript as submitted here.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Answers: 1. c; 2. e; 3. d; 4. d; 5. b
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Charlton, J.R., Baldelomar, E.J., Hyatt, D.M. et al. Nephron number and its determinants: a 2020 update. Pediatr Nephrol 36, 797–807 (2021). https://doi.org/10.1007/s00467-020-04534-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-020-04534-2